Abstract
The present study was to determine the preventive effect of resveratrol (Res) on diabetes-induced cardiac dysfunction and the possible signaling pathway involved. Diabetes was induced in rats by injection of streptozotocin (STZ) at 45 mg/kg. The animals were randomly divided into three groups (10 rats/group): normal group, diabetes groups with or without Res (80 mg/kg) treatment. Biochemistry, cardiac function and fibrosis were detected. Moreover, pro-inflammatory cytokines were evaluated, and heart tissues were homogenized for western blot analysis to analyze the possible mechanisms. The results indicated that Res might regulate glucose and lipid metabolism, ameliorate cardiac function and fibrosis response in STZ-induced diabetic rats. The protective effects were consistent with the inhibition of inflammatory factors such as TNF-α, IL-6 and IL-1β. In addition, Res favorably shifted STZ-induced AT1R, ERK1/2 and p38 MAPK activation in rat heart. In conclusion, the results suggested that Res attenuated diabetes-induced cardiac dysfunction, and the effects were associated with attenuation inflammatory response and down-regulation of AT1R-ERK/p38 MAPK signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is one of the major health problems in the world. It is estimated that the number of diabetic mellitus patients will be up to 300 million by the year 2025 [1]. Importantly, diabetic mellitus patients have a threefold to fivefold increased incidence of developing heart failure, which remains a fatal disease for high morbidity and mortality [2]. Diabetic cardiomyopathy is defined as the ventricular dysfunction that occurs in diabetic mellitus patients independent of another cause, such as hypertension, coronary artery disease or cardiac fibrosis [3]. Although many researches are focusing on these diseases, the exact underlying mechanism of diabetic cardiomyopathy is still very uncertain because its pathophysiology is believed to be multifactorial. Further study for this issue and development of effective agents against this condition are urgently needed.
Mitogen-activated protein kinases (MAPKs) cascades have been reported to be involved in the pathogenesis of cardiac hypertrophy in STZ-induced diabetic condition [4]. Extracellular signal-regulated kinases (ERK1/2) and p38 MAPK, two major members of MAPKs, have been proven to play a dual role, such as increased blood glucose uptake and development of cardiac hypertrophy under high glucose condition. On the other hand, previous study has indicated that renin–angiotensin–aldosterone system (RAAS) has differential signaling effect on ERK1/2/p38 MAPK. Particularly, angiotensin type 1 receptor (AT1R), a crucial compound of RAAS, was found to stimulate ERK 1/2 as well as p38 MAPK in the diabetic state [5]. Moreover, a recent study has revealed that the modulation of AT1R-ERK/p38 MAPK cascade plays an important role in the attenuation of diabetic nephropathy in hyperglycemic condition.
Resveratrol (3,5,4′-trihydroxystilbene, Res, Fig. 1), an atoxic phytoestrogen, has been found in more than 50 plants including berries and grapevine. A promise physiological function of Res is antioxidant, anti-inflammatory and antitumor activities for some therapeutical indications [6]. Epidemiological studies also suggest that Res is associated with improving glucose control [7] and reducing risk of cardiovascular diseases [8]. Moreover, a large body of evidence has challenged the protective effects of Res against ANG II-induced growth and proliferation of cardiac fibroblasts (CFs) by suppressing the ERK1/2 and p38 MAPK phosphorylation [9]. Although the apparent relationship between Res and reduced risk of diabetes mellitus and cardiovascular diseases has been established, the literature on the effects of Res on accelerated macroangiopathy in diabetes mellitus and its mechanisms is poorly understood. Moreover, relationships between the protective effect of Res on diabetic cardiomyopathy and the possible signaling pathway have not been reported previously. In the present study, we investigated the effects of Res on improving diabetes-induced cardiac dysfunction in rat. Furthermore, we examined the effect of Res on AT1R, ERK, p38 MAPK expressions and inflammatory factors to clarify the possible mechanisms of its cardioprotection.
