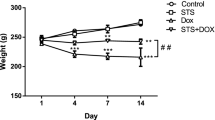
Abstract
In the present study, the preventive effects of orally administered disulfiram (DS) against the doxorubicin (DOX)-induced cardiotoxicity were investigated in rats. DS was orally administered for 7 days at doses of 2, 10, and 50 mg/kg/day. DOX (30 mg/kg) was intraperitoneally administered on the 5th day of the initiation of DS treatment. Within 48 h of injection, DOX treatment significantly altered ECG, elevated the ST height, and increased the QT and QRS intervals. It reduced the cardiac levels of injury markers like creatine kinase isoenzyme-MB and lactate dehydrogenase. DOX elevated the serum levels of SGOT and nitric oxide. Its injection significantly induced lipid peroxidation in the cardiac tissue and reduced the activities of innate antioxidants like super oxide dismutase, catalase, and reduced glutathione in the cardiac tissue. DOX treatment raised the TNF-α level and caused histological alterations in the myocardium like neutrophil infiltrations, myonecrosis, and edema. Pre-treatment of rats with DS (2, 10, and 50 mg/kg p. o. for 7 days) prevented the ECG changes, minimized oxidative stress, and normalized the biochemical indicators of the DOX-induced cardiotoxicity. DS also protected rat heart from DOX-induced histological alterations. Recently, DS is reported to exert chemosensitization of cancer cells. Our in vitro investigation using MCF7 cell line revealed that DS reverses the DOX-induced suppression of NF-κB and Nrf2 expression. These findings about the protective activity of DS against the DOX-induced cardiotoxicity warrant a detailed investigation on its utility as an adjunct therapy to cancer chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Majority of the anticancer chemotherapeutic agents exert dose-dependent and severe multi-organ toxicities. Different approaches to tackle with the organ toxicities of the chemotherapy are under investigation worldwide. The novel anticancer treatments like monoclonal antibodies have shortcomings like immunogenicity, lack of site specificity, difficult formulations, and chemical instability. Hence, even today, chemotherapy remains a mainstay of the cancer treatment. There has been a systematic search for the drugs to reduce the multi-organ toxicities of chemotherapy—chemopreventive, and to simultaneously sensitize the cancer cells to toxicity of the chemotherapeutic agents-chemosensitizers [1].
Doxorubicin (DOX) is a potent broad spectrum anthracycline antibiotic isolated from the Streptomyces peacetius. It is used in treating solid and hematological cancers, breast cancer, sarcomas and leukemia [2]. DOX is the most active drug used in the treatment of metastatic breast cancer [3]. However, DOX exerts dose dependent cardiotoxicity and this adverse effect limits its therapeutic usefulness. It is unequivocally proved, even clinically, that the cardiomyopathy associated with the cumulative doses of DOX leads to potentially fatal congestive heart failure [4]. Due to cardiotoxicity, the recommended maximum dose of DOX is limited to 450 g/m2. At doses beyond this limit, DOX is reported to induce substantial cardiotoxicity [5]. The underlying mechanisms for these devastating adverse effects include mitochondrial damage, free radical generation, and inhibition of the DNA and protein synthesis along with inhibition of the myofibril regeneration [6]. DOX-induced myocardial damage also involves activation of the intrinsic apoptotic pathway [7]. The generation of free radicals is the most frequently reported mechanism of DOX-induced cardiomyopathy [8].
Disulfiram (DS) is routinely used to create alcohol aversion in alcohol dependents and addicted patients. DS was discovered in 1920s and it has been in use for the treatment of alcohol dependence since 1940s. It is the first FDA-approved drug in the treatment of alcohol dependence [9]. DS is a potent inhibitor of aldehyde dehydrogenase, an enzyme responsible for the conversion of alcohol into acetaldehyde. DS is also reported to exert organ protective effects in experimental studies. It exerts cardioprotection against the ketamine and prevents the myocardial damage through inhibition of oxidative stress [10]. DS protects the liver from the paracetamol-induced toxicity by inhibiting cytochrome P450 enzymes and thereby reducing the formation of toxic metabolites of paracetamol [11]. DS is shown to exert protection against the cyclophosphamide-induced urinary bladder hemorrhagic cystitis. DS reduces the cyclophosphamide-induced inflammatory and edematous alterations, prevents the damage of the epithelial lining, lamina propria and muscularis of the bladder. It is important to state here that DS doesn’t alter the anticancer properties of cyclophosphamide and still confers protection to the urinary bladder [12]. The ability of DS to protect pancreatic tissue from alloxan-induced oxidative stress and damage in rats is also reported [13].
Recent investigations have projected DS as an anticancer and a chemosensitizing agent. DS has been reported to exert chemosensitizing effects through multiple mechanism. In the prostate cancer, the tumor suppressor estrogen b receptors (ER-β) having the growth regulatory effects are epigenetically repressed by the hypermethylation of the promoter related to this receptor. An enzyme responsible for hypermethylation-DNA-methyltransferase (DNMT) is over active in the cancer cells. Inhibition of the DNMT leads to re-expression of the tumor suppressors. DS inhibits the DNMT enzyme and thereby inhibits the proliferation of the prostate cancer cells. This effect is selective for the cancer cells in comparison to normal cells [14]. Several studies have indicated that DS and its metabolites potentiate the effects of alkylating agents [15, 16]. Further, DS inhibits the maturation of the p-glycoprotein and increase sensitivity of p-glycoprotein transfected cells to vinca alkaloids [17]. DS and its metabolite, diethyldithiocarbamate, inhibit the NF-κB activation, induce G1/S arrest and apoptosis in human hepatoma HepG2 cells [18].
