Abstract
Chronic mixed toxicant exposure has been implicated in the aetiology of lung and heart failure through prolonged free radical generations. This study was carried out to assess the protective effect of naturally occurring phenolic components from Croton zambesicus (400 mg/kg C-ZAMB) leaves against cardiopulmonary toxicity induced by chronic mixed toxicant (0.5 mL EOMABRSL) in rats. Chronic cardiopulmonary injury via oral administration of 0.5 ml EOMABRSL for 98 days (non-withdrawal) and 70 days (withdrawal) caused unhealthy alteration in the levels of oxidative stress biomarkers [malondialdehyde (MDA), reduced glutathione (GSH), glutathione-S-transferase (GST), superoxide dismutase (SOD) and catalase]. Similarly, both withdrawal and non-withdrawal approaches of EOMABRSL-exposed animals exhibited increase in the activity of eco-51-nucleotidase (51ENT) with corresponding diminution in the activity of lactate dehydrogenase (LDH), i.e. the metabolic fuel for cardiopulmonary wellness. Ultimately, histology examination confirmed hyperplastic, bronchopneumonia and cloudy swelling of cardiovascular cells followed by the accumulation of cellular exudates and haemorrhage in the alveoli and bronchioles. The active antioxidants of 400 mg/kg C-ZAMB leaves were responsible for the biological protection of cardiopulmonary toxicity by modulating the activities of 51ENT and LDH. The oxidative stress was also reversed by 400 mg/kg phenolic C-ZAMB leaves in the heart and lungs. Hence, 400 mg/kg phenolic C-ZAMB leaves may be a natural therapy for the treatment of cardiovascular disorder associated with pulmonary dysfunction in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental contamination and pollution by mixture of ecological toxicants is one of the major challenges in the modern human society [1]. It is also a threat to the biosphere and terrestrial habitat [1, 2]. Due to the evolution of anthropogenic activities, growth of human population and modern expansion of industrial processes, mixture of toxicants are rapidly entered into several environmental compartments; soil, water, air and their interface [3]. Their accumulation in the environment contributes a significant dilemma to all spheres of living organisms [4, 5]. It was reported that numerous industrial activities, including automobile battery production, have often resulted in the accumulation of noxious mixed metal in the environment [6,7,8]. Also, the discharge of heavy metals as by‐products of these activities has been accompanied by large‐scale soil pollution [8, 9]. Ultimately, mixture of toxicants including heavy metals tends to persist in the environment indefinitely due to their non-degradable property [10, 11]. Experimental and epidemiological studies have reported the association between environmental intoxication and increased risks of lungs and vital organs damage [12,13,14]. Mixture of heavy metal toxicity has also been associated with the prostate cancer [13, 15], cardiovascular disease, miscarriage, premature birth and foetal malformations [16]. Further studies also reported behavioural disorders, learning impairments and Alzheimer’s disease on exposure to mixed pollutants [12, 17,18,19].
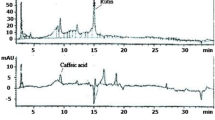
Croton zambesicus(C-ZAMB) has been used against a number of diseases [20]. In-vivo study revealed that the prepared seeds extracts of C-ZAMB have a significant benefit on erythropoiesis and lowering cholesterol level [21]. It has also shown anti-inflammatory, analgesic and antipyretic activity [22, 23]. In the same vein, in-vitro studies have reported its antioxidant activity [24], anticoagulant activity [25] and vascular smooth muscle relaxant activity [26, 27]. Furthermore, aqueous leaf extract and n-butanol fraction of C-ZAMB protected against carbon tetrachloride-induced renal toxicity in rats [20]. It was equally stated that C-ZAMB leaves inhibited hyperlipidemia, hyperglycemia and cardiovascular disorders in a rat model [28]. Added to these findings, HPLC analysis from our recent study had shown the presence of gallic acid, catechin, chlorogenic acid, caffeic acid, orientin, rutin, quercitrin, quercetin and luteolin [29] with essential elements known as thiamin, riboflavin, pyridoxine, niacin, folacin, calcium, iron, sodium, phosphorous, copper, zinc and potassium [30]. Also, monosaccharides such as glucose, rhamnose, xylose and arabinose have been detected in the leaves of C-ZAMB [30]. Finally, the leaves are rich in essential amino acids including arginine, cysteine, lysine, carnitine and fatty acids such as the unsaturated and essential fatty acids [30].
People who experience cardiovascular failure on exposure to mixture of environmental toxicants may demonstrate a multi-complex disease or be at higher risk of pulmonary dysfunction. This is because humans are generally exposed to mixture of chemicals rather than an individual chemical. And, simultaneous exposure to multiple toxicants may occur by additive, antagonistic or synergistic interaction with biomolecules [29]. Due to the excessive creation of small-scale companies coupled with industrial advancement and consequential release of complex mixture of pollutants into the natural environment which exhibits interactive effect on ecosystem thereafter, may alter metabolic processes in mammals. This informs our choice of animal model in this study. Similarly, owing to the multiple pharmacological potentials of C-ZAMB leaves and because its protective effect on chronic mixed metal intoxication related to cardiopulmonary toxicity is not known, an animal model was therefore used in this study to examine the potential protective effects of phenolic C-ZAMB leaves on cardiopulmonary damage induced by chronic mixed toxicant from automobile battery recycling site.
Materials and Methods
Preparation of Plant Materials
Fresh leaves of C-ZAMB were recognized, authenticated at the Department of Plant Biology, University of Ilorin and reported with voucher number of UIH001/1191.
Extraction of the Phenolic C-ZAMB Leaves
Blended leaves (50 g) was weighed into a clean flat bottomed container, 500 ml of methanol was added and kept on shaker and stirring set at room temperature for 24 h. The mixture was then filtered; first through a fresh cotton plug and then with a Whatman No. 1 filters paper. The phenolic fraction was then fractionated by partitioning chromatography. This was done by first suspending it in distilled water. Hexane was added to the suspension in the ratio of 1:2, shaken well and allowed to stand for about 15 min until two layers were formed. The hexane layer was removed and more hexane was added to the aqueous layer. The process was repeated once, and then a colourless hexane layer was seen. The two hexane layers were combined and dried to obtain the hexane fraction. The procedure was repeated with the aqueous layer using ethyl acetate. The filtrate was combined and concentrated using a rotary evaporator at low temperature (40 °C) and pressure. The concentrated product was further dried over a water bath at 40 °C and then kept air-tight for further use.
