Abstract
Among numerous choices in cardiovascular therapies used for the management of hypertension and heart failure, drugs affecting the renin–angiotensin–aldosterone system (RAAS) hold substantial therapeutic roles. Therapies aimed at modifying the RAAS and its overactivation are employed for the management of various insidious disorders. In the pharmacologic perspective, RAAS is one of the frequently manipulated systems for the management of hypertension, heart failure, myocardial infarction, and renal disease. The RAAS pharmacologic interventions principally include the ACE inhibitors, the angiotensin II-AT1 receptor blockers, the mineralocorticoid receptor antagonists, and the direct renin inhibitors. In addition, therapeutic implication of ACE2/angiotensin (1–7)/Mas receptor activation using various ligands is being explored owing to their anti-inflammatory, anti-fibrotic, vasodilatory, and cardiovascular defensive roles. Moreover, being considered as the counter-regulatory arm of AT1 receptor, the potential role of AT2 receptor activation using selective AT2 receptor agonist is currently investigated for its efficacy in pulmonary complications. As an important regulator of fluid volume, blood pressure, and cardiovascular–renal function, the RAAS has been documented as a diversified intricate system with several therapeutic possibilities coupled with their fundamental structural and functional modulatory roles in cardiovascular, renal, and other systems. The RAAS possesses a number of regulatory, deregulatory, and counter-regulatory axes of physiopathologic importance in health and disease. The counter-regulatory arms of the RAAS might play an essential role in mitigating cardiovascular, renal, and pulmonary pathologies. In light of this background, we sought to explore the classical and counter-regulatory axes/arms of the RAAS and their imperative roles in physiologic functions and disease pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant progress has been made in the understanding of the neurohormonal implications of the renin–angiotensin–aldosterone system (RAAS), which plays a fundamental role in cardiovascular and renal health and disease. The RAAS is a century-old diversified system with several therapeutic avenues. This intricate system is conventionally linked with the control of electrolyte balance and regulation of blood pressure. Nevertheless, chronic overactivation and subsequent deregulation of the RAAS may have negative impact on the structure and function of the renal, cardiac, and vascular tissues through hemodynamic changes and direct action [1,2,3]. The multifaceted nature of the RAAS makes it a versatile system based on its fundamental implications in the pathogenesis of the cardiovascular and renal disease [4,5,6,7,8,9]. The cardiovascular–renal negative impact of the chronic RAAS activation is reflected by the potential beneficial outcomes of various RAAS interventions in the management of cardiovascular and renal pathologies [10,11,12].
The RAAS involves various bio-active molecules with each other opposing physiologic effects such as vasoconstriction vs vasodilation, proliferative vs anti-proliferative, and pro-inflammatory vs anti-inflammatory actions. The vasoconstrictive, pro-proliferative, and pro-inflammatory actions are seen with angiotensin-converting enzyme (ACE)–angiotensin II (Ang II)-type 1 (AT1) receptor axis. The RAAS deregulation through overactivation of its classical arm (ACE–Ang-II–AT1 receptor axis) has been linked to the development of cardiovascular pathologies. On the other hand, ACE2–Ang (1–7)–Mas receptor axis might be involved in partially counteracting the potentially cardiovascular harmful actions of the ACE–Ang II–AT1 receptor axis [3, 13]. Of note, ACE2 is recognized a regulatory enzyme of the RAAS having protective functions in several cardiovascular, pulmonary, and metabolic disorders [14]. In addition to its peripheral cardiovascular role, the brain ACE2 has been suggested to play a possible role in the central cardiovascular regulation [15]. Studies employing synthetic compounds that could sustain the elevation of the activity of ACE2 or genetically overexpression of ACE2 in the specific brain regions found some beneficial effects on cardiovascular function [15]. Further to these two well-recognized axes, RAAS involves additional key axes, which are the focus of this review and are evidently discussed. We herein review the existence of different key axes/arms of the RAAS and their imperative roles in the physiologic functions and disease pathogenesis.