The copper complex of DS acts as a proteasome inhibitor and has been shown to induce chemosensitization [19]. A phase-IIb clinical trial on the use of DS in a combination regimen of cisplatin and vinorelbine concluded that DS prolongs the life of patients of metastatic non-small cell lung cancer [20]. Apart from these encouraging clinical results, DS is reported to exert antineoplastic effect by inhibiting the activation of transcription factor/cyclic-AMP-responsive element binding protein [21]. These reports indicate that DS exerts anticancer as well as chemosensitizing actions and also possesses organ protective potentials. There is a need of further substantiation of its organ protective efficacies against the characteristic and specific organ toxicities of chemotherapeutic agents. In present study, we investigated the effects of pre-treatment with DS on the DOX-induced cardiotoxicity in rats.
We carried out a pilot study to determine the dose range for DS to be used in the present study. We tested the cardio protective effect of 7 days oral pre-treatment of DS (25, 50, and 100 mg/kg/day) against the intraperitoneally injected DOX (30 mg/kg) in rats. Karamanakos et al. [22] used a similar dose range for DS to investigate the aldehyde dehydrogenase inhibitory activity and the neurotoxicity of DS in rats. In our pilot study, we found that only 25 and 50 mg/kg doses exerted cardioprotective effects and 100 mg/kg dose of DS worsened the cardiotoxicity induced by DOX. Even the group of rats that received only 100 mg/kg of DS for 7 days revealed mild cardiac toxicities in terms of histological alterations. Hence, for the main study, we selected lower doses of DS as 2, 10 and 50 mg/kg/day.
Materials and Methods
Chemicals
Disulfiram was procured as a pure chemical from Unidrug Innovative Pharma Technologies Ltd., Indore, India. The purity of disulfiram was 99.7%. Doxorubicin lyophilized Powder (Batch# LDXB60608, Khandelwal laboratories, India), Creatine Kinase MB kit (Aspen Lab. India), Lactate Dehydrogenase and SGOT kit (ERBA diagnostics Germany), 5-5-dithio bis-(2-nitrobenzoic acid) and thiobarbituric acid (Loba Chemicals Pvt. Ltd.), Nitro blue tetrazolium, carboxymethyl cellulose (CMC) were bought from local vendors and Sigma Aldrich, USA as available.
Animals
Male Wistar rats (190–200 g) were acquired from the central animal house facility of R.C.Patel Institute of Pharmaceutical Education and Research, Shirpur, India. The animals were kept under standard conditions (12 h light and 12 h dark cycles) at 22 ± 2 °C with a relative humidity of 40–80%. They were fed a standard laboratory diet and water ad libitum. The animals were maintained in compliance with the guidelines of Committee for the Purpose of Control and Supervision of the Experiments on Animals (CPCSEA) established under the prevention of the cruelty to animals Act, 1960, Ministry of Environment and Forests, Government of India. The experimental protocols were permitted by the Institutional Animal Ethics Committee of R.C.Patel Institute of Pharmaceutical Education and Research, Shirpur, Maharashtra, India (Protocol approval # IAEC/CPCSEA/RCPIPER/2017-15).
Experimental Protocol for DOX-Induced Cardiac Toxicity in Rats
DOX was dissolved in the normal saline and was intraperitoneally injected at 30 mg/kg dose. DOX injection was given on the 5th day of the study schedule [23].
Experimental Design
Total 36 rats were randomized and allotted to six groups each containing six rats. The groups and respective treatments were as follows:
Normal group: received 0.5 ml/day of 0.5% CMC solution by oral gavage.
DOX control group: received doxorubicin (30 mg/kg i.p.).
DS (2), DS (10), and DS (50) groups: received DS at 2, 10, 50 mg/kg/day by oral route for a period of 7 days as a suspension in 0.5% CMC by oral gavage. These groups were injected with a single dose DOX (30 mg/kg i.p.) on the 5th day.
DS-alone: received DS at 50 mg/kg/day by oral gavage as a 0.5% CMC suspension for a period of 7 days.
After 48 h of the DOX injection, hemodynamic, and electrocardiographic parameters were recorded for all the groups. The blood samples were collected under a mild anaesthesia and then the rats were sacrificed for isolation of the hearts. A part of the isolated heart from each rat was preserved in 10% formalin for histopathology investigations. The heart tissue homogenates were used for the estimations of the cardiac injury markers, and the extent of oxidative stress.
Surgical Procedures for Recording the Hemodynamic Parameters
Rats were anaesthetized by intraperitoneal injection of 1 g/kg of urethane and placed in the supine position on a wooden board. The ECG was recorded continuously through three lead skin electrodes, with two electrodes towards the heart on right and left forelimbs and the neutral 3rd electrode on the hind limb facing the heart [24]. These electrodes were connected to the data acquisition system (PowerLab, AD Instruments, and Australia). After recording the ECG, the right carotid artery was cannulated with a polyethylene tube (internal diameter 0.30 mm; outer diameter 0.40 mm) attached to a three way cannula filled with heparinized saline solution and linked to the pressure transducer of the data acquisition system (PowerLab Data acquisition system with LabChart 5.0 software). The systolic, diastolic, and mean arterial pressures were recorded [25].
