Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) shows promising result in cancer therapy and induces apoptosis in a wide variety of tumor cells, without causing toxicity to normal cells. However, many tumor cells including acute myeloid leukemia (AML) showed certain degrees of resistance to TRAIL and the mechanism remains largely unknown. Embelin is a potent XIAP inhibitor which has been shown to inhibit the proliferation of tumor cells and cause cell apoptosis. In this study, we investigated the effects of Embelin on the TRAIL-induced apoptosis and the underlying mechanism. Here, we chose an adenovirus vector as the expression vector for TRAIL, which was named Ad-TRAIL. The results in vitro showed that the co-treatment of Embelin and Ad-TRAIL has synergistically suppressed the proliferation of AML cells. Embelin has the ability to enhance TRAIL-induced apoptosis and activate caspase pathway. More interestingly, we found that the underlying mechanism for these talent skills of Embelin is through reducing the TRAIL-mediated activation of NF-κB and decreasing its transcriptional activity. Furthermore, our results in vivo suggest that combined therapy of Embelin and Ad-TRAIL caused significant growth inhibition of HL-60 xenograft tumors. Our results suggested that Embelin could sensitize AML cell to TRAIL through the repression of NF-κB signal pathway in vitro and in vivo, and combined therapy of Ad-TRAIL and Embelin may be the attractive candidate for clinical application in treatment of AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy characterized by rapid growth of a clonal population of neoplastic cells that accumulate in the bone marrow as a result of a blockage in hematopoiesis. In spite of many efforts in the past decades, the outcome for the patients remains poor. AML is predominantly a disease of the elderly. Long-term survival is achieved by approximately 40–45 % of younger patients with AML but less than 10 % of patients aged >60-years [1, 2]. Thus, new therapeutic approaches should be explored with the hope of improving outcomes.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which is also designated as Apo-2, is a typical member of the structurally related tumor necrosis factor (TNF) family. TRAIL has been thought to be a new candidate for anticancer therapy because TRAIL selectively suppresses tumor growth in vivo and in vitro but has little or no effect on normal cells. On the other hand, TRAIL can activate the transcription factor NF-κB leading to transcription of genes known to antagonize the death-signaling pathway [3].
Despite the attractive antitumor activity of TRAIL, many cancer cells show resistance to TRAIL and the mechanism has not been fully elucidated [3]. Recent studies demonstrated that combined treatment of TRAIL and other drugs or chemicals can synergistically increase the cell death and apoptosis of various tumor cells by suppressing survival signals and gene expression as well as activating apoptosis-associated gene expression [4–7].
Embelin has been used for thousands of years to treat fever, inflammatory diseases, and a variety of gastrointestinal ailments. More than 4 decades ago, the active component from this plant was isolated and named Embelin and was later chemically synthesized [8]. Embelin has been shown to have antitumor, anti-inflammatory, and analgesic properties [9]. Furthermore, it is reported that Embelin can block NF-κB signaling pathway leading to suppression of NF-κB-regulated antiapoptotic and metastatic gene products [10, 11]. Based on another report indicating that TRAIL has the ability to activate both the apoptosis signaling pathway and transcription factor NF-κB, which allows transcription of genes known to antagonize the death-signaling pathway [12–15], we hypothesized that Embelin may have this capacity to inhibit NF-κB-dependent transcription which sensitizes AML cells to TRAIL.
In this study, we employed Embelin and Ad-TRAIL to co-operatively work on AML in vitro and in vivo for the first time and examine whether Embelin can synergistically promote TRAIL-induced apoptosis through blocking the NF-κB pathway. Hoping it will provide a promising support for further research on AML in future.
Materials and Methods
Reagents
A 100 mM solution of Embelin (Sigma-Aldrich, St Louis, MO) was prepared in dimethyl sulfoxide (DMSO), stored at −20 °C, and then diluted as needed in cell culture medium. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Hoechst 33342, and propidium iodide (PI) were obtained from Sigma-Aldrich (St Louis, MO). RPMI 1640 medium, penicillin, streptomycin, fetal bovine serum, trypsin, and phosphate-buffered saline (PBS) were purchased from GIBCO (Carlsbad, CA). Antibodies against Caspase-8 and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling (Danvers, MA). Antibodies against Caspase-3, PARP, IkBα, p65, and p50 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Lines and Culture
Human AML cell lines HL-60 and KG-1 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (CBTCCAS, Shanghai, China) and maintained in RPMI 1640 medium supplemented with 10 % heat-inactivated fetal bovine serum at 37 °C in a humidified air atmosphere with 5 % CO2.