Materials and Methods
Animals and Induction of Diabetes
Eight-week-old male Sprague–Dawley (SD) rats, weighing 225 ± 25 g, were purchased from the Experimental Animal Center of Shandong Luye Pharmaceutical Co. Ltd. (China). Rats were maintained on 12-h light/dark cycle at 21–26 °C, fed with a commercial standard rat cube diet and given access to tap water ad libitum. Animals were rendered diabetes by a single tail intravenous injection of streptozotocin (STZ, 45 mg/kg, Sigma) for the study diabetic cardiomyopathy [10]. Only animals with blood glucose levels ≥300 mg/dl 3 days after STZ injection were used in the present study. Age-matched non-diabetic SD rats were injected with sodium citrate buffer (it was used as the solvent of STZ) and used as controls. The animals were randomized into three groups (10 rats/group): normal group, diabetes groups without or with Res (80 mg/kg, i.g.) treatment. Res were dissolved in 0.5 % carboxymethyl cellulose sodium (CMC-Na), and normal group animals were orally given the same volume of CMC-Na. Rats were killed at 12 weeks after STZ injection.
Cardiac Function Assessment
At the end of the experiment, rats were anesthetized and cardiac function was determined by invasive hemodynamic evaluation methods as our previous study [11]. The parameters include left ventricular systolic pressure (LVSP), maximum rate of fall of left ventricle pressure (−dP/dtmax), maximum rate of rise of left ventricle pressure (+dP/dtmax) and left ventricular end-diastolic pressure (LVEDP). Moreover, the heart weight and the heart weight to body weight (WHW/BW) were detected at the end of the experiment.
Biochemistry and Inflammation Factors Assays
Blood for clinical chemistry was placed in tubes devoid of anticoagulant, allowed to clot at room temperature and centrifuged (3000 rpm for 15 min), and then, serum was separated. Glucose (GLU), total cholesterol (T.Cho), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were detected. In addition, the levels of the inflammatory cytokines TNF-α, IL-6 and IL-1β were measured using ELISA kits according to manufacture’s instructions.
Western Blot Analysis
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane and incubated with antibodies to β-actin, ATIR, P38, p-P38, ERK1/2, p-ERK1/2, JNK and p-JNK at 4 °C overnight. Proteins were detected with horseradish peroxidase (HRP)-conjugated secondary antibody and were visualized by ECL method.
Masson’s Trichrome Staining
To determine the effect of Res on diabetes-induced cardiac fibrosis, the histological staining of heart was stained by Masson’s trichrome staining after embedded in paraffin and sectioned (4 µm thick). Briefly, the sections were deparaffinized in Histoclear and then rehydrated using sequential passage through 70–100 % ethanol for 6 min each. The slides were stained with Masson’s trichrome stain for 5 min. The sections were washed in distilled water, stained with phosphomolybdic for 5 min and differentiated for 1 min. After a final distilled water wash, the sections then were dehydrated through alcohol (95 and 100 %, respectively) followed by dehydration and mounting. Interstitial collagen was digitally imaged with a microscope (OLYMPUS DP25). Moreover, fibrosis, for each animal, was determined by five different locations within the left ventricle and was reported as a percent of total tissue area.
Statistical Analysis
Data were reported as mean ± SD. The differences between groups were performed using one-way ANOVA, and p < 0.05 was considered statistically significant.
Results
Res Improved Glucose and Lipid Metabolism in STZ-Induced Diabetic Rats
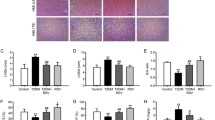
No mortality related to test substance administration was observed in rats during the experiment. At necropsy, neither absolute organ weight (including heart, liver, lung, sleep and kidney), nor relative organ weight, changed due to Res treatment. On histopathological examination, no significant lesions were observed in Res administration group (data not shown). However, the levels of GLU, T.Cho, TG and LDL-C in model rats were considerably higher when compared with control, suggesting that STZ caused glucose and lipid metabolism disorders in rats. Moreover, these were associated with the decrease of HDL-C level in these animals. Res treatment significantly improved GLU level and markedly reduced the associated lipid metabolism (such as T.Cho, TG and LDL-C), but seemed to have no effect on HDL-C level (Table 1).
Res Inhibited TNF-α, IL-6, IL-1β Releases
TNF-α, IL-6 and IL-1β are multi-functional pro-inflammatory factors and generally recognized as markers of vascular inflammation. After 11 weeks, STZ-induced diabetic animals exhibited increased circulating TNF-α, IL-6 and IL-1β concentrations (TNF-α, 49.7 ± 12.7 vs. 15.8 ± 2.6 ng/ml; IL-6, 321.5 ± 12.7 vs. 88.9 ± 15.8 pg/ml; IL-1β, 80.2 ± 9.00 vs. 32.9 ± 4.6 pg/ml, p < 0.01). However, Res treatment led to a significant decrease in these factor levels (Fig. 2). These results suggested a major role for Res in reducing circulating levels of pro-inflammatory cytokines.