Estimation of Cardiac Injury Markers
A 10% tissue homogenate of each isolated heart was prepared using ice-cold 50 mM phosphate buffer saline (pH 7.4). The homogenate was centrifuged at 2000×g for 20 min at 4 °C and the aliquots of the supernatant were used to estimate creatine kinase and lactate dehydrogenase using commercially available kits.
Estimation of Oxidative Stress
The extent of oxidative stress in the myocardial tissue across different groups was estimated in terms of the thiobarbituric acid reactive substances, reduced glutathione, along with the activities of catalase and superoxide dismutase.
Estimation of Extend of Lipid Peroxidation (LPO)
The heart homogenate samples were treated with 3.0 ml of 1% phosphoric acid solution and 1.0 ml of aqueous solution of 0.6% thiobarbituric acid. The reaction mixture was heated at 80 °C for 45 min, cooled in an ice bath and extracted with 4.0 ml of n-butanol. The n-butanol layer was separated and the absorbance of the pink complex formed was estimated at 532 nm as an indicator of extend of lipid peroxidation [26].
Estimation of Reduced Glutathione (GSH)
The GSH content in the heart tissue homogenates was determined by treating the homogenate with DTNB method [27]. Briefly, 20 µl of tissue homogenate was treated with 180 µl of 1 mM DTNB solution at room temperature. The optical density of resulting yellow color was measured at 412 nm using a microplate spectrophotometer (Powerwave XS, Biotek, USA).
Determination of the Catalase Activity
As reported by Gore et al. [28], the heart homogenate (20 µl) was added to 1 ml of 10 mM H2O2 solution in the quartz cuvette. The reduction in optical density of this mixture was monitored using microplate spectrophotometer in UV mode at 240 nm. Rate of decrease in the optical density across 3 min from the addition of heart homogenate was taken as an indicator of the catalase activity present in the homogenate.
Estimation of Superoxide Dismutase (SOD) Activity
The heart homogenate (20 µl) was added to a mixture of 20 µl of 500 mM/1 of Na2CO3, 2 ml of 0.3% Triton X-100, 20 µL of 1.0 mM/1 of EDTA, 5 ml of 10 mM/1 of hydroxylamine, and 178 ml of distilled water. To this mixture, 20 µl of 240 µM/1 of NBT was added. The optical density of this mixture was measured at 560 nm in kinetic mode for 3 min at 1 min intervals. The rate increase in the optical density was determined as indicator of the SOD activity [26].
Estimation of Nitric Oxide
The tissue homogenate was mixed with 50 µl of Griess reagent and the absorbance was determined at 540 nm using Power wave XS microplate spectrophotometer (Biotek, USA). Sodium nitrite was used as a standard for preparation of calibration curve. The NO concentration was expressed as µM/l of NO [29].
Estimation of Aspartate Amino Transferase
The heart homogenates were mixed with 100 µl of working reagent provided in commercial kit (ERBA diagnostics, Germany), and incubated at 37 °C for 5 min. After incubation absorbance was measured at 340 nm and calculated, the mean absorbance change per minute [30].
Estimation of Release of TNF-α
The concentrations of TNF-α in heart tissue homogenate was determined according to the manufacturer’s protocol using ELISA kits.
Histological Examination
The myocardial tissues were fixed in buffered formalin solution and embedded in paraffin. Serial sections (4 µm) were cut using microtome. The sections were stained with haematoxylin and eosin. Sections were examined under the light microscope (Besto Microscopes, India) and photographs were taken. The pathologist evaluating the microscopy photographs was blinded to the treatments. The pathologist evaluated the sections by histology scoring system.
Cell Culture and Reagents
The breast cancer cell line MCF-7 was cultured in DMEM media, supplemented with 10% FBS, 1.5 mM l-glutamine and 1% antibiotic (100 U/ml of penicillin, 10 mg/ml of streptomycin) in a humidified atmosphere of 5% CO2 at 37 °C. A 10 µM stock of Doxorubicin was prepared in distilled water and 10 µM Disulfiram was prepared in DMSO. After cells reached 60–70% confluency, DOX, DS, and their combination treatment was done for 48 h (in case of combination treatment, first the cells were pre-treated with DS for 6 h and then DOX was added and allowed the cells to grow for another 48 h). For western blotting, primary antibody against NFκB (anti-NFκB) (#8242) antibody was from Cell Signaling Technology (MA, USA); anti-NRF2 (sc-722) and anti-GAPDH (sc-25778) antibody were perched from Santa Cruz Biotechnology and Anti-mouse IgG (#7076), Anti-rabbit IgG (#7074) were from Cell Signaling Technology.
MTT Assay
Anchorage dependent viability of cells after treatment with agents was measured as described earlier [31]. In brief, 8000–10,000 cells were seeded in duplicate and grown to 60–70% confluence. Then, cells were exposed to different concentrations of DOX and DS and their combination for 48 h. After treatment, MTT was added and incubated overnight. After the formation of purple formazan crystals, the color intensity was measured spectrophotometrically using a microplate reader (Berthold, Germany) at 570 nm. The data were calculated and presented as percent viability compared to the untreated control.
Western Blotting
Western blotting was performed according to the protocol referred earlier [32]. Approximately, 5 × 105 grown cells were treated with DOX, DS and their combination, respectively, as aforementioned. Then, cellular lysates were prepared using modified RIPA lysis buffer, and 60 µg of proteins were loaded and separated on 10% SDS–PAGE gel electrophoresis. Proteins were transferred onto PVDF membrane, and western blotting was performed using anti-NF-κB, anti-NRF2, anti-GAPDH, antibody using the manufacturer’s protocol. The numerical values above each panel of protein represents the band intensity measured using a UVP GelDoc-It® 310 Imaging system (UVP, Cambridge, UK).