Adenovirus Expression Vectors for TRAIL
An adenovirus expressing TRAIL was constructed as described previously. Briefly, the full-length TRAIL was cloned into the MCS of the pShuttleCMV, leading to the production of pShuttleTRAIL. The recombinant Ad-TRAIL was produced by in vitro recombination of pShuttle TRAIL with pAdEasy as described previously [16].
Cytotoxicity Assay
The effects of Embelin on Ad-TRAIL-induced cytotoxicity were determined by the MTT uptake method. Briefly, cells were seeded in a 96-well plate (Corning) at a density of 1 × 104 cells/well in 100 μl of culture medium and treated with Ad-TRAIL, Embelin, Ad-TRAIL plus Embelin, or PBS. At the indicated time, 10 μl of MTT Reagent (5 mg MTT/ml medium) was added to each well, and plates were incubated for 3 h at 37 °C. The results were quantified by measuring the absorbance at 490 nm with a microplate reader (Tecan, Maennedorf, Switzerland).
Western Blot Analysis
HL-60 cells were cultivated and treated as mentioned above. Following indicated treatments, cells were washed twice with PBS and lysed in RIPA buffer [0.5 M Tris–HCl (pH 7.4), 1.5 M NaCl, 2.5 % deoxycholic acid, 10 %. NP-40, 10 mM EDTA] (20-188, Upstate, CA, USA). Proteins were separated by electrophoresis on 12 % SDS-polyacrylamide gel, transferred to a PVDF (Polyvinylidene Fluoride) membrane, immunostained with appropriate antibody, and visualized. The primary antibodies and their dilutions used were caspase-8 (1:1000), caspase-3 (1:200), PARP (1:200), IkBα (1:200), p65 (1:200), p50 (1:200), and GAPDH (1:1,000). The secondary antibodies used were anti-rabbit (1:10,000) and anti-mouse (1:5,000).
Flow Cytometry Analysis
HL-60 and KG-1 cells were treated with Embelin and/or Ad-TRAIL. After 24 h, apoptotic cells were examined using Annexin V-FITC and PI double staining following the manufacturer’s instructions. Briefly, cells were harvested by careful centrifugation, washed twice with 1× Annexin V binding buffer, resuspended in binding buffer, and stained with Annexin V and PI. Cell apoptosis was detected using the FACS (FACStar cytofluorometer, BD Biosciences).
Animal Experiments
All animal experimental protocols were approved by the Animal Care and Use Committee of the Zhejiang Provincial People’s Hospital and were in compliance with Guidelines by the National Academy Press (NIH Publication No. 85-23, revised 1996). All animals were kept at 23–25 °C with a 12-h light/dark cycle and allowed standard chow and water until the time of the study. Female BALB/c nude mice (4-week-old) were purchased from Shanghai Experimental Animal Center (Shanghai, China). For establishment of xenograft, HL-60 cells were subcutaneously injected into the right flank of each mouse at a dose of 5 × 106 cells in 100 μl DMEM. When tumors reached 100–150 mm3 in size, mice were divided randomly into three groups (six mice per group). Ad-TRAIL (1 × 1010 PFUs per mouse, intra-tumor) and Embelin (30 mg/kg, intraperitoneal) were injected every five days.
Statistical Analysis
All data were expressed as the mean ± standard deviation and analyzed by SPSS 11.0 software (SPSS Inc. Chicago, IL, USA). Data analysis was performed using t tests, and P < 0.05 was considered statistically significant.
Results
Enhanced Antitumor Effect Using a Combination of Ad-TRAIL and Embelin
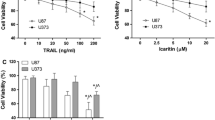
As a novel chemotherapeutic drug, Embelin was reported to block NF-κB-signaling pathway leading to suppression of NF-κB-regulated antiapoptotic and metastatic gene products [13]. To evaluate the cytotoxic effects of Ad-TRAIL and Embelin, two AML cell lines (HL-60, KG-1) were infected with Ad-TRAIL or in combination with Embelin. The MTT assay was performed, and the results are shown in Fig. 1. The results suggest that the combination of Ad-TRAIL and Embelin has an enhanced tumor-killing effect.