An Overview of the RAAS: A Family of Several Biologically Active Components
The primary site for renin release is the kidney even though several other RAAS components are identified in many tissues [16, 17]. The circulatory source of renin is utilized for the local generation (for instance, the perivascular adipose tissue) of several components of the RAAS [18]. This multifaceted system is exclusively activated by a number of stimuli such as sympathetic stimulation, renal artery hypotension, and diminished sodium delivery to the distal tubule [19]. Renin is considered the rate-limiting enzyme in the RAAS, and is secreted into the blood stream only from the juxtaglomerular cells (JGC) of the kidney in response to various stimuli [16]. However, prorenin, which is considered the precursor of renin, has been noted to be synthesized not only in the JGC but also in various tissues [16]. Prorenin is secreted from various tissues into the blood constitutively, while its level in the circulation has been suggested to be 10 times higher than that of renin [16]. The renin precursor is considered inactive because the prosegment region with 43 amino acid residues is known to cover the active site of renin, and prorenin activation takes place either proteolytically or non-proteolytically [16]. The (pro)renin receptor binds both renin and prorenin. The (pro)renin receptor might have two important functions, first as a receptor for renin and prorenin, whereas the second as an accessory subunit of the v-H(+)-ATPase and a cofactor of the Wnt [20]. Although, (pro)renin receptor has been identified as a membrane-bound binding component of renin and prorenin, it is now empathized that most of the effects of (pro)renin receptor are independent of renin and prorenin [21].
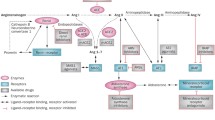
Renin directs the switch of angiotensinogen (AOG) into Ang I, while ACE further converts it into Ang II, the widely studied major effector and vasoconstrictor peptide of the RAAS. The Ang II acting upon AT1 receptors with a high affinity provokes vasopressor effects, oxidative stress, fibrosis, and increase in sympathetic outflow and aldosterone release, among other key actions [4]. Besides, Ang I also acts as a key substrate for ACE2, which generates Ang (1–9) from it. The Ang (1–9) thus formed is converted to Ang (1–7) by the involvement of ACE. In addition, the ACE2 exerts a prominent action on Ang II, converting it directly into Ang (1–7), which is recognized as an agonist of the Mas, a G protein-coupled receptor [4]. The recently explored other imperative RAAS components and their physiopathologic significance are discussed in subsequent sections. The schematic representation of various key components of the RAAS is shown in Fig. 1.
Differential Axes of the RAAS with Several Efficacious Members
Ang II, one of the major components of the RAAS, via activation of the AT1 receptor promotes vasoconstriction and sodium retention to maintain blood pressure, and mediates oxidative stress, inflammation, fibrosis, organ hypertrophy, cellular growth, and proliferation in various pathologic conditions [22, 23]. Importantly, Ang (1–7) has opposite functional roles to that of Ang II, counteracting possibly the adverse actions of Ang II on the blood vessels, the kidney, and the heart [4, 22, 23]. This leaves the RAAS to have two major axes, but may not be limited to, such as (i) the ACE–Ang II–AT1 receptor axis, which plays a fundamental role in mediating vasoconstriction, proliferation, and inflammation; and (ii) the ACE2–Ang (1–7)–Mas receptor axis, which has abilities to counterbalance the adverse actions of Ang II, affording Ang (1–7)-mediated endothelial protective, vasodilatory, anti-proliferative, antihypertrophic, and cardio-renal protective actions [4, 22,23,24]. Increased understanding of the fundamental role of RAAS has led to the exploration of novel approaches with an aim of upregulating the ACE2–Ang (1–7)–Mas receptor axis to counterbalance the harmful actions provoked by the activation of ACE–Ang II–AT1 receptor axis [25]. This opens up new avenues for the development of potential pharmacologic agents modulating the RAAS in optimal direction to more efficiently treat hypertension and heart failure. The antihypertensive role of ACE2–Ang (1–7)–Mas receptor axis generated interest in investigating its potential cardioprotective effects against a group of cardiovascular disorders such as hypertensive heart disease, left ventricular hypertrophy, heart failure, and ischemic heart disease [26, 27]. In contrast to the ACE–Ang II–AT1 receptor axis, which activates multifaceted cell functions and several signal transduction pathways related to tissue injury, inflammation, and fibrosis, the counterpart ACE2–Ang (1–7)–Mas receptor axis exerts opposite effects in relation to inflammation and tissue fibrosis [28]. Evidence showed that the ACE2–Ang (1–7)–Mas receptor axis has abilities to reduce cytokine release and inhibit signaling pathways of tissue fibrosis in experimental disease models including atherosclerosis, obesity, chronic kidney disease, and asthma [28]. Moreover, the ACE2–Ang (1–7)–Mas receptor axis is emerging as a potential pharmacologic target for treating cardiopulmonary diseases [29]. It is worthwhile to mention that the maintenance of Ang (1–7)/Ang II balance might represent a valuable criterion for monitoring the outcomes of concerned therapeutic interventions in disease pathogenesis. However, more clinical pharmacologic evidence in different pathologic conditions is needed to support this contention.