Statistical Analysis
Data were expressed as mean ± standard error mean (SEM) for each group. Statistical analysis was performed using the one way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test with comparison of all column pairs. The data were analyzed using a graph pad, prism software, version 6.0, USA. The value of p < 0.05 was considered as a significant.
Results
Effect of DS on the ST-Height Alterations Induced by DOX Cardiotoxicity
ECG patterns and averaged waveforms were found to be irregular in DOX-treated rats as compared to normal rats as given in Fig. 1. In DOX-treated rats, there was a significant rise in the ST height up to 0.1 ± 0.003 mV and a rise in the QRS interval 0.016 ± 0.001 as compared with the normal rats having average ST height of 0.06 ± 0.003 mV and QRS interval of 0.011 ± 0.006 s. The pre-treatment with DS at 2, 10, and 50 mg/kg doses reduced the DOX-induced rise in the ST-height and also reduced the QRS interval. The average ST height in the DS-treated rats was different from that of the control group. The effect DS at the dose of 50 mg/kg was more prominent (0.062 ± 0.005 mV). DS alone did not affect the ST-height and QRS levels.
Effect of DS on the DOX-induced ECG alterations. (A) Normal rats, (B) DS control, (C) DS (2) + DOX, (D) DS (10) + DOX, (E) DS (50) + DOX, (F) DS alone, (G) ST-height, (H) QRS interval. Data were analyzed by one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. ###p < 0.001 as compared with normal rats, *p < 0.05, ***p < 0.001 as compared with doxorubicin treated rats
Effect of DS on Arterial Pressure and Heart Rate in DOX-Induced Cardiotoxicity in Rats
DOX-treated rats had a significant upsurge in the systolic arterial pressure, diastolic arterial pressure, mean arterial pressure and heart rate as compared to the normal rats (p < 0.001). Seven days treatment with the DS at doses of 2, 10, and 50 mg/kg reduced the DOX-induced rise in the systolic, diastolic and mean arterial pressure and heart rate. DS treatment significantly inhibited the effects of DOX on the hemodynamic parameters (p < 0.001). These effects of DS were dose dependent and maximum protection observed at the dose of 50 mg/kg is given in Fig. 2.
Effect of DS on DOX-induced hemodynamic changes. a Systolic arterial pressure, b Diastolic arterial pressure, c Mean arterial pressure, d Heart rate. Data were analyzed by one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. ###p < 0.001 as compared with normal rats, *p < 0.05, ***p < 0.001 as compared with doxorubicin treated rats
Effect of DS on CK-MB and LDH in DOX-Induced Cardiotoxicity in Rats
A significant decline in the myocardial injury marker enzymes, CK-MB isoenzyme, and LDH was detected in the DOX-treated rats (p < 0.001) as compared to normal group (Fig. 3). All the tested doses of DS significantly attenuated the DOX-induced depletion of myocardial enzymes (p < 0.001). DS at 50 mg/kg dose exerted maximum protection against DOX-induced alterations in the myocardial injury markers.
Effect of DS on Reactive Oxygen Species in DOX-Induced Cardiotoxicity in Rats
We used the heart homogenate to estimate the extent oxidative stress and. DOX induced a state of marked oxidative stress as evident from a significant increase in MDA level (75.42 ± 9.20 mg/g of wet tissue) and a significant decrease in the GSHup to 3.428 ± 0.5122 mg/g of wet tissue (p < 0.001). The activities of SOD (9.408 ± 1.184 U/mg of protein) and catalase (7.481 ± 1.495 U/g of protein) in the heart tissue homogenates of DOX receiving rats was significantly lower than the normal rats (p < 0.001). The DS treatment protected the rat hearts from the lipid peroxidation induced by DOX. In tune with the reduced lipid peroxidation, there was a significant rise in the GSH levels and the activities of catalase and SOD in the rats receiving DS (Fig. 4). The treatment with DS alone did not alter the oxidative stress significantly.
Effect of DS on reactive oxygen species in DOX-induced cardiac toxicity rats. a SOD, b MDA content, c GSH and d catalase. Data were analyzed by one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. ###p < 0.001 as compared with normal rats, *p < 0.05, ***p < 0.001 as compared with doxorubicin treated rats
Effect of DS on Nitric Oxide and SGOT in DOX-Induced Cardiotoxicity in Rats
DOX injection significantly increased the serum nitric oxide (756.6 ± 46.54 µM/l) and SGOT levels (182.6 ± 5.58 U/l) as compared to normal rats (NO: 418.6 ± 52.50 µM/l; SGOT: 79.81 ± 4.46 U/l) and prior treatment of rats with DS attenuated these changes significantly and dose dependently (p < 0.001). At the dose of 50 mg/kg per day, DS-alone did not affect the levels of nitric oxide and SGOT (Fig. 5).
Effect of DS on the Release of TNF-α in DOX-Induced Cardiotoxicity in Rats
DOX significantly increased the levels of TNF-α (134.4 ± 9.79 pg/mg of protein) in the heart tissue homogenate as compared to the normal rats (88.99 ± 2.214 pg/mg of protein, p < 0.001). All the tested doses of DS reduced the DOX-induced TNF-α elevation. The highest dose of 50 mg/kg of DS-alone did not affect the TNF-α level in heart tissue given in Fig. 6.
