Abstract
Data on the association between −1607 1G > 2G polymorphism in the promoter region of matrix metalloproteinase-1 (MMP1) and nasopharyngeal carcinoma (NPC) are conflicting. The aim of this study was to confirm whether this polymorphism was a causative factor of NPC. We searched PubMed, Embase, and China National Knowledge Infrastructure (CNKI) for studies on the present topic. A total of four publications (1,044 NPC patients and 1,284 healthy control subjects) were included and meta-analysis was performed to assess the association between −1607 1G > 2G polymorphism and NPC risk. Odds ratio (OR) with 95 % confidence interval (95 % CI) was calculated for 1G1G versus 2G2G, 1G1G + 1G2G versus 2G2G, 1G1G versus 1G2G + 2G2G, 1G versus 2G, and 1G2G versus 2G2G contrast models. Meta-analysis results showed significantly reduced risk of NPC associated with the 1G1G versus 2G2G, 1G versus 2G and 1G2G versus 2G2G contrast models (OR = 0.61, 95 % CI 0.49–0.77; OR = 0.78, 95 % CI 0.65–0.92; OR = 0.86, 95 % CI 0.74–0.99, respectively). When we continued to perform subgroup analysis by ethnicity, the significant association persisted in Asian population and was most pronounced under the 1G2G versus 2G2G model (OR = 0.85, 95 % CI 0.73–0.99). These data suggested that MMP1 −1607 1G > 2G polymorphism was associated with reduced risk of NPC, particularly in the population of Asian descent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a generally rare cancer worldwide, with the incidence rate of 1 per 100,000 people each year. Southern China, nevertheless, is suffering an obviously higher prevalence rate. The average incidence increases to 30 per 100,000 people [1, 2]. Environmental factors, such as Epstein-Barr virus, tobacco use, dietary habits, and occupational exposure to poisonous chemicals accelerate the development of NPC [3], but only a small fraction of individuals who are exposed to these factors develop NPC, implicating an effective role of genetic susceptibility in this cancer. Familial aggregation is a remarkable epidemiological feature of NPC. There is sufficient evidence for a positive association between high-incidence families and high-incidence [4–6].
Matrix metalloproteinase-1 (MMP1) is a member of MMP family that is characterized by breaking down extracellular matrix components and basement membranes, and facilitating cell invasion and metastasis [7]. MMP1 affects many stages of tumorigenesis, promoting tumor growth through stimulating cellular proliferation, invasion and migration, angiogenesis, and suppressing tumor cells apoptosis [8–10]. Molecular and biochemical expression of MMP1 are relevant to clinical outcomes of numerous cancers. High expression could lead to a poor prognosis of colorectal cancer, head and neck squamous cell cancer and lung cancer [11–13]. Single nucleotide polymorphisms (SNPs) in the promoter region serve as mediators for MMP1 expression. 1G/2G polymorphic site at the 1,607 locus is a common deletion/insertion polymorphism. MMP1 expression can be up-regulated by insertion of −1607 2G, consequently resulting in increased cancer risk [14–17].
Recently, −1607 1G > 2G polymorphism (rs1799750) within the promoter region of MMP1 has been extensively investigated in the field of NPC, but reported with controversial results [18, 19]. Most importantly, as far as we know, no previous meta-analysis has reported the association of −1607 1G > 2G polymorphism and NPC. In view of these problems, we performed a meta-analysis to confirm whether this polymorphism was a causative factor of NPC.
Materials and Methods
Publication Search
To cover all publications on the relationship between −1607 1G > 2G polymorphism and NPC risk, two investigators systematically searched
-
PubMed (http://www.ncbi.nlm.nih.gov/pubmed).
-
China National Knowledge Infrastructure (CNKI, http://www.cnki.net/) using the keywords: ‘‘nasopharyngeal carcinoma,’’ ‘‘NPC,’’ ‘‘Matrix metalloproteinase-1,’’ ‘‘MMP1,’ ‘‘polymorphism,’’ “polymorphisms,” “genotypes,” and “variants.” We updated the last search on February, 2014. All eligible studies were retrieved and their bibliographies were manually screened for additional relevant publications.
Inclusion Criteria
Selection of studies eligible for the current meta-analysis was based on
-
(a)
Publication date was prior to December, 2013;
-
(b)
The association of −1607 1G > 2G polymorphism and NPC risk must be addressed;
-
(c)
Healthy unrelated subjects were selected as control population;
-
(d)
The author must publish allele and genotype frequency that could estimate the risk of NPC.
