Abstract
Extracorporeal membrane oxygenation (ECMO) use in poisoned patients is increasing, but is rare post cardiac arrest. We report a case of ECMO use with complete recovery in a patient who arrested twice after a cardiotoxicant overdose. A 17-year-old male presented after an unknown overdose. He rapidly became hypotensive and bradycardic and received aggressive supportive care without improvement. He was transferred to our institution and suffered a cardiac arrest shortly after arrival. Six minutes of advanced cardiac life support resulted in return of spontaneous circulation. High-dose insulin, lipid emulsion, and ECMO were initiated. While awaiting ECMO deployment, he again became pulseless. Compressions resumed, and after 30 min, ROSC was achieved, and he was cannulated for veno-arterial ECMO. Within 48 h, he was decannulated, and then weaned off epinephrine 2 days later. Upon extubation, he was neurologically intact. Amlodipine and metoprolol were later confirmed in serum. Adolescent poisoned patients represent an ideal population for ECMO due to lack of comorbidities. As experience with ECMO in overdose increases, additional research is needed to determine appropriate indications and timing for its use. ECMO is an option for patients poisoned with a cardiotoxicant drug, even following witnessed cardiac arrest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular medication overdoses can be the most serious and refractory poisonings in humans [1]. This can be especially true when multiple agents with synergistic mechanisms of action are ingested together. Management of calcium channel and beta-blocker overdose can be very challenging and requires multiple interventions throughout the resuscitation. We report a case of an adolescent who ingested amlodipine and metoprolol and experienced subsequent cardiovascular collapse, but survived neurologically intact after treatment with extracorporeal membrane oxygenation (ECMO).
Narrative
A 17-year-old male with a past medical history significant for bipolar disorder and attention deficit hyperactivity disorder presented to an outside hospital emergency department (ED) with concerns for overdose of unknown substances in an apparent suicide attempt. He arrived with family who brought him to the ED after finding him stumbling and slurring his speech. The patient told the ED physician that he had taken trazodone and “another medication.” His family reported multiple medications in the house including amlodipine, citalopram, diphenhydramine, furosemide, levothyroxine, metoprolol, trazodone, valium, warfarin, and zolpidem. His initial vital signs were heart rate 68 beats per minute, blood pressure (BP) 196/127 mmHg, and oxygen saturation 98 % on room air. Physical exam is documented as normal except for altered mental status (AMS) due to lethargy, though he was arousable to voice. Laboratory results were unremarkable except for blood glucose of 197 mg/dL. Within approximately 30 min, he became acutely hypotensive with BP 50–80 s/20–60 s mmHg. He received three normal saline (NS) boluses of one liter each with minimal resolution of hypotension. He received intravenous naloxone due to concern for opiate ingestion though no significant response was seen. Given his ongoing hypotension, he was given calcium and glucagon due to concern for beta-blocker and/or calcium channel blocker ingestion.
The patient continued to have altered mental status and hypotension; our institution was contacted for transfer. Upon our recommendation, he was started on an epinephrine infusion for volume-resistant hypotension. Our critical care transport team was dispatched and transported the patient directly to the pediatric intensive care unit (PICU). During the transport, the patient remained hypotensive despite titration of epinephrine. He was, however, responsive, answering questions, and protecting his airway.
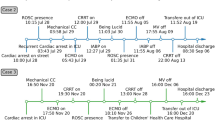
Upon arrival to the PICU, the patient’s blood pressure was 60–80/20–40 s mmHg and his initial electrocardiogram showed a sinus rhythm, rate 76, with a first-degree atrioventricular block (PR 222 ms) and a prolonged QTc of 479 ms. He received another 2 l of NS for resuscitation along with increasing epinephrine infusion. Laboratory studies on arrival were significant for a creatinine of 1.0 g/dL, blood glucose > 500 mg/dL, and lactate 8.4 mmol/L. Shortly after arrival to the PICU, the patient became acutely bradycardic and then pulseless. Cardiopulmonary resuscitation (CPR) was immediately started; he was oxygenated and ventilated via bag-valve mask and received two bolus doses of 1 mg epinephrine along with repeated boluses of 1 g calcium chloride, 50 mEq sodium bicarbonate, and 5 mg glucagon. An intraosseous line was placed, and the patient was intubated during active resuscitation. Return of spontaneous circulation (ROSC) was achieved after 6 min of CPR. With the consultation of toxicology, repeated doses of calcium chloride, sodium bicarbonate for acidemia, and glucagon were given. In addition, the recommendation was made for high-dose insulin bolus and infusion (1 unit/kg followed by 1 unit/kg/h) and lipid emulsion bolus and infusion (1.5 ml/kg followed by 0.5 ml/kg/min) given concern for calcium channel and/or beta-blocker overdose. A right femoral central venous line was placed for improved access. Epinephrine infusion was titrated up to 1.5 mcg/kg/min, and norepinephrine infusion was added and titrated up to 20 mcg/min. Due to ongoing cardiovascular collapse despite maximum medical management, the decision was made to support the patient with ECMO. While awaiting ECMO deployment, the patient again became pulseless approximately 1 h after previous arrest. CPR was immediately resumed, and again the patient received multiple boluses of epinephrine, calcium chloride, sodium bicarbonate, and glucagon. He was also continued on epinephrine, norepinephrine, and insulin infusions; he received vasopressin and atropine boluses as well. After approximately 30 min of CPR, ROSC was achieved with a normal sinus rhythm with HR in the 80 s. The patient was then cannulated for veno-arterial ECMO by pediatric surgery. Following ECMO cannulation, a chest radiograph was performed which confirmed support apparatus in appropriate positions and also showed nonspecific low lung volumes with diffuse haziness.