Determination of Essential Phenolic Compounds in C-ZAMB Leaves
The total phenol content of C-ZAMB leaves was determined according to the Folin-Ciocalteu method used by Chan et al. [31]. Total flavonoid was assessed as reported by Kale et al. [32] Determination of tannin content was done using the method of Padmaja [33]. Also, total saponins were determined by the modified method of Makkar et al. [34] Vitamin C level was estimated using the method of Benderitter et al. [35]
EOMABRSL Preparation
Leachate from Elewi Odo Municipal Auto-Battery Recycling Site was prepared according to our previous finding [18]. It was designated as EOMABRSL.
Characterization of Heavy Metals and Organic Pollutants in EOMABRSL
Characterization of heavy metals and identification of organic pollutants in EOMABRSL using Fourier infrared chromatography have been documented in our previous study [20].
Experimental Animal
Sixty (60) Adult Female Wistar rats (130–170 g) were obtained from the Central Animal House of the Kwara State University, Nigeria, and were kept in well-aerated cages at a room temperature on a 12-h light/dark cycle with water and standard fed was giving ad libitum for 2 weeks before the commencement of the study.
Animal Grouping
After acclimatization, the animals were randomly divided into five (5) groups of ten animals each (n = 10).
-
Group 1 (Control) was given 0.5 ml of distilled water only via oral route.
-
Group 2 (NWD-EOMABRSL) was exposed to 0.5 ml of leachate from EOMABRSL for 98 days (chronic exposure).
-
Group 3 (WD-EOMABRSL) was exposed to 0.5 ml of leachate from EOMABRSL for 70 days and the leachate was withdrawn for 28 days.
-
Group 4 (EOMABRSL + C-ZAMB) was exposed to 0.5 ml of leachate from EOMABRSL for 70 days and post-treated with 400 mg/kg body weight of phenolic C-ZAMB leaves for 28 days.
-
Group 5 (C-ZAMB only) was treated with 400 mg/kg body weight of phenolic C-ZAMB leaves for 28 days.
Dose Selection
The dose of 400 mg/kg of phenolic C-ZAMB leaves and 0.5 ml EOMABRSL was selected following the method of Ashwell et al. [36] and Akintunde et al. [37], respectively. The control and treated rats were subjected to the same condition. The treatment period for the experiment was 98 days. For uniformity in the exposure period, we choose exposure time 70 days while the treatment period was 28 days. Furthermore, we selected this exposure period because 70 days and 98 days reflect similar outcome in toxicity and the treatment for 28 days advocates its management. This model of exposure was justified by previous study [38].
Preparation of Heart and Lung Homogenates
The rats were anesthetized using N-hexane gas medium and sacrifice by cervical dislocation. The hearts and lungs were removed and cleared of adhering connective tissues. Each rat’s heart and lung was weighed and then homogenized in 50 mM Tris–HCl buffer (0.1 M, pH 7.4). Thereafter, each heart and lung tissue homogenate was centrifuged at 10, 000×g for 15 min at 4 °C. Afterward, the heart and lung supernatants were immediately separated into various aliquots for different biochemical assays (pellets were discarded).
Eco-51-nucleotidase activity assay
The eco- 51-nucleotidase activity (heart and lung) was determined essentially by the method of Heymann et al. [39].
Lactate Dehydrogenase (LDH) Assay
LDH activity was determined using commercially available kit (Randox Laboratories UK). It was carried out according to the manufacturer’s guidelines [40].
Biological Antioxidant Assays
Superoxide dismutase (SOD) activity carried out according to the method described by Misra and Fridovich [41]. Catalase activity was also measured in the homogenate collected using hydrogen peroxide as the substrate as described by Clairborne [42]. Glutathione-S-transferase (GST) activity was assayed using 1-chloro-2, 4-dinitrobenzene as the substrate according to the method of Habig [43]. Reduced glutathione (GSH) was determined using the method described by Jollow et al. [44]. Lipid peroxidation was quantified as malondialdehyde (MDA) according to our previous method [45] and expressed as nmoles/mg protein.
Histopathological Examination
Each heart and lung was fixed in Bouin’s fluid for 24 h before they were cut longitudinally into two equal halves and again post-fixed in fresh Bouin’s fluid for an additional 24 h. The tissues were dehydrated in ascending strengths of alcohol and cleared in xylene. Infiltrated and embedded in paraffin wax, tissue blocks were made by cutting them into 5-μm thick sections with the help of a rotatory microtome. The sections were mounted on albumenized glass slides and stained with eosin and hematoxylin. Morphological study of each heart and lung was performed with the help of ocular micrometer scale under the microscope.
Statistical Analysis
All data were expressed as mean ± SD (standard deviation). The statistical analysis used was one-way ANOVA, followed by Duncan's multiple range tests at the significance level α = 0.05.
Result
Active Antioxidants
The active antioxidants from C-ZAMB leaves responsible for the protection of cardiopulmonary tissue were quantified (Table 1) as phenols (348 mg/100 g), tannin (248 mg/100 g), flavonoids (125 mg/100 g), saponins (303 mg/100 g) and carotenoids (643 mg/100 g).
Effect of Antioxidant C-ZAMB Leaves on Eco-51-Nucleotidase Activity in Cardiopulmonary-Induced Toxicity Rat
The effect of C-ZAMB leaves on eco-51-nucleotidase activity in EOMABRSL-induced cardiopulmonary toxicity is presented in Fig. 1. Post hoc study revealed that EOMABRSL-exposed animals for 98 days (non-withdrawal) and 70 days (withdrawal) significantly (p < 0.05) increased eco-51-nucleotidase activity when compared to the corresponding control. The increased eco-51-nucleotidase activity was substantially (p < 0.05) reversed on post-treatment with 400 mg/kg C-ZAMB phenolic fraction in relation to the EOMABRSL groups. A better significant (p < 0.05) reduction of the eco-51-nucleotidase activity occurred in C-ZAMB- treated animals in relation to post-treatment rats.