Effect of DS on the Histology in DOX-Induced Cardiotoxicity in Rats
The DOX administration induced inflammatory changes in the myocardium and there was edema, infiltration of neutrophils and other inflammatory cells (Fig. 7). The Table 1 represents the extent of these inflammatory changes and infiltrations. The 50 mg/kg dose of DS reduced the neutrophil infiltration in to the myocardium. The group treated with DS-alone did not have any signs of toxicity and had a normal microstructure.
DS Prevents DOX-Induced Cell Death in MCF-7 Cell Line
To check the cytotoxic effect of DOX in MCF-7 cells, we performed an MTT cell viability assay. For this, cells were treated with an increasing concentration of DOX (0–250 nM) for 48 h and then the viability was determined. A dose dependent reduction of the cell viability with IC50 (50% cell death) of 170 nM (91.9 ng/ml) was noticed (Fig. 8a). Next, we checked the cell viability after treating with increasing concentrations of DS (0–1000 nM) for 48 h and we got a 50% cell death (IC50 value) at 480 nM (144 ng/ml). In combination treatment, cells were pre-treated with a fixed concentration of DS (300 nM, 90 ng/ml) for 6 h and then medium was aspirated and increasing concentrations of DOX were added in each concentration of DS and incubated for another 48 h. In this case, the IC50 value was found to be 310 nM (167.6 ng/ml of DOX) which is much lower than DS-treated cells, but higher than the individual treatment of DOX (Fig. 8a).
To confirm the enhanced cell viability in DS-treated cells, we further carried out western blot of NFκB and Nrf2 after the treatment of DOX, DS, and their combination treatment (DS + DOX) according to their IC50 values. Here, 2.01 times increased expression of NFκB was found in case of DS-treated cells as compared to untreated control and in case of Nrf2, it was 1.59 times higher. Increased NFκB/ Nrf2 in DS-treated cells compared to individual treatment of DOX and the combination treatment of DS and DOX, indicated that cell proliferation was enhanced after the exposure of DS in MCF-7 cells (Fig. 8b).
Discussion
In this study, the protective efficacy of DS was determined against the DOX-induced cardiotoxicity in rats. DOX is one of the most potent anticancer agents but exerts dose dependent cardiotoxicity [33]. The currently available drugs for prevention and treatment of DOX-induced cardiotoxicity include dexrazoxane, amifostine, co-enzyme Q, and lipid lowering drugs [34]. However, even these chemoprotectants have their own limitations in terms of the adverse effects. Hence, other drugs for preventing the organ toxicities of chemotherapy are continually being searched.
An age-old drug, DS, has recently attracted attention due to new revelations on its anti-tumor and chemosensitizing activities. This drug is emerging as a redox modulator and NF-κB inhibitor which may have a role in treatment of breast cancer [35]. Across the last 2 decades, there have been reports that hinted regarding multiple activities of DS other than its aldehyde dehydrogenase inhibitory activity. DS reduces the lipid peroxidation in cell membrane and inhibits the irradiation-induced DNA damage [36]. It protects the innate antioxidative mechanisms [10] and also inhibits the release of pro-inflammatory mediators from immune cells [37,38,39]. DS also sensitizes the cancer cells to cytotoxicity of anticancer agents through inhibition of proteasome [40]. These activities may contribute to chemopreventive therapy. Considering these reports, we explored how DS affects the DOX-induced cardiotoxicity in rats.
In the preliminary investigations, we used two rats per group and only determined SGOT and histopathology as study parameters. We tested the prevention of DOX-induced cardiotoxicity by 25, 50, and 100 mg/kg/day doses of DS given for 7 days prior to DOX. We observed that 100 mg/kg dose of DS itself elevated serum SGOT levels and induced inflammatory alterations in the rat’s heart. We speculated that this worsening of the DOX-induced cardiotoxicity by DS may involve its pro-apoptotic activity at higher doses [41]. The 25 and 50 mg/kg doses of DS prevented the DOX toxicities. Hence, in the further investigation, we used 2, 10, and 50 mg/kg doses of DS and DOX at intraperitoneal dose of 30 mg/kg.
In case of ECG changes induced by DOX, there are diverse reports as far as ST height is concerned [42, 43]. In our laboratory, the 40 mg/kg intraperitoneal DOX injections resulted in significant mortality (> 50%). However, this dose induced an increase in the ST height. Such rise in ST height is reported by others and this is projected as an indication of the transmural myocardial damage [44]. We titrated the dose of DOX and found that, at 30 mg/kg, it induced significant alternation in the biochemical indicators of cardiotoxicity and reduced the ST height. As described by Mustafa et al. [44], a fall in the ST height indicated partial damage to the myocardial wall. Other electrocardiographic abnormalities induced by DOX including QRS, QT prolongation, and R–R interval reduction were in congruence with the report by Abushouk et al. [45].
DOX is reported to increase systolic and diastolic blood pressure [46] and this is attributed to the DOX-induced catecholamine release from adrenergic nerve endings [22]. The DOX toxicity may involve the catecholamine induced decrease in the myocytes viability through cyclic AMP-mediated calcium overload. Catecholamine is also a potential source of oxygen-derived free radicals [47]. The DOX-induced ST height and T wave changes are also involved the role of catecholamines. In this study, DS inhibited the DOX-induced increase in the ST height and restored the QT interval. The anti-arrhythmic effect of DS is also reported to ameliorate epinephrine-induced cardiotoxicity [48]. It is speculated that these effects might have contributed to the observed cardioprotection by DS.