When the same case series was repeatedly studied in more than one publication, the one with more complete data was considered.
Data Extraction
A standardized form according to the inclusion criteria was designed before data extraction, which was performed by two investigators independently. Information gathered from the studies included (a) last name of the first author; (b) publication year; (c) study country; (d) ethnicity; (f) matching criteria; (g) source of controls; and (h) genotype frequencies of −1607 1G > 2G polymorphism. In a case that a single study contained two or more independent case–control populations, we retrieved them separately and categorized them into their own ethnic groups.
Statistical Analysis
In order to demonstrate if the studies deviated from Hardy–Weinberg equilibrium (HWE), we evaluated HWE for the control populations using the goodness-of-fit χ 2 test. Association strength of −1607 1G > 2G polymorphism and NPC risk was measured by an odds ratio (OR) with 95 % confidence interval (95 % CI). The ORs were calculated for 1G1G versus 2G2G, 1G1G + 1G2G versus 2G2G, 1G1G versus 1G2G + 2G2G, 1G versus 2G, and 1G2G versus 2G2G contrast models. Heterogeneity assumption was evaluated by the χ 2-based Q test and I 2 index [20, 21], with a P value above 0.10 or I 2 < 50 % being considered non-significant. To assess the effective size for each study, the fixed-effect model (the Mantel-Haenszel method) was employed if the studies were homogeneous [22]. Otherwise, the ORs were summarized using the random-effect model (the DerSimonian and Laird method) [23]. To further detect the heterogeneity, subgroup analyses were performed by ethnicity. Stability of the combined results was determined by performing sensitivity analysis. Publication bias in the literature was estimated by funnel plot and Egger’s linear regression test [24]. Asymmetric funnel plots or P values of Egger’s test below 0.10 were suggestive of significant publication bias. STATA version 12.0 software (Stata Corporation, College Station, TX) was used to deal with all statistical data. All tests were two-sided and P < 0.10 was deemed statistically significant.
Results
Characteristics of the Included Studies
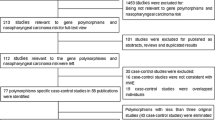
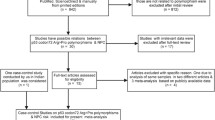
We initially retrieved 42 publications matching the search terms. We then verified their eligibility according to the pre-described inclusion criteria, and finally selected 4 full-text articles with complete data [18, 19, 25, 26]. The selection process is described in Fig. 1.
Table 1 summarizes the characteristics of the four publications, including all extracted information listed previously (data extraction). In the studies of Kondo et al. [25] and Zhou et al. [19], the genotype frequencies were completely presented for two independent case–control populations; thus, our meta-analysis included six case–control studies involving 1 044 NPC patients and 1,284 healthy control subjects. All of these studies were carried out in Asian countries except for Nasr et al. [18], who initiated their study in Africa. This was the only study deviating from HWE.
Meta-Analysis Results
We first compared the minor allele (1G) frequency between patients and controls. The 1G allele frequency was 0.26 in cancer patients, which was lower compared to the control subjects (0.35). Main meta-analysis results are shown in Table 2. As obvious heterogeneity was observed in part of the contrast models, both the fixed-effect model and the random-effect model were performed for pooled ORs. Overall, we observed significantly reduced NPC risk related to −1607 1G > 2G polymorphism under the 1G1G versus 2G2G, 1G versus 2G and 1G2G versus 2G2G contrast models (OR = 0.61, 95 % CI 0.49–0.77; OR = 0.78, 95 % CI 0.65–0.92; OR = 0.86, 95 % CI 0.74–0.99, respectively) (Fig. 2).
Forest plot of nasopharyngeal carcinoma (NPC) risk associated with MMP1 −1607 1G > 2G polymorphism stratified by ethnicity under 1G1G versus 2G2G model. The boxes and horizontal lines represent the OR and the corresponding 95 % CI. The area of the boxes indicates the weight (inverse of the variance). The diamond correspond to the summary OR and 95 % CI. Significant association between MMP1 −1607 1G > 2G polymorphism and NPC risk was observed
Since data were sufficiently provided for Asians, we then evaluated the effects in subgroup of Asian population and obtained an OR of 0.63 under the 1G1G versus 2G2G contrast model (OR = 0.63, 95 % CI 0.49–0.80) (Fig. 2). The results also provided an OR of 0.77 for the 1G versus 2G contrast model (OR = 0.77, 95 % CI 0.62–0.95) and 0.85 for the 1G2G versus 2G2G contrast model (OR = 0.85, 95 % CI 0.73–0.99).