Effect of Phenolic C-ZAMB Leaves on LDH Activity in EOMABRSL Cardiopulmonary-Induced Toxicity Rat
The protective effect of phenolic C-ZAMB leaves on activity of LDH enzyme in EOMABRSL-induced cardiopulmonary toxicity of rats is shown in Fig. 2. Exposure to 0.5 ml EOMABRSL 98 days (non-withdrawal) and 70 days (withdrawal) significantly (p < 0.05) decreased the metabolic marker of ATP (LDH) in both heart and lung of rat when compared to their corresponding controls. Contrariwise, post-treatment with 400 mg/kg phenolic C-ZAMB leaves significantly (p < 0.05) elevated the depleted activity of LDH cardiopulmonary homogenates.
Effect of Phenolic C-ZAMB Leaves on MDA Level in EOMABRSL-Induced Cardiopulmonary Toxicity Rat
Cardiac and pulmonary MDA reflect the extent of cell membrane damage. As shown in Fig. 3, exposure of animals to EOMABRSL for 98 days (non-withdrawal) and 70 days (withdrawal) did not proffer effect on MDA content in relation to control, whereas there was significant (p < 0.05) high MDA content in the lungs of rats exposed to chronic EOMABRSL for 98 days (non-withdrawal) and 70 days (withdrawal) when compared with the control (Fig. 3) thus, signifying pulmonary membrane damage. Noticeably, post-treatment of rats with 400 mg/kg C-ZAMB antioxidant fraction significantly (p < 0.05) prevented the cell membrane damage by depleting MDA level in the heart and lung. Cardiopulmonary MDA content was restored below normal (Fig. 3).
Effect of C-ZAMB Antioxidant Fraction on Induced Alterations in Cardiac and Pulmonary Oxidative Stress Biomarkers (GSH, GST, SOD and Catalase)
Figure 4 depicts considerable reduction (p < 0.05) in GSH in animal intoxicated with EOMABRSL for 90 days (non-withdrawal) and 70 days (withdrawal) in relation to the control. Post-treatment of rats with C-ZAMB phenolic fraction at 400 mg/kg significantly (p < 0.05) restored GSH in both cardiac and pulmonary tissue. Pulmonary GSH content was upregulated better by 400 mg/kg C-ZAMB than cardiac GSH level (Fig. 4). Figure 5 shows that the heart and lung GST were remarkably (p < 0.05) depleted on exposure to EOMABRSL, while post-treatment of rats with 400 mg/kg C-ZAMB antioxidant fraction evidently (p < 0.05) restored the cardiac and pulmonary GST activities. Furthermore, post hoc analysis showed that pulmonary SOD activity was significantly (p < 0.05) depleted on exposure to 0.5 ml EOMABRSL for 98 days (non-withdrawal) and 70 days (withdrawal) (Fig. 6) when compared to the corresponding control. Non-withdrawal exposure to EOMABRSL for 98 days significantly (p < 0.05) amplified cardiac SOD activity in relation to control (Fig. 6), whereas EOMABRSL withdrawal animals did not show significant (p > 0.05) rise in cardiac SOD activity when compared with the control (Fig. 6). The treatment with phenolic C-ZAMB leaves prevented the alteration of SOD activity in the examined tissues, although post-treatment with 400 mg/kg C-ZAMB did not show significant reduction (p > 0.05) in cardiac SOD activity in respect to the control (Fig. 6). Finally, exposure of rats to EOMABRSL for 98 and 70 days remarkably (p < 0.05) increased cardiac catalase activity when compared with the control (Fig. 7). Conversely, exposure of rats to EOMABRSL for 98 and 70 days remarkably (p < 0.05) decreased pulmonary catalase activity when compared with the control (Fig. 7). However, administration of 400 mg/kg C-ZAMB leaf fraction prohibited the alteration of cardiopulmonary catalase activity in relation to the control (Fig. 7).
Effect of C-ZAMB Antioxidant Fraction on Histopathological Changes in Cardiac and Pulmonary Tissues
Histological examination (Fig. 8) shows no visible lesions to cardiac tissue in the control animals. Whereas, exposure of animals to EOMABRSL for 98 days (NWD-EOMABRSL) and 70 days (WD-EOMABRSL) caused cloudy swelling and degeneration of the cardiomyocytes, indicating cardiovascular dysfunction. Post-treatment with 400 mg/kg C-ZAMB showed no visible lesions to the cardiac cells. Figure 9 shows histologic picture of lung rats intoxicated with EOMABRSL for 98 days (NWD-EOMABRSL) and 70 days (WD-EOMABRSL). Exposure to 0.5 ml EOMABRSL caused multiple foci accumulation of cellular exudates and haemorrhage in the alveoli and bronchioles. The bronchiolar epithelium was characterized by hyperplastic, bronchopneumonia, pulmonary haemorrhages and emphysema. Post-treatment with 400 mg/kg C-ZAMB repaired the pathological changes as evidenced by normal alveoli and bronchioles (Fig. 9).
Cardiac histopathology changes on rat treated with C-ZAMB in EOMABRSL-induced cardiopulmonary toxicity (original magnification × 400). Control: The cardiomyocytes show no visible lesion. NWD-EOMABRSL: The cardiomyocytes show a few foci of mild cloudy swelling (arrows). These are indicative of cardiovascular dysfunctions. WD-EOMABRSL: The cardiomyocytes show few foci of moderate degeneration (arrows). These are suggestive of cardiovascular injuries EOMABRSL + C-ZAMB: The cardiomyocytes depict no visible lesion (NVL). C-ZAMB only: The cardiomyocytes portray no visible lesion
Pulmonary (lung) histopathology changes on rat treated with C-ZAMB in EOMABRSL-induced cardiopulmonary toxicity (original magnification × 400). Control: There are foci of accumulation of cellular exudates into the alveoli (star) and bronchioles (arrow). NWD-EOMABRSL: There are multiple foci of accumulation of cellular exudates and haemorrhage into the alveoli and bronchioles (thick arrow). The bronchiolar epithelium (thin arrow) is mildly hyperplastic. These are suggestive of a bronchopneumonia WD-EOMABRSL: There are multiple extensive foci (star) of haemorrhages interspersed between overtly-distended alveoli (arrows). These are indicative of pulmonary haemorrhages and emphysema EOMABRSL + C-ZAMB: The alveoli and bronchioles appear normal, i.e. no visible lesions (NVL) C-ZAMB only: There are foci (star) of accumulation of cellular exudates into the alveoli and bronchioles
Discussion
Previously, Cu (0.34 ppm), Zn (0.01 ppm), Cd (0.006 ppm), Mn (7.842 ppm), Co (0.049 ppm), Cr (0.068 ppm), Fe (2.667 ppm), Ni (0.051 ppm) and Pb (0.015 ppm) were discovered as major constituents of EOMABRSL [29]. Mixture of metals which constitute an important class of toxic substances is encountered on daily basis during occupational and environmental activities [46,47,48]. Subchronic exposure to mixture of environmental toxicants in this study may cause injury [49, 50] to the cardiovascular [23] and pulmonary organs [51, 52] because the entering of mixed metal into the animals’ body [53] caused the diffusion of contaminated blood into the alveolar airways to initiate inflammatory responses in the respiratory tract [54, 55]. However, the biotransformation of the metals in the target organs (heart and lung) may trigger cardiopulmonary toxicity [56, 57]. This observation is similar to previous findings which reported that mixture of environmental metals has the potential to interact with biomolecules [58] to cause cardiotoxicity [59] and pulmonary dysfunction [60] in both animals and humans. Organic pollutants including acetonitrile, ethyl-4-chloro-2-cyanoacetoacetate, 3-methoxyphenyl acetonitrile, 2,4,6 tri-hydroxyl acetoacetate, phenyl sulfonyl acetonitrile, 3-methylacetophenone, ethyl aceto-hydroxamate, 1-acetonaphthone and benzyl acetone acetonitrile were equally detected in EOMABRSL [29]. Findings have reported that organic pollutants can be activated into highly toxic metabolites by phase 1 enzyme (CYP450) to implicate organ toxicity [61, 62] and free radical toxicity [63, 64]. The antioxidant compounds in C-ZAMB leaves particularly phenols, flavonoids, and carotenoids may proffer protection against cardiopulmonary toxicity [13]. Former studies have reported that antioxidants and mineral elements including sodium, potassium, calcium, magnesium, zinc, iron, copper and manganese may cause organ resuscitation [65, 66].