DOX has quinone and hydroquinone moieties in its structure and these can form semiquinone radical intermediates [49] leading to increased oxidative stress. The DOX-induced cardiotoxicity and nephrotoxicity have also been attributed to such increase in oxidative stress induced by DOX [50]. DS has been reported to possess anti-oxidant activity [10] and lower specific activities of cytochrome C oxidase in mitochondria. The antioxidative effects of DS also might have contributed to the observed protection conferred by DS. DOX induces NO synthesis and also binds to eNOS reductase domain and induces O2− generation. The resultant peroxy and peroxynitrite radicals are also responsible for DOX toxicity [51]. The cardiac muscles contain low levels of free-radical detoxifying enzymes/molecules like superoxide dismutase, GSH catalase and lipid peroxidation and hence, they are particularly susceptible to free-radical injury [37]. In present study, we found that DS reduced the lipid peroxidation, spare the GSH levels and also the activities of SOD and catalase in the similar manner as described by Cetin et al. [10]. These effects indicate the versatility with which DS may exert protective effect against DOX cardiotoxicity.
This study confirmed the preventive efficacy of DS against DOX-induced toxicity by substantiating the effects of DS on the CK-MB, LDH and SGOT. These enzymes are found in the myocardium and are used to evaluate the myocytes injury. WHO has recognized CK-MB and LDH as gold standard indicative of myocardial damage [52]. The DOX-induced free-radical generation and resultant lipid peroxidation leads to cardiomyocyte damage. This causes increased release of CK-MB and LDH in to the serum [53]. In this study, we observed that the 2, 10, and 50 mg/kg doses of DS significantly elevated the DOX-suppressed CK-MB and LDH in the heart homogenate. Even the serum levels of SGOT were normalized by DS.
The histopathology examination of the heart tissue revealed that DOX-induced inflammatory changes in the myocardium. More detailed investigations reporting the DOX-induced inflammatory alterations have been reported by Fard et al. [54]. The inflammatory changes were also confirmed by a significant rise in the tissue levels of TNF-α, and it is a major inflammatory mediator. In the present study, DS suppressed the DOX-induced TNF-α elevations in the cardiac tissue. This finding further reinforces the protective efficacy.
Furthermore, the in vitro study of DS also supported with the fact that it has cell protective property against DOX-induced toxicity in breast cancer cell line MCF-7.
Conclusion
In conclusion, the orally administered DS at 2, 10, and 50 mg/kg doses exerts significant and dose dependent protective effects against the DOX-induced cardiotoxicity in rats. In the light of the very recent findings that DS in combination with Cu exerts chemosensitization, it is suggested that DS may have a role in the cancer chemotherapy. Our study results point towards the possibility that DS can be considered for further detailed investigations as an adjunct therapy to DOX chemotherapy. This repositioning of DS may improve the chances of utilizing DOX at higher doses than recommended in absence of a safe organ protective adjuvant.
References
Kamble, S. M., Goyal, S. N., & Patil, C. R. (2014). Multifunctional pentacyclic triterpenoids as adjuvants in cancer chemotherapy: A review. RSC Advances, 4, 33370–33382.
Peiris, D., Spector, A. F., Lomax-Browne, H., Azimi, T., Ramesh, B., Loizidou, M., et al. (2017). Cellular glycosylation affects herceptin binding and sensitivity of breast cancer cells to doxorubicin and growth factors. Scientific Reports, 7, 43006.
Moreno-Aspitia, A., & Perez, E. A. (2009) Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clinic Proceedings, 84, 533–545.
Hahn, V. S., Lenihan, D. J., & Ky, B.(2014) Cancer therapy-induced cardiotoxicity: Basic mechanisms and potential cardioprotective therapies. Journal of the American Heart Association, 3, e000665.
Swain, S. M., Whaley, F. S., & Ewer, M. S. (2003). Congestive heart failure in patients treated with doxorubicin. Cancer, 97, 2869–2879.
Yang, S. D., Ma, L., Gu, T. X., Ding, W. Y., Zhang, F., Shen, Y., et al. (2014). 17β-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis, 19, 789–800.
Li, S., Wang, W., Niu, T., Wang, H., Li, B., Shao, L., et al.(2014) Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxidative Medicine and Cellular Longevity, 2014, 1–15.
El-Sayed, E. S. M., Mansour, A. M., & Abdul-Hameed, M. S. (2016). Thymol and carvacrol prevent doxorubicin-induced cardiotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. Journal of Biochemical and Molecular Toxicology, 30, 37–44.
Petrakis, I. L., Nich, C., & Ralevski, E. (2006). Psychotic spectrum disorders and alcohol abuse: A review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophrenia Bulletin, 32, 644–654.
Cetin, N., Suleyman, B., Altuner, D., Kuyrukluyildiz, U., Ozcicek, F., Coskun, R., et al. (2015). Effect of disulfiram on ketamine-induced cardiotoxicity in rats. International Journal of Clinical and Experimental Medicine, 8, 13540.
Jorgensen, L., Thomsen, P., & Poulsen, H. (1988). Disulfiram prevents acetaminophen hepatotoxicity in rats. Basic & Clinical Pharmacology & Toxicology, 62, 267–271.
Ishikawa, M., Aoki, T., Yomogida, S., Takayanagi, Y., & Sasaki, K. (1994). Drug interaction effects on antitumour drugs (XV): Disulfiram as protective agent against cyclophosphamide-induced urotoxicity without compromising antitumour activity in mice. Basic & Clinical Pharmacology & Toxicology, 74, 255–261.