We observed obvious heterogeneity between studies in overall comparisons and subgroup analyses (Table 2). Sensitivity analysis was subsequently carried out and identified the Guangxi population studied by Zhou et al. [19] as the origin. The heterogeneity decreased drastically when we removed the study from meta-analysis. In addition, we found that the primary ORs were significantly altered (data not shown).
Publication Bias
By performing Begg’s test and Egger’s test, we evaluated publication bias across studies. Neither asymmetry was indicated in the funnel plots nor the P values of Egger’s test less than 0.10 were observed, indicating no significant publication bias in this meta-analysis (P > 0.10). Figure 3 shows the funnel plot for the 1G1G versus 2G2G contrast model.
Discussion
It is well established that NPC susceptibility is determined by environmental factors. For example, a meta-analysis of six case–control studies associated preserved vegetable consumption in adulthood with NPC risk, and suggested 2.04 fold higher risk compared to the lowest intake. Conversely, fresh vegetables intake decreased the risk of NPC by nearly 40 % [27]. However, the etiology of NPC is still incompletely understood. In the past decade, much attention has been directed to the research of host genetic factors and initiation of NPC. A number of susceptibility genes, such as Interleukin-18, NFKB1, Cyclin D1, and HSP70-2, have been investigated across different populations and recognized as risk factors [28–31]. Therefore, identification of candidate genes may facilitate an extended understanding of pathogenesis of this malignancy.
Several population-based, clinical, and physiological studies have investigated the relation of −1607 1G > 2G polymorphism and NPC risk. Evidence from these studies, however, failed to suggest a definitive role. Nasr et al. investigated the MMP1 polymorphism in 174 patients with NPC and 171 healthy control subjects, and found a significantly increased risk of NPC associated with the 2G2G genotype [18]. Afterward, a replication study conducted by Zhou et al. who genotyped two larger independent case–control populations (593 patients with NPC and 480 controls; 239 patients and 286 controls, respectively) reported no association [19]. Several possible factors may cause the discordance, such as insufficient sample size, diverse genetic backgrounds, and different laboratory methods used for each study.
In the present study, we summarized all data from four research publications and performed a meta-analysis in an attempt to examine the relationship between −1607 1G > 2G polymorphism in the promoter region of the MMP1 gene with the risk of NPC. The overall comparisons showed obviously reduced risk of NPC in the carriage of 1G1G genotype or 1G2G genotype or 1G allele compared to 2G2G genotype and 2G allele. When we continued to perform subgroup analysis by ethnicity, the significant association persisted in Asian population. A recent meta-analysis of head and neck cancer risk detected significant increased risk associated with −1607 1G > 2G polymorphism [32]. Head and neck cancer comprises multiple types of tumors, and NPC is one of them. The etiology of NPC is polygenic in nature and a single genetic polymorphism is typically inadequate to predict the risk of the malignancy. Moreover, the present sample limits us to derive a precise estimate; hence, the association remains to be further verified.
In our meta-analysis, we included four research publications involving six independent case–control studies. But the insufficient sample for overall comparisons as well as subgroup analysis may lack statistical power to precisely detect the association. Besides, heterogeneity was observed between studies. Even though heterogeneity was drastically deceased when eliminating the source, the original combined effects were significantly changed. Also, we did not assess the impact of gene–gene or gene-environment interactions on the development of NPC, because no usable data were allowed for extraction. So now we look forward to performing larger studies to confirm the association in the near future.
To draw a conclusion, this meta-analysis suggested that −1607 1G > 2G polymorphism within the promoter region of MMP1 could reduce the risk of NPC in the population of Asian descent. Additional research in thousands of subjects with various ethnicities is warranted to further validate the findings in the present study.
References
Parkin, D. M., Whelan, S., Ferlay, J., Teppo, L., Thomas, D. B., et al. (2002). Cancer incidence in five continents, IARC Scientific Publications No. 155 (Vol. III). Lyon: IARC.
Cao, S. M., Simons, M. J., & Qian, C. N. (2011). The prevalence and prevention of nasopharyngeal carcinoma in China. Chinese Journal Of Cancer, 30(2), 114–119.
Chang, E. T., & Adami, H. O. (2006). The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiology Biomarkers & Prevention, 15(10), 1765–1777.
Jung, P. F. (1965). Familial tendency of nasopharyngeal carcinoma; a report of cases. Pacific Medicine and Surgery, 73, 242–243.
Ko, J. Y., et al. (1998). Familial clustering of nasopharyngeal carcinoma. Otolaryngology-Head and Neck Surgery, 118(5), 736–737.