The major target of RAAS interventions to treat cardiovascular and renal disorders is to primarily block the detrimental effects of the aforementioned classical arm (i) the ACE–Ang II–AT1 receptor axis, which may also be represented as the renin–AOG–ACE–Ang II–AT1 receptor–aldosterone axis. At the same time, pharmacologic strategies to potentiate the effects or to enhance the formation of key components of axis (ii) i.e., ACE2–Ang (1–7)–Mas receptor axis are of potential therapeutic importance in the relevant disorders. Apart from these two key axes, additional potential axes of the RAAS are described in recent studies (Fig. 2). These include (iii) the Ang II/aminopeptidase A (APA)/Ang III/AT2 receptor/NO/cGMP axis [30,31,32], (iv) the prorenin/renin/(pro)renin receptor/MAP kinases ERK1/2/V-ATPase axis [30, 32, 33], and (v) the Ang III/aminopeptidase N (APN)/Ang IV/insulin-regulated aminopeptidase (IRAP)/AT4 receptor axis, suggesting that RAAS has at least five axes [30, 31, 33]. On functional perspectives, the aforesaid axes (i) and (iv) represent principally the vasopressor systems, playing the fundamental physiologic role in maintaining cardiovascular, blood pressure, and renal homeostasis, while their overactivation contributes to the development of cardio-renal and vascular diseases [30]. On the other hand, axes (ii) and (iii) might essentially serve as the vasodepressor and cardio-renal protective arms of the RAAS, possibly counteracting some of the detrimental effects of the axes (i) and (iv) on the cardio-renal and vascular system [30]. Besides, the Ang III/APN/Ang IV/IRAP/AT4 receptor axis is unlikely falling exclusively under either the vasopressor or the vasodepressor system, but it appears to have a possible role in learning and memory [30, 34, 35].
Differential axes of the renin–angiotensin–aldosterone system with vasopressor and vasodepressor/defensive arms. ACE angiotensin-converting enzyme; AOG angiotensinogen; APA aminopeptidase A; APN aminopeptidase N; IRAP insulin-regulated aminopeptidase; MAP kinases Mitogen-activated protein kinases; MrgD Mas-related G protein-coupled receptor, member D
Does RAAS have Additional Axes of Therapeutic Importance?
Essentially, the category of angiotensin class of peptides is growing with the findings of angioprotectin, alamandine, and angiotensin A. Of note, angioprotectin is an Ang II-like peptide but causing the vasodilatory effect, whereas it has the affinity to the Mas receptor [36]. The physiologic antagonism of vasoconstrictor actions of Ang II by angioprotectin has been suggested to be mediated by the Mas receptor [36]. Since its identification in 2011, there have been only a few studies with regard to its key actions, and hence the cellular and physiopathologic roles and the therapeutic implications of angioprotectin–Mas receptor axis remain elusive, necessitating further studies. Alamandine, another vasodepressor component of the RAAS family, is a vasoactive peptide suggested to have protective actions like Ang (1–7) [37, 38]. Alamandine produces physiologic actions resembling to those produced by Ang (1–7), including vasodilation, antifibrosis, and antihypertensive effects [37]. However, unlike Ang (1–7) (a Mas receptor agonist), alamandine acts through the Mas-related G protein-coupled receptor, member D (MrgD) [37]. On the other hand, angiotensin A is a vasoconstrictive Ang II-derived peptide that is an agonist for AT1 receptor [39, 40]. The physiopathologic role and therapeutic implications of angiotensin A in cardiovascular and renal diseases are not precisely understood. Taken together, Ang (1–7) and angioprotectin through Mas receptor activation, and alamandine through MrgD activation might counterbalance some of the adverse actions of Ang II mediated by the AT1 receptor overactivation (Fig. 1); however, additional studies are required in this setting to precisely understand the counter-regulatory mechanisms of the RAAS.