Masukawa, T., & Nakanishi, K. (1994). Protection against alloxan-induced diabetes by diethyldithiocarbamate and disulfiram in mice. The Japanese Journal of Pharmacology, 64, 141–146.
Sharma, V., Verma, V., Lal, N., Yadav, S. K., Sarkar, S., Mandalapu, D., et al. (2016). Disulfiram and its novel derivative sensitize prostate cancer cells to the growth regulatory mechanisms of the cell by re-expressing the epigenetically repressed tumor suppressor-estrogen receptor β. Molecular Carcinogenesis, 55(11), 1843–1857.
Kohn, F. R., Landkamer, G. J., Manthey, C. L., Ramsay, N. K., & Sladek, N. E. (1987). Effect of aldehyde dehydrogenase inhibitors on the ex vivo sensitivity of human multipotent and committed hematopoietic progenitor cells and malignant blood cells to oxazaphosphorines. Cancer Research, 47, 3180–3185.
Valeriote, F., & Grates, H. E. (1989). Potentiation of nitrogen mustard cytotoxicity by disulfiram, diethyldithiocarbamic acid, and diethylamine in mice. Cancer Research, 49, 6658–6661.
Loo, T. W., & Clarke, D. M. (2000). Blockage of drug resistance in vitro by disulfiram, a drug used to treat alcoholism. Journal of the National Cancer Institute, 92, 898–902.
Liu, G. Y., Frank, N., Bartsch, H., & Lin, J. K. (1998). Induction of apoptosis by thiuramdisulfides, the reactive metabolites of dithiocarbamates, through coordinative modulation of NF_B, c-fos/c-jun, and p53 proteins. Molecular Carcinogenesis, 22, 235–246.
Cvek, B., & Dvorak, Z. (2008). The value of proteasome inhibition in cancer: Can the old drug, disulfiram, have a bright new future as a novel proteasome inhibitor? Drug Discovery Today, 13, 716–722.
Nechushtan, H., Hamamreh, Y., Nidal, S., Gotfried, M., Baron, A., Shalev, Y. I., et al., (2015). A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. The Oncologist, 20, 366–367.
Sauna, Z. E., Shukla, S., & Ambudkar, S.V. (2005). Disulfiram, an old drug with new potential therapeutic uses for human cancers and fungal infections. Molecular bioSystems, 1, 127–134.
Karamanakos, P. N., Pappas, P., Stephanou, P., & Marselos, M. (2001). Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Basic & Clinical Pharmacology & Toxicology, 88, 106–110.
Henninger, C., & Fritz, G. (2017) Statins in anthracycline-induced cardiotoxicity: Rac and rho, and the heartbreakers. Cell death & Disease, 8, e2564.
Goyal, S. N., Sharma, C., Mahajan, U. B., Patil, C. R., Agrawal, Y. O., Kumari, S., et al. (2015). Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. International Journal of Molecular Sciences, 16, 27457–27469.
Mahajan, U. B., Chandrayan, G., Patil, C. R., Arya, D. S., Suchal, K., Agrawal, Y. O., et al. (2017). The protective effect of apigenin on myocardial injury in diabetic rats mediating activation of the PPAR-γ pathway. International Journal of Molecular Sciences, 18, 756.
Chaudhary, G., Mahajan, U. B., Goyal, S. N., Ojha, S., Patil, C. R., & Subramanya, S. B. (2017). Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. American Journal of Translational Research, 9, 1792.
Reddy, N. M., Mahajan, U. B., Patil, C. R., Agrawal, Y. O., Ojha, S., & Goyal, S. N. (2015). Eplerenone attenuates cardiac dysfunction and oxidative stress in β-receptor stimulated myocardial infarcted rats. American Journal of Translational Research, 7, 1602.
Gore, P. R., Prajapati, C. P., Mahajan, U. B., Goyal, S. N., Belemkar, S., Ojha, S., et al. (2016). Protective effect of thymoquinone against cyclophosphamide-induced hemorrhagic cystitis through inhibiting DNA damage and upregulation of Nrf2 expression. International Journal of Biological Sciences, 12, 944.
Anu, G., & Usha, P. (2017). Phytochemical screening and in vitro antioxidant study of chloroform soluble fraction of Thespesia populnea bark extract. Journal of Livestock Science, 8, 77–80.
Goyal, S. N., Mahajan, U. B., Chandrayan, G., Kumawat, V. S., Kamble, S., Patil, P., et al. (2016). Protective effect of oleanolic acid on oxidative injury and cellular abnormalities in doxorubicin induced cardiac toxicity in rats. American Journal of Translational Research, 8, 60.
Mohapatra, P., Preet, R., Das, D., Satapathy, S. R., Siddharth, S., & Choudhuri, T. (2014). The contribution of heavy metals in cigarette smoke condensate to malignant transformation of breast epithelial cells and in vivo initiation of neoplasia through induction of a PI3K-AKT-NFkappaB cascade. Toxicology and Applied Pharmacology, 274(1), 168–179.
Siddharth, S., Mohapatra, P., Preet, R., Das, D., Satapathy, S. R., & Choudhuri, T. (2013). Induction of apoptosis by 4-(3-(tert-butylamino)imidazo[1,2-alpha]pyridine-2-yl) benzoic acid in breast cancer cells via upregulation of PTEN. Oncology Research, 21(1), 1–13.