TNF-α, IL-1 and IL-6 levels were measured using ELISA kits according to manufacture’s instructions. Res inhibited diabetes-induced inflammatory cytokines releases. Data were reported as mean ± SD. The differences between groups were performed using one-way ANOVA. ## p < 0.01 versus control group; *p < 0.05; **p < 0.01 versus model group
Res Ameliorated Diabetes-Induced Cardiac Dysfunction
To assess cardiac function, a pressure-detecting catheter was positioned into the carotid artery and the left ventricle. As shown in Fig. 3, STZ-induced diabetic animals exhibited a decreased body weight (298.0 ± 19.8 vs. 488.1 ± 38.4 g, p < 0.01) along with an increased WHW/BW (4.12 ± 0.21 vs. 3.05 ± 0.16, p < 0.01). Moreover, STZ-treated groups also exhibited markedly impaired systolic function as indicated by the decrease of LVSP and +dp/dtmax, obviously deteriorated diastolic function as shown by an increase of LVEDP and a decrease of −dp/dtmax. In contrast, Res treatment significantly reversed WHW/BW and hemodynamic values to the near normal levels. Moreover, there was no statistical difference in animal body weight between Res treatment group and model group (Fig. 3).
Measurement of body weight, WHW/BW and cardiac dysfunction. Cardiac function assessment was calculated through carotid artery cannulation. Data were reported as mean ± SD. The differences between groups were performed using one-way ANOVA. ## p < 0.01 versus control group; *p < 0.05; **p < 0.01 versus model group
Res Attenuated Diabetes-Induced Cardiac Fibrosis
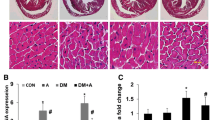
Masson’s trichrome staining was used to examine whether Res affected diabetes-induced myocardial fibrosis. As shown in Fig. 4, diabetes-induced cardiac fibrosis (as determined by blue staining) was obvious in the STZ-treated rats when compared to control animals, indicating the presence of cardiomyopathy in diabetic rats. However, Res markedly ameliorated this fibrosis development. These results indicated that Res had the potential to attenuate diabetes-induced myocardial interstitial fibrosis.
Res attenuated diabetes-induced cardiac fibrosis (×200 magnification). Representative images of Masson’s trichrome staining are shown. (a) Control group, (b) model group and (c) Res group. Data were reported as mean ± SD (d). The differences between groups were performed using one-way ANOVA. ## p < 0.01 versus control group; *p < 0.05; **p < 0.01 versus model group
Res Decreased AT1R, ERK1/2 and p38 MAPK Phosphorylation
To clarify the signaling pathway of Res on diabetes-induced cardiac dysfunction, we used western blot to analyze the phosphorylation of AT1R, ERK1/2, JNK and p38 MAPK in myocardial tissues of rats. Figure 5 shows that the phosphorylation of these proteins was considerably increased in model rats, in comparison with the normal animals. However, most of these changes were obviously blocked by treatment with Res (Fig. 5). Meanwhile, Res did not change the phosphorylation of JNK induced by diabetes (data not shown), suggesting that the beneficial effects of Res were mediated at least partly by AT1R-ERK/p38 MAPK signaling pathway.
Phosphorylation of AT1R, ERK1/2, JNK and p38 MAPK in myocardial tissues of rats was analyzed by western blot. Representative western blot images are shown, and data were reported as mean ± SD. The differences between groups were performed using one-way ANOVA. ## p < 0.01 versus control group; *p < 0.01 versus model group
Discussion
The sharply increasing incidence of diabetes mellitus has been represented an important burden for the society and for patients as well due to macro- and microvascular complications (such as diabetic cardiomyopathy and diabetic nephropathy). Factors underlying this complication risk include poor glycemic control, dyslipidemia, endothelial dysfunction and inflammation response. However, up to now, the mechanisms linking diabetes mellitus to these factors are not well understood. Res is a natural polyphenolic compound which is the chemopreventive agent of heart and cerebrovascular diseases. Res, fortunately, also has been proved to be closely linked these factors. For example, Novaes et al. have indicated that Res causes antihyperglycemic [12], antiatherogenic [13] and antihypercholesterolemic [14] effects. Meanwhile, the possible mechanisms are still poorly understand.