The therapeutic potentials of alamandine/MrgD axis in various pathologic conditions have been demonstrated in recent experimental studies. In an aged, spontaneous hypertensive rat model, Wang et al. [41] investigated the potential therapeutic effects of alamandine on long‑term hypertension‑induced cardiac fibrosis. The findings of this study have suggested that alamandine is an effective antihypertensive peptide that has a potential to attenuate cardiac dysfunction and fibrosis induced by chronic hypertension independently to blood pressure action [41]. A bench study showed that alamandine via MrgD induced AMP-activated protein kinase/nitric oxide (AMPK/NO) signaling to counter-regulate Ang II-induced experimental hypertrophy, highlighting the therapeutic potential of an additional arm of the RAAS, the alamandine/MrgD axis in the heart [42]. In addition, alamandine has been shown to attenuate Ang II-induced vascular fibrosis via inhibiting p38 MAPK pathway, suggesting that alamandine/MrgD axis could be a potential therapeutic target for the treatment of vascular remodeling [43]. Likewise, alamandine has been reported to attenuate arterial remodeling in mice induced by transverse aortic constriction, in part, through its anti-fibrotic and anti-inflammatory effects, opening new avenues in potential therapeutic targets for vascular disease [44]. Moreover, a recent study by Silva et al. [45] suggested that alamandine has a potential to improve a number of aspects of pressure overload-induced experimental cardiac remodeling, including oxidative stress markers, cell hypertrophy, and fibrosis. Interestingly, a bench study by Fernandes et al. [46] reported that alamandine treatment attenuated the development of fibrosis in fibrotic rats. This experimental in vivo study demonstrated the therapeutic potential of alamandine in the alleviation of pulmonary fibrosis and the improvement of respiratory system mechanics [46]. Adding further, in line with these findings, Liu et al. [47] demonstrated that alamandine via MrgD receptor reduced the experimental pulmonary fibrosis through attenuation of oxidative injury and induction of autophagy [47]. These studies collectively justify the inclusion of alamandine/MrgD axis as one of the protective arms of the RAAS. Considering its potential beneficial effects, alamandine/MrgD axis might therefore be a promising therapeutic target for the management of cardiovascular and pulmonary fibrotic disease. Exploration of the biologic importance of such key RAAS components might expand the number of aforementioned axes of the RAAS, a never-ending vibrant system of the biologic and pharmacologic importance. The differential arms of the RAAS are depicted in Fig. 2.
Concluding Remarks and Future Perspectives
Although ACE inhibitors, ang II-AT1 receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), and direct renin inhibitors interrupt RAAS effectively at its various points, their clinical efficacies are often considered different. For instance, ACE inhibitors may not halt the entire generation of Ang II on account of the presence of alternate pathways for Ang II production. In contrast to ARB therapies, clinical use of ACE inhibitors often leads to the elevation of bradykinin levels, which might influence the overall clinical efficacy of ACE inhibitors on blood pressure management, and also account for adverse effects such as dry cough and angioedema. The ACE inhibitors and ARBs often lead to compensatory increases in the plasma renin activity, the effect of which is not shared by the renin inhibitor. While efficiently lowering blood pressure in patients afflicted with essential hypertension, the comparative effectiveness of the pharmacologic RAAS interventions such as ACE inhibitors, ARBs, MRAs, and direct renin inhibitors is not precisely known. The existence of different RAAS axes and their potential modulation by RAAS interventions might improve our understanding of the differential efficacies of pharmacologic agents interrupting RAAS at various levels.