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., & Gianni, L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews, 56, 185–229.
Granados-Principal, S., Quiles, J. L., Ramirez-Tortosa, C. L., Sanchez-Rovira, P., & Ramirez-Tortosa, M. (2010). New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food and Chemical Toxicology, 48, 1425–1438.
Allensworth, J. L., Evans, M. K., Bertucci, F., Aldrich, A. J., Festa, R. A., Finetti, P., et al. (2015). Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Molecular Oncology, 9, 1155–1168.
Gandhi, N. M., Gopalaswamy, U. V., & Nair, C. K. K. (2003). Radiation protection by disulfiram: Protection of membrane and DNA in vitro and in vivo against γ-radiation. Journal of Radiation Research, 44, 255–259.
Zhao, M., Sun, D., Guan, Y., Wang, Z., Sang, D., Liu, M., et al. (2016). Disulfiram and diphenhydramine hydrochloride upregulate miR-30a to suppress IL-17-associated autoimmune inflammation. Journal of Neuroscience, 36, 9253–9266.
Kanai, K., Itoh, N., Yoshioka, K., Yonezawa, T., Ikadai, H., Hori, Y., et al. (2010). Inhibitory effects of oral disulfiram on endotoxin-induced uveitis in rats. Current Eye Research, 35, 892–899.
Zhao, A., Wu, Z.-Q., Pollack, M., Rollwagen, F. M., Hirszel, P., & Zhou, X. (2000). Disulfiram inhibits TNF-α-induced cell death. Cytokine, 12, 1356–1367.
Cvek, B., Milacic, V., Taraba, J., & Dou, Q. P. (2008). Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. Journal of Medicinal Chemistry, 51, 6256–6258.
Cen, D., Brayton, D., Shahandeh, B., Meyskens, F. L., & Farmer, P. J. (2004). Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. Journal of Medicinal Chemistry, 47, 6914–6920.
Kelleni, M. T., Amin, E. F., & Abdelrahman, A. M. (2015) Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: Impact of oxidative stress, inflammation, and apoptosis. Journal of Toxicology. https://doi.org/10.1155/2015/424813.
Hydock, D. S., Lien, C.-Y., Jensen, B. T., Parry, T. L., Schneider, C. M., Hayward, R. (2012). Rehabilitative exercise in a rat model of doxorubicin cardiotoxicity. Experimental Biology and Medicine, 237, 1483–1492.
Mustafa, H. N., Hegazy, G. A., Awdan, S. A., AbdelBaset, M. (2017). Protective role of CoQ10 or l-carnitine on the integrity of the myocardium in doxorubicin induced toxicity. Tissue and Cell, 49, 410–426.
Abushouk, A. I., Ismail, A., Salem, A. M. A., Afifi, A. M., & Abdel-Daim, M. M. (2017). Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomedicine & Pharmacotherapy, 90, 935–946.
Yagmurca, M., Fadillioglu, E., Erdogan, H., Ucar, M., Sogut, S., & Irmak, M. K. (2003). Erdosteine prevents doxorubicin-induced cardiotoxicity in rats. Pharmacological Research, 48, 377–382.
Yang, Y.-N., Chuang, Y.-C., Yin, W.-H., & Young, M. S. (2007). Catecholamine-induced cardiomyopathy secondary to pheochromocytoma mimicking fulminant acute myocarditis. Acta Cardiologica Sinica, 23, 125–130.
Fossa, A. A., White, J. F., & Carlson, G. P. (1982). Antiarrhythmic effects of disulfiram on epinephrine-induced cardiac arrhythmias in rabbits exposed to trichloroethylene. Toxicology and Applied Pharmacology, 66, 109–117.
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., & Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology, 52, 1213–1225.
Bolaman, Z., Cicek, C., Kadikoylu, G., Barutca, S., Serter, M., Yenisey, C., & Alper, G. (2005). The protective effects of amifostine on adriamycin-induced acute cardiotoxicity in rats. The Tohoku Journal of Experimental Medicine, 207, 249–253.
Bahadır, A., Kurucu, N., Kadıoğlu, M., & Yenilme, E. (2014). The role of nitric oxide in doxorubicin-induced cardiotoxicity: Experimental study. Turkish Journal of Hematology, 31, 68.
Adams, J. E., Sicard, G. A., Allen, B. T., Bridwell, K. H., Lenke, L. G., Davila-Roman, V. G. et al. (1994) Diagnosis of perioperative myocardial infarction with measurement of cardiac troponin I. New England Journal of Medicine, 330, 670–674.
Liu, P., Brown, S., Goktug, T., Channathodiyil, P., Kannappan, V., Hugnot, J. P. et al. (2012). Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. British Journal of Cancer, 107, 1488.
Fard, M., Naseh, G., Bodhankar, S., & Dikshit, M. (2010). Cardioprotective effect of Lagenaria siceraria (Molina) Standley (Cucurbitaceae) fruit juice on doxorubicin induced cardiotoxicity in rats. American Journal of Pharmacology and Toxicology, 5, 103–108.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Handling Editor: Rajiv Janardhanan
Rights and permissions
About this article
Cite this article
Sonawane, V.K., Mahajan, U.B., Shinde, S.D. et al. A Chemosensitizer Drug: Disulfiram Prevents Doxorubicin-Induced Cardiac Dysfunction and Oxidative Stress in Rats. Cardiovasc Toxicol 18, 459–470 (2018). https://doi.org/10.1007/s12012-018-9458-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-9458-y