Ectonucleotidases are uncontrollably produced in the body on exposure to exotoxins during the purinergic cascade of many cells [32]. Result from this study showed that EOMABRSL upregulated the activity of eco-51-nucleotidase enzyme in cardiac and pulmonary tissues, indicating rapid ATP hydrolysis which decimate heart and lungs capacity or reperfusion injury. The reduced activity of eco-51-nucleotidase enzyme in cardiac and pulmonary tissues by post-treatment with 400 mg/kg C-ZAMB phenolic fraction suggested a remarkable fall in ATP hydrolysis, causing more extracellular production of adenosine and ATP for organ’s efficiency [32, 67]. Report has shown that extracellular ATP protects endothelial cells against DNA damage induced by irradiation or chemically induced damage [68]. Also, the low activity of ectonucleotidase enzyme in rats treated with C-ZAMB leaves indicated proper workability of cardiac function [69] and controlled synthesis of adenine nucleotides in the lungs [70]. This outcome suggested abolishment of lung inflammation through NOS/cGMP/PKG signalling pathway on treatment with flavonoids found in C-ZAMB leaves [71]. Although this speculation calls for confirmatory studies. Ultimately, increased cardiopulmonary LDH activity in animals post-treated with phenolic C-ZAMB leaves confirmed that the heart and lungs have supported extra-hepatic biotransformation and detoxification of toxic substances. This is consistent with previous finding which stated that reduced ATP weakens the heart, causing ischaemia or myocardial infarction [72].
Oxidative damage has been associated with mixture of metal exposure in experimental animal models and humans [73, 74]. This finding shows that enhanced MDA particularly in the lung may possibly be the result of a joint effort of the build-up of reactive oxygen species, causing dysfunctional GSH, and overproduction of free radicals during subchronic exposure to metal mixture [70]. Additive or synergistic interaction of the activated mixed metals (Cu2+, Zn2+, Cd2+ Mn2+, Cr3+, Fe2+, Ni+ and Pb2+) may induce oxidative stress by inhibiting the enzymes, which results in accumulation of O2−, •OH and H2O2. Also, Pb2+ may stimulate Fe2+-mediated membrane lipid peroxidation by eliciting changes in membrane physical properties. However, the group of divalent metals, namely Cu2+, Zn2+, Cd2+ Mn2+, Fe2+ and Pb2+ competitively bind exclusively to thiol groups, thus, decreasing glutathione’s reductive potency to interfere with antioxidant activity [74]. They may also block the activation of Ca2+/phospholipid-dependent protein kinase C (PKC) which is closely associated with cardiopulmonary functions [75]. Report has suggested that the organ toxicity of metals is due to their interference with normal Ca2+ signalling in cells [76]. It is categorically reported here that one of the potential mechanisms of mixed metal intoxication may include their capacity to affect cell membrane biophysics, cause oxidative stress, and trigger oxidant-sensitive transcription factors. However, this study revealed the positive effects of phenolic C-ZAMB leaves on cell membrane of cardiopulmonary cells. In accordance with this, it has been reported that phenols and flavonoid-tocopherol inhibit free radical formation and PKC in epithelial cells [12, 77, 78].
Recently, chronic exposure to mixture of environmental and organic toxicants has generated a lot of public trauma [79]. Following chronic exposure, the organic pollutants may undergo extra-hepatic biotransformation via glucuronidation and sulfation reactions occurring principally in the heart and result in air-soluble metabolites that are excreted in the respiratory tract of the lungs [80]. Due to the metabolic transformation of environmental toxicants by the microsomal CYP450 enzyme scheme, many mixtures of highly reactive intermediates are produced [68]. The complex mixture of highly toxic metabolites directly reacts with GSH at chronic state, and the diminution of lung and cardiac GSH occurs. Composite toxic metabolites additively bind to cellular proteins and initiate cellular damage, causing cardiac and pulmonary injury [74, 81, 82]. Remarkably, the generation of free radicals or reacting oxygen species may have heralded the exhaustion of GSH and cardiopulmonary toxicity [73]. This finding further suggests that intracellular GSH plays a crucial role in the elimination of complex toxic metabolites and prevention of EOMABRSL-induced cardiopulmonary toxicity. Glutathione redox cycle contributes to intracellular antioxidant structure in the body due to its maintenance of cell metabolism and integral stability [12]. Substantial drop in the pulmonary tissue level of glutathione was associated with EOMABRSL-mediated pulmonary toxicity. Consistent with these studies [79, 83, 84], chronic exposure to 0.5 ml EOMABRSL for 98 days (non-withdrawal) and 70 days (withdrawal) in our study led to the fall in GST and alterations of the cardiac as well as pulmonary antioxidant pool viz., SOD and catalase. The decline in GST status with corresponding alterations of SOD and catalase activities partially explains the mechanism of cardiopulmonary toxicity induced by chronic exposure to EOMABRSL and may be due to the free radical generations which have been confirmed to have damaging effects on the cells, tissues and organs [12, 78]. Polyphenols, natural and composite antioxidants, can ligate with the free radicals in several media to enhance their detoxification from the body [67]. The post-treatment of C-ZAMB, a composite phenol fraction, to rats, however, significantly restored the activity of GST alongside with the inhibition of altered SOD and catalase activities both in the heart and in the lungs. The recovery in the activities of these antioxidant enzymes amplifies the protection by the phenolic C-ZAMB leaves in rats against cardiopulmonary toxicity on exposure to EOMABRSL. Some findings have confirmed composite phenolic compound to be a strong antioxidant, showing robust protection against cytotoxicity [65], oculopathy [85], diabetes [86] and cancer progression [87].