Since the breakthrough finding of renin by Tigerstedt and Bergman in 1898, there has been a tremendous evolution toward a better understanding of the role of diverse components of the RAAS in health and disease. In fact, remarkable developments and conceptual changes have occurred over the past decades in the multipronged field of the RAAS research at the levels of bench to bedside. This results in significant changes to our understanding of this important biologic system. The RAAS is composed of different arms, including vasopressor and vasodepressor axes, that are certainly considered physiologic and physiopathologic importance in health and disease pathogenesis. Increased understanding of the intricate role of RAAS components in pathologic states has led to the exploration of novel approaches aiming at upregulating the ACE2–Ang (1–7)–Mas receptor axis to counteract the detrimental effects of the ACE/Ang II/AT1 receptor axis. This opens up new avenues for the identification and development of potential pharmacologic interventions for ameliorating the abnormal activation of harmful arms of the RAAS to treat optimally the cardiovascular disorders such as hypertension and heart failure. Though our knowledge on the diverse role of RAAS, its different axes, and its multiple peptide components continues to distinctly advance, a comprehensive understanding of their intricate central and peripheral implications in various disease pathogenesis remains incomplete, necessitating additional studies.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- AMPK:
-
AMP-activated protein kinase
- Ang II:
-
Angiotensin II
- AOG:
-
Angiotensinogen
- APA:
-
Aminopeptidase A
- APN:
-
Aminopeptidase N
- AT1 receptor:
-
Ang II-type 1 receptor
- IRAP:
-
Insulin-regulated aminopeptidase
- JGC:
-
Juxtaglomerular cells
- MRA:
-
Mineralocorticoid receptor antagonist
- MrgD:
-
Mas-related G protein-coupled receptor, member D
- NO:
-
Nitric oxide
- RAAS:
-
Renin–angiotensin–aldosterone system
References
Sutanto, H., Dobrev, D., & Heijman, J. (2021). Angiotensin receptor-neprilysin inhibitor (ARNI) and cardiac arrhythmias. International Journal of Molecular Sciences, 22(16), 8994.
Balakumar, P., & Jagadeesh, G. (2021). The renin-angiotensin-aldosterone system: A century-old diversified system with several therapeutic avenues. Pharmacological Research, 174, 105929.
Adamcova, M., Kawano, I., & Simko, F. (2021). The impact of microRNAs in renin-angiotensin-system-induced cardiac remodelling. International Journal of Molecular Sciences, 22(9), 4762.
Balakumar, P., & Jagadeesh, G. (2014). A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cellular Signalling, 26(10), 2147–2160.
Balakumar, P., & Jagadeesh, G. (2010). Cardiovascular and renal pathologic implications of prorenin, renin, and the (pro)renin receptor: Promising young players from the old renin-angiotensin-aldosterone system. Journal of Cardiovascular Pharmacology, 56(5), 570–579.
Balakumar, P., & Jagadeesh, G. (2014). Structural determinants for binding, activation, and functional selectivity of the angiotensin AT1 receptor. Journal of Molecular Endocrinology, 53(2), R71-92.
Jagadeesh, G., Balakumar, P., & Stockbridge, N. (2012). How well do aliskiren’s purported mechanisms track its effects on cardiovascular and renal disorders? Cellular Signalling, 24(8), 1583–1591.
Balakumar, P., Anand-Srivastava, M. B., & Jagadeesh, G. (2017). Renin-angiotensin-aldosterone: An inclusive, an invigorative, an interactive and an interminable system. Pharmacological Research, 125(Pt A), 1–3.
Balakumar, P., Sambathkumar, R., Mahadevan, N., Muhsinah, A. B., Alsayari, A., Venkateswaramurthy, N., & Jagadeesh, G. (2019). A potential role of the renin-angiotensin-aldosterone system in epithelial-to-mesenchymal transition-induced renal abnormalities: Mechanisms and therapeutic implications. Pharmacological Research, 146, 104314.