Within 48 h, he was able to be decannulated, and then was weaned off epinephrine 2 days later. He was then extubated the following day. He had no neurologic deficits and was at his neurologic baseline. During the course of the patient’s hospitalization, the toxicology laboratory qualitatively confirmed the presence of amlodipine, caffeine, and metoprolol in his serum. No other coingestants were identified on routine toxicologic screening. He was discharged to an inpatient pediatric psychiatric facility 7 days after admission.
Discussion
Poisoned patients are often excellent candidates for extracorporeal life support [2]. These patients have a defined condition which in most cases will resolve with time as the body eliminates the xenobiotic. This is especially true in the case of young, otherwise healthy patients who have few if any comorbidities and have the physiologic reserves to facilitate recovery. This patient survived neurologically intact after a severe polysubstance ingestion including lab-confirmed metoprolol and amlodipine. While he responded intermittently to multiple therapies including high-dose insulin and lipids, he ultimately experienced cardiac arrest twice and required ECMO for hemodynamic stabilization. This case adds to the growing literature supporting use of ECMO in poisoned patients, particularly in the setting of overdose of cardiovascular medications.
Previous work from an in-patient toxicology consultation registry confirms that ECMO is still very rarely used in poisoned patients—10 out of 26,271 patients reported over a 3-year period [3]. In this small subset of patients, ECMO was typically started prior to cardiac arrest. Our case highlights that these patients can survive after ECMO support despite prolonged arrests prior to ECMO initiation.
One significant limitation of ECMO is that it is very resource intensive. It is not readily available at all centers, especially on an emergency basis [4]. Establishing an ECMO program able to respond to emergent scenarios requires communication and coordination across a spectrum of disciplines and departments. Despite these challenges and costs, there is evidence of overall value and efficacy from the use of ECMO in poisoned patients. The survival rate in one meta-analysis of cardiotoxic overdose patients who received ECMO was 66 %. While limited by confounders such as publication bias, this is a strikingly high proportion given that ECMO is typically used in moribund patients [2]. Further, a recent cost-effectiveness analysis of veno-arterial ECMO in cardiotoxicant patients in Canada was favorable, showing increased costs of only $7185 per life-year gained, $5151 in post-arrest patients [5].
The use of ECMO in poisonings is still relatively novel and additional work is needed. While it is currently used in the most severe overdoses, there are not yet clearly established indications for when and why it is best implemented in a given patient. The interplay between ECMO and other extracorporeal therapies such as hemodialysis would also benefit from further investigation, especially in the multiply poisoned patient [6].
Conclusions
ECMO is a viable option for patients who have overdosed on a cardiotoxicant drug, even following witnessed cardiac arrest.
References
Mowry, J. B., Spyker, D. A., Cantilena, L. R, Jr, McMillan, N., & Ford, M. (2014). 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clinical Toxicology (Philadelphia), 52(10), 1032–1283.
Johnson, N. J., Gaieski, D. F., Allen, S. R., Perrone, J., & DeRoos, F. (2013). A review of emergency cardiopulmonary bypass for severe poisoning by cardiotoxic drugs. Journal of Medical Toxicology, 9(1), 54–60.
Wang, G. S., Levitan, R., Wiegand, T. J., Lowry, J., Schult, R. F., & Yin, S. et al. (2015). Extracorporeal Membrane Oxygenation (ECMO) for severe toxicological exposures: Review of the toxicology investigators consortium (ToxIC). Journal of Medical Toxicology (Epub ahead of print)
de Lange, D. W., Sikma, M. A., & Meulenbelt, J. (2013). Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clinical Toxicology (Philadelphia), 51(5), 385–393.
St-Onge, M., Fan, E., Megarbane, B., Hancock-Howard, R., & Coyte, P. C. (2015). Venoarterial extracorporeal membrane oxygenation for patients in shock or cardiac arrest secondary to cardiotoxicant poisoning: A cost-effectiveness analysis. Journal of Critical Care, 30(2), 437.e7–437.e14.
Koschny, R., Lutz, M., Seckinger, J., Schwenger, V., Stremmel, W., & Eisenbach, C. (2014). Extracorporeal life support and plasmapheresis in a case of severe polyintoxication. Journal of Emergency Medicine, 47(5), 527–531.
Acknowledgments
The authors would like to acknowledge Dr. Carl Wolf for completing the toxicology lab analysis and confirming the presence of amlodipine and metoprolol.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Rights and permissions
About this article
Cite this article
Maskell, K.F., Ferguson, N.M., Bain, J. et al. Survival After Cardiac Arrest: ECMO Rescue Therapy After Amlodipine and Metoprolol Overdose. Cardiovasc Toxicol 17, 223–225 (2017). https://doi.org/10.1007/s12012-016-9362-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-016-9362-2