Enhanced suppression of tumor cell proliferation using the combination of Ad-TRAIL and Embelin. The tumor cells HL-60 (a) and KG-1 (c) were treated with Ad-TRAIL, Embelin, or Ad-TRAIL + Embelin at the indicated concentration for 48 h. Data are presented as mean ± SD of three independent experiments. Synergistic effect of Ad-TRAIL combined with Embelin on HL-60 (b) and KG-1 (d) was quantified by combination index (CIN) analysis and expressed as lg (CIN) versus fraction affected. Where calculable, 95 % confidence intervals are shown
Apoptosis Induction by Treatment with Ad-TRAIL and/or Embelin
Ad-TRAIL could efficiently mediate TRAIL expression in tumor cells, as shown in our previous study. We detected TRAIL protein expression in HL-60 cells by Western Blot. To determine whether Embelin affects TRAIL expression, we treated the cancer cell line and HL-60, with Ad-TRAIL alone or Ad-TRAIL plus Embelin. The results indicated that Embelin did not attenuate the expression of TRAIL (Fig. 2a). To determine the underlying mechanism by which Ad-TRAIL, Embelin alone, or a combination of Ad-TRAIL and Embelin can induce apoptosis in cancer cells, the activation of caspase-3 and death substrate, poly(ADP-ribose)polymerase (PARP), was detected by Western Blotting analysis (Fig. 2b). The results showed that the cleavage of caspase-3 and PARP was observed in the tumor cells, HL-60 after treatment with Ad-TRAIL plus Embelin for 48 h.
Apoptosis induction by treatment with Ad-TRAIL and/or Embelin. a Effect of Embelin on the expression of the TRAIL gene. TRAIL was detected by Western blot analysis when HL-60 cells were treated with Ad-TRAIL (20 MOI) or Ad-TRAIL (20 MOI) plus Embelin (10 μM). b HL-60 cells were treated with Ad-TRAIL (20 MOI), Embelin (10 μM), or a combination of Ad-TRAIL (20 MOI) and Embelin (10 μM) for 48 h. Whole cell extracts were prepared and immunoblotted for the detection of activation of caspase pathway. GAPDH was used as a loading control
Flow cytometry analysis was performed to confirm the enhanced apoptosis of tumor cells after treatment with combined Ad-TRAIL and Embelin therapy. The percentage of apoptosis was determined by Annexin V and PI staining. The combination of Ad-TRAIL with Embelin showed a higher percentage of cell apoptosis in HL-60 cells compared with other groups (Fig. 3). These observations were consistent with the morphological features. Most tumor cells died during treatment with combined Ad-TRAIL and Embelin, as shown by cell surface blebbing and the formation of apoptotic bodies.
Apoptosis detection by Annexin V-FITC and PI staining assay after treatment with combined Ad-TRAIL and Embelin therapy. a Percent apoptotic cell death was determined by FACS 48 h after treatment of Ad-TRAIL, Embelin alone, or Ad-TRAIL plus Embelin. b A column graph representing the mean values of three cytometric assays. *P < 0.01
Taken together, Ad-TRAIL could lead to efficient tumor-specific replication and TRAIL expression and significantly induce tumor cell apoptosis. The combination of Ad-TRAIL and Embelin resulted in a significantly enhanced antitumor effect.
Embelin Inhibits TRAIL-Induced Activation of NF-κB in AML Cells
To testify the hypothesis proposed in the introduction, we detected the IκBα, p65, and p50 changes in the indicated treatments using Western blot. As the results shown in Fig. 4, the reduced expression of IκBα, p65, and p50 in the combined treatment of Embelin and Ad-TRAIL is much more dramatic than that of the TRAIL-induced alone, while less than that of Embelin treatment, which implied that Embelin can reduce the TRAIL-mediated activation of NF-κB and decrease its transcriptional activity which promotes TRAIL-induced apoptosis (Fig. 4).
Embelin inhibits TRAIL-induced activation of NF-κB in AML cells. HL-60 cells were treated with Ad-TRAIL (20 MOI) or Ad-TRAIL (20 MOI) plus Embelin (10 μM). After 48 h, cell lysates were collected and Western blot assay was performed to examine the changes of IκBa, p65, and p50. GAPDH was used as a loading control
Animal Experiments to Evaluate Therapeutic Efficacy
To investigate the therapeutic effects of combination of Ad-TRAIL and Embelin in vivo, HL-60 cells were used to establish a model of AML tumor on athymic nude mice. As shown in Fig. 5a, various degrees of tumor growth inhibition were observed after Ad-TRAIL & Embelin-treated or Ad-TRAIL-treated. The average volume of Ad-TRAIL & Embelin-treated HL-60 xenograft tumors was about 432 mm3 at the end of the experiment compared to that of in the Ad-TRAIL-treated group (1803 mm3) and in the PBS-treated group (2549 mm3).