Several reports reveal that mixture of environmental toxicants causes cellular dysfunctions and alterations in tissue histology [23, 88, 89] in Wistar rats. Chronic exposure of animals to EOMABRSL for 98 days (NWD-EOMABRSL) and 70 days (WD-EOMABRSL) enhanced cloudy swelling and degeneration of the cardiomyocytes, indicating cardiovascular dysfunction and histologic lesions in the heart [89]. Similarly, chronic exposure to 0.5 ml EOMABRSL caused multiple foci accumulation of cellular exudates and haemorrhage in the alveoli and bronchioles. The bronchiolar epithelium lesion was augmented by hyperplastic, bronchopneumonia, pulmonary haemorrhages and emphysema in EOMABRSL-exposed animals. The EOMABRSL-mediated bronchiolar dysfunction and cardiac structural alterations are in agreement with other studies [23, 88] and these may relate to cardiopulmonary disorder. However, post-treatment with 400 mg/kg C-ZAMB leaf fraction attenuated the EOMABRSL-mediated functional and histological alterations, showing no visible lesions and its potential to ameliorate EOMABRSL-induced cardiopulmonary dysfunction in rats. Thus, the prevention of EOMABRSL-stimulated cardiopulmonary toxicity and downregulation of pericarditis, myocardial infarction, atherosclerosis, bronchopneumonia, pulmonary haemorrhages and emphysema may follow other mechanisms by which composite phenol of C-ZAMB leaves normalizes the development of cardiopulmonary dysfunction in rats. This finding needs further studies to elucidate the inflammatory/chemokines modulation in the EOMABRSL/phenolic-treated rats.
Finally, our findings show that subchronic exposure to EOMABRSL for 70 days, i.e. withdrawal for 28 days similarly reflected cardiopulmonary lesions in relation to animals chronically exposed for 98 days. This provides evidence that environmental metals bio-accumulate and bio-magnify in the living tissues [90] on exposure to mammals. The cardiopulmonary toxicity induced by EOMABRSL for 70 days likewise indicated that heavy metals as well as organic toxicants have freely and identically diffused into compartmentalized body cells and tissues for bio-accumulation. This signifies that there is imbalance between bio-accumulation and detoxification processes of mixed metal exposure [12, 91]. This result is supported by earlier finding [92] which reported that toxicant exposure for short (subchronic) and long (chronic) duration was associated with prolonged liver damage and respiratory cell death [93,94,95]. Thus, it is rational to report here that subchronic (70 days) and chronic (90 days) exposures to mixed environmental toxicants showed no significant difference in the outcome of cardiopulmonary toxicity.
Conclusion
Subchronic (70 days) and chronic (90 days) exposure of rats to EOMABRSL caused cardiovascular and bronchiolar dysfunctions as evident by increased activity of eco-51-nucleotidase and oxidative damage with corresponding depletion of cellular ATP in the lungs and heart. A dose of 400 mg/kg phenolic C-ZAMB leaves showed a strong protective potential to ameliorate EOMABRSL-mediated cardiopulmonary dysfunction by inhibiting eco-51-nucleotidase activity and oxidative stress with improving cellular ATP and cardiopulmonary histology. Hence, this finding needs further studies to elucidate the inflammatory/chemokines modulation in the EOMABRSL/phenolic-treated rats.
References
Ali, H., Khan, E., & Sajad, M. A. (2013). Phytoremediation of heavy metals-concepts and applications. Chemoshere, 91(7), 869–881.
Hashem, M. A., Nur-A-Tomal, M. S., Mondal, N. R., & Rahman, M. A. (2017). Hair burning and liming in tanneries is a source of pollution by arsenic, lead, zinc, manganese and iron. Environmental Chemistry and Letters, 15(3), 501–506.
Ogaga, A. A., Faith, A. M., & Sylvester, C. I. (2018). Impacts of anthropogenic activities on heavy metal levels in surface water of Nun River around Gbarantoru and Tombia Towns, Bayelsa State, Nigeria. Annals of Ecology and Environmental Science, 2(2), 1–8.
Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8(3), 199–216.
Jaishankar, M., Mathew, B. B., Shah, M. S., & Gowda, K. R. S. (2014). Biosorption of few heavy metal ions using agricultural wastes. Journal of Environmental Pollution and Human Health, 2(1), 1–6.
He, Z. L., Yang, X. E., & Stoffella, P. J. (2005). Trace elements in agroecosystems and impacts on the environment. Journal of Trace Elementary Medicine and Biology, 19(2–3), 125–140.
Stern, B. R. (2010). Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. Toxicological Environmental Health A, 73(2), 114–127.
Beyersmann, D., & Hartwig, A. (2008). Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Archive of Toxicology, 82(8), 493–512.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metals toxicity and the environment. NHS Public Access, 101, 133–164.
Sheng, J. J., Wang, X. P., Gong, P., Tian, L. D., & Yao, T. D. (2012). Heavy metals of the Tibetan top soils Level, source, spatial distribution, temporal variation and risk assessment. Environmental Science and Pollution Resources, 19, 3362–3370.
Wieczorek-Dąbrowska, M., Tomza-Marciniak, A., Pilarczyk, B., & Balicka-Ramisz, A. (2013). Roe and red deer as bioindicators of heavy metals contamination in north-western Poland. Chemical Ecology, 29(2), 100–110.
Monisha, J., Tenzin, T., Naresh, A., Blessy, B. M., & Krishnamurthy, N. B. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60–72.