Zhang, F., & Zhang, J. (1999). Clinical hereditary characteristics in nasopharyngeal carcinoma through Ye-Liang’s family cluster. Chinese Medical Journal, 112(2), 185–187.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174.
Stetler-Stevenson, W. G., Liotta, L. A., & Kleiner, D. E, Jr. (1993). Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB Journal, 7(15), 1434–1441.
Liotta, L. A., DeVita, V. T., Helman, S., and Rosenberg, S. A., (Eds.). (1989) Cancer: Principles and Practice of Oncology (Vol I, pp 98–115). New York: J.B. Lippincott.
Fink, K., & Boratynski, J. (2012). The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis. Postepy Higieny I Medycyny Doswiadczalnej (Online), 66, 609–628.
Tahara, K., et al. (2010). Serum matrix-metalloproteinase-1 is a bona fide prognostic marker for colorectal cancer. Annals of Surgical Oncology, 17(12), 3362–3369.
Pradhan-Palikhe, P., et al. (2010). Plasma level of tissue inhibitor of matrix metalloproteinase-1 but not that of matrix metalloproteinase-8 predicts survival in head and neck squamous cell cancer. Oral Oncology, 46(7), 514–518.
Li, M., et al. (2010). Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer, 69(3), 341–347.
Rutter, J. L., et al. (1998). A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Research, 58(23), 5321–5325.
Hinoda, Y., et al. (2002). Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. International Journal of Cancer, 102(5), 526–529.
Su, L., et al. (2006). Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis, 27(5), 1024–1029.
Nishizawa, R., et al. (2007). The 2G allele of promoter region of matrix metalloproteinase-1 as an essential pre-condition for the early onset of oral squamous cell carcinoma. BMC Cancer, 7, 187.
Nasr, H. B., et al. (2007). Matrix metalloproteinase-1 (−1607) 1G/2G and −9 (−1562) C/T promoter polymorphisms: susceptibility and prognostic implications in nasopharyngeal carcinomas. Clinica Chimica Acta, 384(1–2), 57–63.
Zhou, G., et al. (2007). Functional polymorphisms and haplotypes in the promoter of the MMP2 gene are associated with risk of nasopharyngeal carcinoma. Human Mutation, 28(11), 1091–1097.
Lau, J., Ioannidis, J. P., & Schmid, C. H. (1997). Quantitative synthesis in systematic reviews. Annals of Internal Medicine, 127(9), 820–826.
Higgins, J. P., et al. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560.
Mantel, N., & Haenszel, W. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute, 22(4), 719–748.
DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188.
Egger, M., et al. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634.
Kondo, S., et al. (2005). Epstein-Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. International Journal of Cancer, 115(3), 368–376.
Gao, W., Sui, J., Wang, B., Li, X., Zhang, C., Wen, S., et al. (2010). MMP-1(-1607)1G/2G gene polymorphism and susceptibility to nasopharyngeal carcinoma in Han population in Yunnan China. Chinese Archives of Otolaryngology-Head and Neck Surgery, 17(3), 116–120.
Gallicchio, L., et al. (2006). Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. International Journal of Cancer, 119(5), 1125–1135.
Zhou, B., et al. (2009). A functional insertion/deletion polymorphism in the promoter region of NFKB1 gene increases susceptibility for nasopharyngeal carcinoma. Cancer Letters, 275(1), 72–76.
Nong, L. G., et al. (2009). Interleukin-18 gene promoter polymorphism and the risk of nasopharyngeal carcinoma in a Chinese population. DNA and Cell Biology, 28(10), 507–513.
Deng, L., et al. (2002). Cyclin D1 polymorphism and the susceptibility to NPC using DHPLC. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai), 34(1), 16–20.
Jalbout, M., et al. (2003). Polymorphism of the stress protein HSP70-2 gene is associated with the susceptibility to the nasopharyngeal carcinoma. Cancer Letters, 193(1), 75–81.
Zhang, C., et al. (2013). Association between MMP1 −1607 1G > 2G polymorphism and head and neck cancer risk: a meta-analysis. PLoS One, 8(2), e56294.
Conflict of interest
The authors have not declared any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhen Li and Hongzhou Ge have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Li, Z., Ge, H., Xie, YG. et al. Matrix Metalloproteinase-1 (MMP1) Polymorphism is Associated with Lowered Risk of Nasopharyngeal Carcinoma in Asian Population. Cell Biochem Biophys 71, 999–1004 (2015). https://doi.org/10.1007/s12013-014-0299-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0299-4