Antitumor efficacy in established HL-60 tumors in vivo. Mice bearing subcutaneous HL-60 xenografts (n = 8) were randomized to receive one of the following intratumoral injections on 5 consecutive days: PBS, Ad-TRAIL, Embelin, and Ad-TRAIL & Embelin. a Time courses of changes in tumor volume in each group are shown. b Survival curve analysis. The percentage of surviving mice was determined by monitoring the death of mice over a period of 43 days. Mice treated with combination therapy showed significant survival advantage over mice treated with other groups
87.5 % mice treated with the combination agents survived up to 43 days after implantation with tumor cells, while the Ad-TRAIL- and Embelin-treated groups had 12.5 and 62.5 %, respectively; animals alive after 43 days compared with PBS-treated group with no animals surviving (Fig. 5b).
Discussion
TRAIL is thought to be an attractive antitumor gene for its ability to induce apoptosis in various tumor cells, but elicits little cytotoxicty against normal cells. It induces tumor cell apoptosis through extrinsic apoptotic signal pathway which includes caspase-8 activation, Bid cleavage, cytochrome c release, caspase-3 activation, and cell apoptosis. On the other hand, TRAIL can activate another signal mediated by the transcription factor NF-κB, leading to transcription of genes known to antagonize the death signaling pathway [15] These two signal pathways function in opposite. Thus, TRAIL-induced apoptosis can be significantly augmented by inhibition of the NF-κB activation pathway [17, 18].
Although TRAIL is thought to be a highly promising candidate for cancer treatment, the use of TRAIL has major limits in clinical application for its resistance in a variety of cancer cells [18]. Recent studies demonstrated that combination treatment of TRAIL and other drugs or chemicals can synergistically increase cell death and apoptosis of various tumor cells by suppressing survival signals and gene expression as well as activating apoptosis-associated gene expression [4, 19]. Many studies have shown that conventional cytotoxic drugs such as antioxidants and novel molecular-targeted agents or irradiation markedly sensitize TRAIL-induced apoptosis in TRAIL-resistant cancer cells [20, 21]. In fact, it is estimated that the small molecule XIAP inhibitor can increase TRAIL-induced apoptosis by down-regulating Bcl-2 and subsequently increasing caspase-3 activation [22]. We herein hypothesized that other small molecule XIAP inhibitors, such as Embelin, can also increase the TRAIL-induced cell apoptosis and may have a different triggering mechanism. Our results indicated that Embelin co-treated with Ad-TRAIL can synergistically suppress the survival of AML cells in vitro and in vivo and the combination of these two agents can remarkably increase the activation of apoptotic elements, such as caspase-3 and PARP, thus enhancing the tumor cells apoptosis. These results suggested that Embelin can significantly relieve TRAIL-induced apoptosis from TRAIL-resistant cancer cells and increase TRAIL-induced apoptosis in these resistant tumor cells.
Based on the reports that Embelin can block NF-κB-signaling pathway leading to suppression of NF-κB-regulated antiapoptotic and metastatic gene products, we hypothesized that the underlying mechanism of Embelin sensitizing AML cells to TRAIL-induced apoptosis is probably to inhibit NF-κB-dependent transcription. So we detected the expression of NF-κB-signaling pathway in AML cells co-treated with Embelin and Ad-TRAIL. The results showed that Embelin can indeed assist TRAIL to reduce the expression level of members in NF-κB pathway, such as IκBα, p65, and p50, which suggested that Embelin can inhibit the TRAIL-mediated activation of NF-κB and decrease its transcriptional activity which promotes TRAIL-induced apoptosis.
In conclusion, our study demonstrated that Embelin can increase the TRAIL-induced apoptosis in AML cells in vitro and in vivo. The underlying mechanism is to suppress the NF-κB-dependent survival pathway. In other words, Embelin can significantly relieve TRAIL-induced apoptosis from the resistance against TRAIL in cancer cells, thus sensitizing tumor cells to TRAIL-induced apoptosis, which indicates a potent therapeutic strategy for cancer therapy in future.
References
Burnett, A. K., et al. (2010). Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. Journal of Clinical Oncology, 28(4), 586–595.
Buchner, T., et al. (2009). Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. Journal of Clinical Oncology, 27(1), 61–69.