Akintunde, J. K., & Oboh, G. (2015). Depletion of cellular adenosine triphosphate and hepatocellular damage in rat after sub-chronic exposure to leachate from anthropogenicrecycling site. Human and Experimental Toxicology, 34(11), 1083–1095.
Aleksandra, B., David, W., Vesna, M., Amie, S., Branislav, O., Dusan, M., & Vladimir, D. (2017). Cadmium exposure as a putative risk factor for the development of pancreatic Cancer: Three different lines of evidence. Biomedical Resources International, 1, 1981837.
Krishna, A. K., & Mohan, K. R. (2016). Distribution, correlation, ecological and health risk assessment of heavy metal contamination in surface soils around an industrial area, Hyderabad, India. Environmental Earth Science, 75, 411.
World Health Organization. (2017). Fact sheet: Lead poisoning and health. Retrieved December 10, 2017, from https://www.who.int/mediacentre/factsheets/fs379/en/.
Sogl, M., Taeger, D., Pallapies, D., Brüning, T., Dufey, F., Schnelzer, M., et al. (2012). Quantitative relationship between silica exposure and lung cancer mortality in German uranium miners, 1946–2003. British Journal of Cancer, 107(7), 1188–1194.
Akintunde, J. K., Oboh, G., & Akindahunsi, A. A. (2015). Inhibition of key markers linked with spermatogenesis and cellular ATP by sub-chronic exposure to leachate in a rat model. Archive Environmental Contamination and Toxicology, 68(1), 159–168.
French, I. T., & Muthusamy, K. A. (2018). A review of the pedunculopontine nucleus in parkinson’s disease. Frontier of Aging Neuroscience, 10, 99.
Ayanniyi, R. O., Olumoh-Abdul, H. A., Ojuade, F. I., Abdullahi, R., & Anafi, S. B. (2019). The protective effect of Croton zambesicus against carbon tetrachloride-induced renal toxicity in rats. International Journal of Toxicology, 1, 5–8.
Adam-Sharma, I. Y., & Ebtihal, A. S. (2013). Investigations on the effects of various oral doses of Croton zambesicus seeds’ in wistar rats. Journal of Pharmacology and Toxicology, 8(1), 19–27.
Okokon, J. E., & Nwafor, P. A. (2010). Anti-inflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pakistan Journal of Pharmaceutical Science, 92(4), 385–392.
Ndhlala, A. R., Aderogba, M. A., Ncube, B., & Van Staden, J. (2013). Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related Croton species. Molecules, 18(2), 1916–1932.
Aderogba, M. A., McGaw, L. J., Bezabih, M., & Abegaz, B. M. (2011). Isolation and characterisation of novel antioxidant constituents of Croton zambesicus leaf extract. Natural Product Resources, 25(13), 1224–1233.
Robert, S., Baccelli, C., Devel, P., Dogne, J. M., & Quetin-Leclercq, J. (2010). Effects of leaf extracts from Croton zambesicus Muell Arg. on hemostasis. Journal of Ethnopharmacology, 128(3), 641–648.
Baccelli, C., Navarro, I., Block, S., Abad, A., Morel, N., & Quetin-Leclercq, J. (2007). Vasorelaxant activity of diterpenes from Croton zambesicus and synthetic trachylobanes and their structure-activity relationships. Journal of Natural Products, 70(6), 910–917.
Martinsen, A., Baccelli, C., Navarro, I., Abad, A., Quetin-Leclercq, J., & Morel, N. (2010). Vascular activity of a natural diterpene isolated from Croton zambesicus and of a structurally similar synthetic trachylobane. Vascular Pharmacology, 52(1), 63–69.
Asare, G. A., Adjei, S., Afriyie, D., Appiah-Danquah, A. B., Asia, J., Asiedu, B., et al. (2015). Croton membranaceus improves some biomarkers of cardiovascular disease and diabetes in genetic animal models. Journal Clinical Diagnosis Resources, 9(12), 1–5.
Akintunde, J., Ayeni, S., Adeoye, M., & Shittu, A. (2020). Rat liver and kidney post-mitochondrial dysfunction by addition of chronic mixed metal intoxication and hepatorenal wellness mediated by phenolic components from Croton zambiscus leaves. Environmental Toxicology and Pharmacology, 74, 103293.
Bello, M. O., Abdul-Hammed, M., Adekunle, S. A., & Fasogbon, O. T. (2014). Nutrient contents and fatty acids profiles of leaves and seeds of Croton zambesicus. Advance Journal of Food Science and Technology, 6(3), 398–402.
Chan, E. W. C., Lim, Y. Y., & Chew, Y. L. (2006). Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chemistry, 102, 1214–1222.
Kale, A., Gaikwad, S., Mundhe, K., Deshpande, N., & Salvekar, J. (2010). Quantification of phenolics and flavonoids by spectrophotometer from Juglans regia. International Journal of Pharmaceutical Bioscience, 1, 1–4.
Padmaja, G. (1989). Evaluation of techniques to reduce assayable tannin and cyanide in cassava leaves. Journal of Agriculture and Food Chemistry, 137, 712–716.
Makkar, H. P. S., Siddhuraju, P., & Becker, K. (2007). Plant secondary metabolites. Totowa, NJ: Humana Press.
Benderitter, M., Maupoil, V., Vergely, C., Dalloz, F., Briot, F., & Rochette, L. (1998). Studies by electron paramagnetic resonance of the importance of iron in the hydroxyl scavenging properties of ascorbic acid in plasma: Effects of iron chelators. Fundamental Clinical Pharmacology, 12, 510–516.
Ashwell, R. N., Mutalib, A. A., Bhekumthetho, N., & Johannes, V. S. (2013). Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related croton species. Molecules, 18, 1916–1932.
Akintunde, J. K., Irondi, A. E., Ajani, E. O., & Olayemi, T. V. (2018). Neuroprotective effect of dietary black seed flour on key enzymes linked with neuronal signaling molecules in rats exposed to mixture of environmental metals. Journal of Food Biochemistry, 42(5), e12573.
Dauwe, T., Janssens, E., Kempernaers, B., & Eens, M. (2004). The effect of heavy metal exposure on egg size, egg shell thickness and the number of spermatozoa in blue tit parus caeruleus eggs. Environmental Pollution, 129, 125–129.