Feng, Y., Huang, R., Kavanagh, J., Li, L., Zeng, X., Li, Y., & Fu, P. (2019). Efficacy and safety of dual blockade of the renin-angiotensin-aldosterone system in diabetic kidney disease: A meta-analysis. American Journal of Cardiovascular Drugs, 19(3), 259–286.
Zhao, M., Qu, H., Wang, R., Yu, Y., Chang, M., Ma, S., Zhang, H., Wang, Y., & Zhang, Y. (2021). Efficacy and safety of dual vs single renin-angiotensin-aldosterone system blockade in chronic kidney disease: An updated meta-analysis of randomized controlled trials. Medicine (Baltimore), 100(35), e26544.
Strauss, M. H., Hall, A. S., & Narkiewicz, K. (2021). The combination of beta-blockers and ACE inhibitors across the spectrum of cardiovascular diseases. Cardiovascular Drugs and Therapy. https://doi.org/10.1007/s10557-021-07248-1
Sepúlveda-Fragoso, V., Alexandre-Santos, B., Salles, A. C. P., Proença, A. B., de Paula Alves, A. P., Vázquez-Carrera, M., Nóbrega, A. C. L., Frantz, E. D. C., & Magliano, D. C. (2021). Crosstalk between the renin-angiotensin system and the endoplasmic reticulum stress in the cardiovascular system: Lessons learned so far. Life Science, 284, 119919.
Jia, H., Yue, X., & Lazartigues, E. (2020). ACE2 mouse models: A toolbox for cardiovascular and pulmonary research. Nature Communications, 11(1), 5165.
Mohammed, M., Berdasco, C., & Lazartigues, E. (2020). Brain angiotensin converting enzyme-2 in central cardiovascular regulation. Clinical Science (London, England), 134(19), 2535–2547.
Nabi, A. H., & Suzuki, F. (2010). Biochemical properties of renin and prorenin binding to the (pro)renin receptor. Hypertension Research, 33(2), 91–97.
Kurtz, A. (2012). Control of renin synthesis and secretion. American Journal of Hypertension, 25(8), 839–847.
Balakumar, P., Alqahtani, A., Khan, N. A., Alqahtani, T., & Jagadeesh, G. (2021). The physiologic and physiopathologic roles of perivascular adipose tissue and its interactions with blood vessels and the renin-angiotensin system. Pharmacological Research, 173, 105890.
Yim, H. E., & Yoo, K. H. (2008). Renin-Angiotensin system—Considerations for hypertension and kidney. Electrolyte Blood Press, 6(1), 42–50.
Balakumar, P., & Jagadeesh, G. (2011). Potential cross-talk between (pro)renin receptors and Wnt/frizzled receptors in cardiovascular and renal disorders. Hypertension Research, 34(11), 1161–1170.
Hoffmann, N., & Peters, J. (2021). Functions of the (pro)renin receptor (Atp6ap2) at molecular and system levels: Pathological implications in hypertension, renal and brain development, inflammation, and fibrosis. Pharmacological Research. https://doi.org/10.1016/j.phrs.2021.105922
Chappell, M. C., Marshall, A. C., Alzayadneh, E. M., Shaltout, H. A., & Diz, D. I. (2014). Update on the angiotensin converting enzyme 2-angiotensin (1–7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne)., 4, 201.
Nunes-Silva, A., Rocha, G. C., Magalhaes, D. M., Vaz, L. N., Salviano de Faria, M. H., & Simoes E Silva, A. C. (2017). Physical exercise and ACE2-angiotensin-(1–7)-Mas receptor axis of the renin angiotensin system. Protein & Peptide Letters, 24(9), 809–816.
Iwai, M., & Horiuchi, M. (2009). Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs ACE2-angiotensin-(1–7)-Mas receptor axis. Hypertension Research, 32(7), 533–536.
Arendse, L. B., Danser, A. H. J., Poglitsch, M., Touyz, R. M., Burnett, J. C., Jr., Llorens-Cortes, C., Ehlers, M. R., & Sturrock, E. D. (2019). Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacological Reviews, 71(4), 539–570.