Seo, O. W., et al. (2012). Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-kappaB-dependent cFLIP expression in HeLa cells. Experimental & Molecular Medicine, 44(11), 653–664.
Neuzil, J., Swettenham, E., & Gellert, N. (2004). Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of Bcl-xL down-regulation. Biochemical and Biophysical Research Communications, 314(1), 186–191.
Griffith, T. S., et al. (2011). Sensitization of human bladder tumor cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis with a small molecule IAP antagonist. Apoptosis, 16(1), 13–26.
Gupta, S. C., et al. (2011). Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. Journal of Biological Chemistry, 286(2), 1134–1146.
Jung, Y. H., et al. (2012). Tunicamycin sensitizes human prostate cells to TRAIL-induced apoptosis by upregulation of TRAIL receptors and downregulation of cIAP2. International Journal of Oncology, 40(6), 1941–1948.
Du, Y. C., & Wie, J. S. (1963). Study of Vermifuges. I. Isolation of Embelin from the fruit of Mugua-Wha (Embelia Oblongifolia Hemsl.). Yao Xue Xue Bao, 10, 578–580.
Dallacker, F., & Lohnert, G. (1972). Derivatives of methylenedioxybenzene. 35. A novel synthesis of 3,6 dihydroxy-2-ethyl-1,4-benzoquinone, embelin, vilangin, rapanone, dihydromaesaquinone, bhogatin, spinulosin and oosporein. Chemische Berichte, 105(2), 614–624.
Wang, S. B., et al. (2012). Suppression of hepatoma carcinoma cell Bel-7404 by small molecule XIAP inhibitor Embelin through blocking NF-kappa B signaling pathway. Progress in Biochemistry and Biophysics, 39(2), 161–167.
Franzoso, G., et al. (1997). Requirement for NF-kappaB in osteoclast and B-cell development. Genes & Development, 11(24), 3482–3496.
Guo, A. K., et al. (2014). Loss of p53 enhances NF-κB—dependent lamellipodia formation. Journal of Cellular Physiology, 229(6), 696–704.
Magrangeas, F., et al. (2013). Low level of NF-κb activity is associated with higher response rate to bortezomib-based induction therapy in patients with newly diagnosed multiple myeloma. Blood, 122(21), 3106.
Cobler, L., et al. (2013). Activation of the NF-κB pathway downregulates TFF-1 in gastric carcinogenesis. Virchows Archiv, 463(4), 497–507.
Seo, O. W., et al. (2012). Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-kappa B-dependent cFLIP expression in HeLa cells. Experimental & Molecular Medicine, 44(11), 653–664.
Wang, S. B., et al. (2012). Complete eradication of xenograft hepatoma by oncolytic adenovirus ZD55 Harboring TRAIL-IETD-smac gene with broad antitumor effect. Human Gene Therapy, 23(9), 992–1002.
Han, J., et al. (2007). Kushen flavonoids induce apoptosis in tumor cells by inhibition of NF-kappa B activation and multiple receptor tyrosine kinase activities. Phytotherapy Research, 21(3), 262–268.
Walczak, H., et al. (1999). Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Medicine, 5(2), 157–163.
Wang, S. B., et al. (2012). Complete eradication of xenograft hepatoma by oncolytic adenovirus ZD55 harboring TRAIL-IETD-Smac gene with broad antitumor effect. Human Gene Therapy, 23(9), 992–1002.
Falschlehner, C., et al. (2007). TRAIL signalling: decisions between life and death. International Journal of Biochemistry & Cell Biology, 39(7–8), 1462–1475.
Ganten, T. M., et al. (2005). Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology, 42(3), 588–597.
Dean, E. J., et al. (2010). A small molecule inhibitor of XIAP induces apoptosis and synergises with vinorelbine and cisplatin in NSCLC. British Journal of Cancer, 102(1), 97–103.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LY14H160041 and LY13H080005), National Natural Science Foundation of China (Nos. 81201783, 81372463 and 81201089), Funds of Science Technology Department of Zhejiang Province (No. 2014C37101) and Open Fund of Zhejiang Provincial Top Key Discipline of Biology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, T., Lan, J., Huang, Q. et al. Embelin Sensitizes Acute Myeloid Leukemia Cells to TRAIL through XIAP Inhibition and NF-κB Inactivation. Cell Biochem Biophys 71, 291–297 (2015). https://doi.org/10.1007/s12013-014-0197-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0197-9