Heymann, D., Reddington, M., & Kreutzberg, G. (1984). Sub-cellular localization of 5I-nucleotidase in rat brain. Journal of Neurochemistry, 43, 971–978.
Weisshaar, H. D., Prasad, M. C., & Parker, R. S. (1975). Estimation of lactate dehydrogenase in serum/plasma. Medicine Welt, 26, 387.
Misra, H., & Fridovich, I. (1989). The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay of superoxide dismutase. Toxicology and Biological Chemistry, 2417, 3170.
Clairborne, A. (1995). Catalase activity. In A. Greewald (Ed.), Handbook of methods for oxygen radical research (pp. 237–242). Florida: CRC Press.
Habig, W., Pabst, M., & Jacoby, W. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biochemistry, 249, 7130–7139.
Jollow, D., Mitchell, J., Zampaglione, N., & Gillette, J. (1974). Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology, 11, 151–169.
Akintunde, J. K., Akintola, T. E., Adenuga, G. O., Odugbemi, Z. A., Adetoye, R. O., & Akintunde, O. G. (2020). Naringin attenuates Bisphenol-A mediated neurotoxicity in hypertensive rats by abrogation of cerebral nucleotide depletion, oxidative damage and neuroinflammation. Nuerotoxicology, 81, 18–33.
Guan, D. S., Chen, Y. J., & Ruan, G. D. (2001). Study on heavy metal concentrations and the impact of human activity on them in urban and suburb soils of Guangzhou. Acta Scientiarum Naturalium Unversitatis Sunyatseni, 40(4), 93–101.
Yan, J., Ye, Z. X., Yan, Y., & Huang, X. P. (2008). Study on heavy metals distribution in atmospheric particulate matter on both sides of the Cheng-Ya Expressway. Sichuan Environment, 2(2), 19–21.
Christoforidis, A., & Stamatis, N. (2009). Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geodermadrology, 151, 257–263.
Geiss, O., Bianchi, I., & Barrero-Moreno, J. (2016). Lung-deposited surface area concentration measurements in selected occupational and non-occupational environments. Journal of Aerosol Science, 96, 24–37.
Badding, M. A., Fix, N. R., Antonini, J. M., & Leonard, S. S. (2014). A comparison of cytotoxicity and oxidative stress from welding fumes generated with a new nickel-, copper-based consumable versus mild and stainless steel-based welding in RAW 264.7 mouse macrophages. PLoS ONE, 9(6), e101310.
Young-Seoub, H., Ki-Hoon, S., & Jin-Yong, C. (2014). Health effects of chronic arsenic exposure. Journal of Preventive Medicine and Public Health, 47(5), 245–252.
Richter, P., Faroon, O., & Pappas, R. S. (2017). Cadmium and cadmium/zinc ratios and tobacco related morbidities. International Journal of Environmental Resources and Public Health, 29, 10–14.
Duffus, J. H. (2002). Heavy metals-a meaningless term? Pure and Applied Chemistry, 74(5), 793–807.
Terzano, C., Di Stefano, F., Conti, V., Graziani, E., & Petroianni, A. (2010). Air pollution ultrafine particles: Toxicity beyond the lung. European Review Medicine and Pharmacological Science, 14, 809–821.
Grahame, T. J. (2011). Distinguishing health effects among different PM 2.5 components in urban airborne particulate matter. Eds Fathi Z, 1, 575–597.
Lu, X., Zhu, Y., Bai, R., Li, S., & Teng, X. (2015). The effect of manganese-induced toxicity on the cytokine mRNA expression of chicken spleen lymphocytes in vitro. Resources and Veterinary Science, 101, 165–167.
Li, Q., Liu, H., Alattar, M., Jiang, S., Han, J., Ma, Y., et al. (2015). The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats. Scientific Reports, 5(1), 1–12.
Valko, M., Morris, H., & Cronin, M. T. D. (2005). Metals, toxicity and oxidative stress. Current Medicine and Chemistry, 12(10), 1161–1208.
Patra, R. C., Rautray, A. K., & Swarup, D. (2011). Oxidative stress in lead and cadmium toxicity and its amelioration. Veterinary Medicine International, 2011, 457327.
Leonard, S. S., Harris, G. K., & Shi, X. (2002). Metal-induced oxidative stress and signal transduction. Free Radical Biological Medicine, 37(12), 1921–1942.
Wagner, C., Vargas, A. P., Roos, D. H., Morel, A. F., Farina, M., Nogueira, C. W., et al. (2010). Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Archive Toxicology, 84, 89–97.
Carvalho, C. M., Lu, J., Zhang, X., Arner, E. S., & Holmgren, A. (2010). Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: Implications for treatment of mercury poisoning. FASEB Journal, 25, 370–381.
Bechan, S., Shweta, S., & Nikhat, J. S. (2014). Biomedical implications of heavy metals induced imbalances in redox systems. Biomedical Resources International, 2014, 640754.
Arif, T. J., Mudsser, A., Kehkashan, S., Arif, A., Inho, C., Qazi, M., et al. (2015). Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. International Journal of Molecular Science, 2015(16), 29592–29630.
Mohamed, N. A., Amna, A., & Ahmed, S. K. (2016). In vitro antioxidant activity and phytochemical screening of Croton zambesicus. Journal of Pharmacognosy and Phytochemistry, 5(6), 12–16.
Ajayi, A. O., & Omomagiowawi, I. E. (2018). Antimicrobial activity of Croton zambesicus on Staphylococcus aureus and Streptococcus species. SYLWAN, 160, 10.
Akintunde, J. K. (2017). Functional oil from black seed differentially inhibits aldose-reductase and ectonucleotidase activities by up-regulating cellular energy in haloperidol-induced hepatic toxicity in rat liver. Journal of Oleo Science, 66(9), 1051–1060.
Aho, J., Helenius, M., Vattulainen-Collanus, S., Alastalo, T. P., & Koskenvuo, J. (2016). Extracellular ATP protects endothelial cells against DNA damage. Purinergic Signalling, 12, 575–581.
Takahashi-Sato, K., Murakawa, M., & Kimura, J. (2013). Loss of ectonucleotidases from the coronary vascular bed after ischemia-reperfusion in isolated rat heart. BMC Cardiovascular Disorder, 13, 53.
Burnstock, G. (2015). Blood cells: An historical account of the roles of purinergic signalling. Purinergic Signalling, 11(4), 411–434.