Patel, V. B., Zhong, J. C., Grant, M. B., & Oudit, G. Y. (2016). Role of the ACE2/Angiotensin 1–7 Axis of the renin-angiotensin system in heart failure. Circulation Research, 118(8), 1313–1326.
Han, W., Wang, M., Zhai, X., Gan, Q., Guan, S., & Qu, X. (2020). Chemical renal denervation-induced upregulation of the ACE2/Ang (1–7)/Mas axis attenuates blood pressure elevation in spontaneously hypertensive rats. Clinical and Experimental Hypertension, 42(7), 661–668.
Rodrigues Prestes, T. R., Rocha, N. P., Miranda, A. S., Teixeira, A. L., & Simoes-E-Silva, A. C. (2017). The anti-inflammatory potential of ACE2/Angiotensin-(1–7)/Mas receptor axis: Evidence from basic and clinical research. Current Drug Targets, 18(11), 1301–1313.
Gupta, D., Kumar, A., Mandloi, A., & Shenoy, V. (2021). Renin angiotensin aldosterone system in pulmonary fibrosis: Pathogenesis to therapeutic possibilities. Pharmacological Research, 174, 105924.
Li, X. C., Zhang, J., & Zhuo, J. L. (2017). The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacological Research, 125(Pt A), 21–38.
Yao, G., Li, W., Liu, W., Xing, J., & Zhang, C. (2021). The level and significance of circulating angiotensin-III in patients with coronary atherosclerosis. Journal of the Renin-Angiotensin-Aldosterone System, 2021, 1704762.
Zhuo, J. L., & Li, X. C. (2019). Angiotensin III/AT2 receptor/NHE3 signaling pathway in the proximal tubules of the kidney: A novel natriuretic and antihypertensive mechanism in hypertension. Journal of the American Heart Association, 8(9), e012644.
Zhuo, J. L., Ferrao, F. M., Zheng, Y., & Li, X. C. (2013). New frontiers in the intrarenal renin-angiotensin system: A critical review of classical and new paradigms. Front Endocrinol (Lausanne)., 4, 166.
Chai, S. Y., Fernando, R., Peck, G., Ye, S. Y., Mendelsohn, F. A., Jenkins, T. A., & Albiston, A. L. (2004). The angiotensin IV/AT4 receptor. Cellular and Molecular Life Sciences, 61(21), 2728–2737.
Wong, M. K. S. (2016). Other angiotensins. Handbook of Hormones, 261, e29C-3.
Jankowski, V., Tölle, M., Santos, R. A., Günthner, T., Krause, E., Beyermann, M., Welker, P., Bader, M., Pinheiro, S. V., Sampaio, W. O., Lautner, R., Kretschmer, A., van der Giet, M., Zidek, W., & Jankowski, J. (2011). Angioprotectin: An angiotensin II-like peptide causing vasodilatory effects. The FASEB Journal, 25(9), 2987–2995.
Lautner, R. Q., Villela, D. C., Fraga-Silva, R. A., Silva, N., Verano-Braga, T., Costa-Fraga, F., Jankowski, J., Jankowski, V., Sousa, F., Alzamora, A., Soares, E., Barbosa, C., Kjeldsen, F., Oliveira, A., Braga, J., Savergnini, S., Maia, G., Peluso, A. B., Passos-Silva, D., … Santos, R. A. (2013). Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circulation Research, 112(8), 1104–1111.
Villela, D. C., Passos-Silva, D. G., & Santos, R. A. (2014). Alamandine: A new member of the angiotensin family. Current Opinion in Nephrology and Hypertension, 23(2), 130–134.
Jankowski, V., Vanholder, R., van der Giet, M., Tölle, M., Karadogan, S., Gobom, J., Furkert, J., Oksche, A., Krause, E., Tran, T. N., Tepel, M., Schuchardt, M., Schlüter, H., Wiedon, A., Beyermann, M., Bader, M., Todiras, M., Zidek, W., & Jankowski, J. (2007). Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arteriosclerosis, Thrombosis, and Vascular Biology, 27(2), 297–302.