Akintunde, J. K., Akintola, T. E., Aliu, F. H., Fajoye, M. O., & Adimchi, S. O. (2020). Naringin regulates erectile dysfunction by abolition of apoptosis and inflammation through NOS/cGMP/PKG signalling pathway on exposure to Bisphenol-A in hypertensive rat model. Reproductive Toxicology, 95, 123–136.
Deepak, J., Raju, K. P., & Hari, K. (2013). Non-respiratory functions of the lung, continuing education in anaesthesia. Critical Care & Pain, 13(3), 98–102.
Sha, L., Hor-Yue, T., Ning, W., Zhang-Jin, Z., Lixing, L., Chi-Woon, W., et al. (2015). The role of oxidative stress and antioxidants in liver diseases. International Journal of Molecular Sciences, 16(11), 26087–26124.
Akintunde, J. K., & Oboh, G. (2016). Nephritic cell damage and antioxidant status in rats exposed to leachate from battery recycling industry. Interdisciplinary Toxicology, 9(1), 1–11.
Jadhav, S. H., Sarkar, S. N., Patil, R. D., & Tripathi, H. C. (2007). Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: A biochemical and histopathological study in male rats. Archive Environmental Contamination and Toxicology, 53, 667–677.
Hwang, K. Y., Schwartz, B. S., Lee, B. K., Strickland, P. T., Todd, A. C., & Bressler, J. P. (2001). Associations of lead exposure and dose measures with erythrocyte protein kinase C activity in 212 current Korean lead workers. Toxicological Science, 62, 280–288.
Westerink, R. H., Klompmkers, A. A., Westenberg, H. G., & Vijverberg, H. P. (2002). Signaling pathways involved in Ca and Pb-induced vesicular catecholamine release from rat PC12 cells. Brain Research, 957, 25–36.
Ndhlala, A., Ncube, B., & Van Staden, J. (2014). Antioxidants versus reactive oxygen species: A tug of war for human benefits? In I. Laher (Ed.), Systems biology of free radicals and antioxidants (pp. 3987–4002). New York: Springer.
Bernard, A. (2008). Cadmium and its adverse effects on human health. Indian Journal of Medicine Resources, 128, 557–564.
Bhattacharyya, A., Chattopadhyay, R., Mitra, S., & Crowe, S. E. (2014). Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiological Review, 94, 329–354.
Chen, J. R., Ko, J., Yeh, W. J., Huang, W. C., & Yang, H. Y. (2018). Renoprotective effects of Antroquinonol in rats with Nω-Nitro-l-Arginine methyl ester-induced hypertension. Nutrients, 10(10), E1521.
Akintunde, J. K., Oboh, G., & Akindahunsi, A. A. (2013). Testicular membrane lipid damage by complex mixture of leachate from municipal battery recycling site as indication of idiopathic male infertility in rat. Interdisciplinary Toxicology, 6(4), 192–197.
Akintunde, J. K. A., & J.B., Ebinama O.N, . (2020). Potential protective effects of naringin on oculo-pulmonary injury induced by PM 10 (wood smoke) exposure by modulation of oxidative damage and acetylcholine esterase activity in a rat mode. Current Therapeutic Research, 92, 1005861.
Fernández-Martínez, E., Jiménez-Santana, M., Centeno-Álvarez, M., Torres-Valencia, J. M., Shibayama, M., & Cariño-Cortés, R. (2017). Hepatoprotective effects of nonpolar extracts from inflorescences of thistles Cirsium vulgare and Cirsium ehrenbergii on acute liver damage in rat. Pharmacognosy Magazine, 13(4), S860–S867.
Akintunde, J. K., Akintola, T. E., Hammed, M. O., Amoo, C. O., Adegoke, A. M., & Ajisafe, L. O. (2020). Naringin protects against Bisphenol-A induced oculopathy as implication of cataract in hypertensive rat model. Biomedical Pharmacotherapy, 126, 110043.
Murni, N. S., Qamar, U. A., Siti, Z., Mat, S., Alhassan, M. A., & Suganya, M. (2017). Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. Biomedical Research International, 2017, 8386065.
Priya, B., & Anil, K. S. (2013). Anti-cancer potential of flavonoids: Recent trends and future perspectives. Biotechnology, 3(6), 439–459.
Mahmoud, A. M., Hernández Bautista, R. J., Sandhu, M. A., & Hussein, O. E. (2019). Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Medicine Cellular Longevity, 1, 1–19.
Riaz, M. A., Nisa, Z. U., & Anjum, M. S. (2020). Assessment of metals induced histopathological and gene expression changes in different organs of non-diabetic and diabetic rats. Science Reports, 10, 5897.
Reena, S., Neetu, G., Anurag, Mi., & Rajiv, G. (2011). Heavy metals and living systems: An overview. Indian Journal of Pharmacology, 43(3), 246–253.
Bell, M. L., Zanobetti, A., & Dominici, F. (2013). Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: A systematic review and meta-analysis. American Journal Epidemiology, 178(6), 865–876.
Markiewicz-Górka, I., Januszewska, L., Michalak, A., Prokopowicz, A., Januszewska, E., Pawlas, N., et al. (2018). Effects of chronic exposure to lead, cadmium, and manganese mixtures on oxidative stress in rat liver and heart. Archive High Rada Toksikology, 66, 51–62.
Cope, G., & Hodgson, E. (2010). Classes of toxicants: Use classes. In E. Hodgson (Ed.), Modern Toxicology (4th ed., pp. 49–50). New Jersey: Wiley.
Carneiro, M. F. H., Oliveira-Souza, J. M., Grotto, D., Batista, B. L., De Oliveira Souza, V. C., & Barbosa, F. (2014). A systemic study of the deposition and metabolism of mercury species in mice after exposure to low levels of Thimerosal (ethylmercury). Environmental Research, 134, 218–227.
Jan, A., Azam, M., Siddiqui, K., Ali, A., Choi, I., & Haq, Q. (2015). Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. International Journal of Molecular Science, 16(12), 29592–29630.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Handling Editor: Vittorio Fineschi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akintunde, J.K., Oyedibu, G.O., Olanipekun, N.J. et al. Modulation of Cardiopulmonary Toxicity and Oxidative Stress by Phenolic-Rich Fraction of Croton zambiscus Leaves in Rat Exposed to Chronic Mixture of Environmental Toxicants. Cardiovasc Toxicol 21, 272–285 (2021). https://doi.org/10.1007/s12012-020-09618-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-020-09618-x