Yang, R., Smolders, I., Vanderheyden, P., Demaegdt, H., Van Eeckhaut, A., Vauquelin, G., Lukaszuk, A., Tourwé, D., Chai, S. Y., Albiston, A. L., Nahmias, C., Walther, T., & Dupont, A. G. (2011). Pressor and renal hemodynamic effects of the novel angiotensin A peptide are angiotensin II type 1A receptor dependent. Hypertension, 57(5), 956–964.
Wang, L., Liu, C., Chen, X., & Li, P. (2019). Alamandine attenuates long-term hypertension-induced cardiac fibrosis independent of blood pressure. Molecular Medicine Reports, 19(6), 4553–4560.
Jesus, I. C. G., Scalzo, S., Alves, F., Marques, K., Rocha-Resende, C., Bader, M., Santos, R. A. S., & Guatimosim, S. (2018). Alamandine acts via MrgD to induce AMPK/NO activation against ANG II hypertrophy in cardiomyocytes. American Journal of Physiology. Cell Physiology, 314(6), C702–C711.
Yang, C., Wu, X., Shen, Y., Liu, C., Kong, X., & Li, P. (2020). Alamandine attenuates angiotensin II-induced vascular fibrosis via inhibiting p38 MAPK pathway. European Journal of Pharmacology, 883, 173384.
de Souza-Neto, F. P., Silva, M. M. E., Santuchi, M. C., de Alcântara-Leonídio, T. C., Motta-Santos, D., Oliveira, A. C., Melo, M. B., Canta, G. N., de Souza, L. E., Irigoyen, M. C. C., Campagnole-Santos, M. J., Guatimosim, S., Santos, R. A. S., & da Silva, R. F. (2019). Alamandine attenuates arterial remodelling induced by transverse aortic constriction in mice. Clinical Science (London, England), 133(5), 629–643.
Silva, M. M., de Souza-Neto, F. P., Jesus, I. C. G., Gonçalves, G. K., Santuchi, M. C., Sanches, B. L., de Alcântara-Leonídio, T. C., Melo, M. B., Vieira, M. A. R., Guatimosim, S., Santos, R. A. S., & da Silva, R. F. (2021). Alamandine improves cardiac remodeling induced by transverse aortic constriction in mice. American Journal of Physiology. Heart and Circulatory Physiology, 320(1), H352–H363.
Fernandes, R. S., Dias, H. B., de Souza Jaques, W. A., Becker, T., & Rigatto, K. (2021). Assessment of alamandine in pulmonary fibrosis and respiratory mechanics in rodents. Journal of the Renin-Angiotensin-Aldosterone System, 2021, 9975315.
Liu, Q., Zheng, B., Zhang, Y., Huang, W., Hong, Q., & Meng, Y. (2021). Alamandine via MrgD receptor attenuates pulmonary fibrosis via NOX4 and autophagy pathway. Canadian Journal of Physiology and Pharmacology, 99(9), 885–893.
Acknowledgements
The authors convey their gratitude to CA. Dr. Pannai M. Karthikeyan, Ph.D, Chairman of Pannai College of Pharmacy, Dindigul, India for his constant support. The authors extend their thankfulness to Er. M. Praveen Kanna of Pannai College of Pharmacy, India for the editorial support.
Funding
This review work did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have substantially contributed to the conception and design of the article and interpreting the literature pertaining to the topic. PB, RSL, and AT contributed in drafting the article. PB, RSL, and VS prepared the figures. PB, SH, AA, TA, NAK, KS, and VS revised the article for important intellectual content. All authors have read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work.
Additional information
Handling Editor: Kurt J. Varner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balakumar, P., Handa, S., Alqahtani, A. et al. Unraveling the Differentially Articulated Axes of the Century-Old Renin–Angiotensin–Aldosterone System: Potential Therapeutic Implications. Cardiovasc Toxicol 22, 246–253 (2022). https://doi.org/10.1007/s12012-022-09724-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09724-y