It is well known that hyperglycemia and blood lipid disorder have been proposed as major drivers of diabetic cardiomyopathy [15]. Consistent with previous studies, our data showed in Table 1 that GLU level and blood biochemical parameters, including T.Cho, TG, HDL-C and LDL-C, aggravated along with weight gain decrease after the rats were treated with TZD. This also showed the animal model was well established in the present study. However, Res significantly decreased GLU level, improved T.Cho, TG and LDL-C contents. Similarly, previous clinical and preclinical studies have suggested that treatment with Res can potentially delay or attenuate GLU level [12, 16]. These results indicated that the protective effects of Res might be due, in large part, to the regulation of glycosphingolipid metabolism disorder. Unfortunately, Res did not significantly influence HDL-C content and body weight. Diabetic cardiomyopathy, damage to the heart muscle, leads to impaired filling and relaxation of the heart diastolic dysfunction and eventually heart failure. Heart weight, LVSP, LVEDP and ± dp/dt max, as cardiac performance parameters, have been widely used in animal models and human clinical trials. In the current study, our results indicated that STZ injection successfully induced diabetes and cardiac dysfunction as indicated by the decrease of LVSP and ±dp/dtmax, the increase of LVEDP and WHW/BW, and myocardial fibrosis. Interestingly, all of these diabetes-induced cardiac dysfunctions were improved after administration of Res treatment. As expected, we have also found that the acceleration of cardiac fibrosis, as indicated by Masson’s trichrome staining, was ameliorated by Res treatment.
Inflammatory mediators, as well as glycosphingolipid metabolism disorder, also occur in the myocardium of diabetic patients. The abnormal inflammation response and blood biochemistry result in functional problems such as diastolic and systolic dysfunctions, which may cause symptoms of cardiac failure and myocardial fibrosis [17]. On the other hand, a depression of cardiac contractile function, such as diabetic cardiomyopathy, induces pro-inflammatory cytokine release, such as TNF-α, IL-6 and IL-1β [18]. Therefore, more and more researchers regard anti-inflammatory therapy as a promising strategy against these diseases. Recently, Das et al. have reported that the cardioprotection of Res is likely attributable, at least in part, to its anti-inflammatory properties [19]. In the current study, our data also indicated that Res not only ameliorated STZ-induced cardiac dysfunctions and myocardial fibrosis, but also attenuated inflammatory mediator releases (TNF-α, IL-6 and IL-1β), suggesting great promise for the prevention of vascular complications.
Though the exact mechanism is still unknown, extensive studies for last several decades have shown that RAAS plays a vital role in the process of diabetic cardiomyopathy [20, 21]. The RAAS has also been implicated in cardiac hypertrophy and atherosclerosis, in which it is considered to induce cardiovascular system remodeling via ACE-Ang II-AT1R axis [22]. AMPKs, an important conserved fuel-sensing enzyme group, are present in almost all mammalian cells. MAPKs include three major subfamilies, such as c-Jun-N-terminal kinase (JNK), ERK1/2 and p38 MAPK [23]. These major members are found in cardiac tissue, and they are most strongly activated by hyperglycemia and inflammatory cytokines [24]. Particularly, there is overwhelming evidence that AT1R-ERK/p38 MAPK signaling pathway plays a crucial role in the development of accelerated diabetes-induced cardiac dysfunction in diabetic cardiomyopathy [25].
RAAS and MAPK also influence cardiac functions in many diseases. Some scholars suggested that RAAS causes and accelerates the development and progression of diabetic-induced restenosis after arterial and venous reconstructions to some extent, and thus regarded RAAS as signal transducers of diabetic cardiomyopathy induced by high glucose levels [26]. Li et al. [27] have found that the activation of the MAPK signaling pathways is involved in a series of changes associated with diabetic cardiomyopathy such as cardiac dysfunction and fibrosis and is considered to be the primary cause of these diseases. Our results were in agreement with these reports, demonstrating enhanced activation of AT1R, JNK, ERK1/2 and p38 MAPK in diabetes-induced cardiac dysfunction. However, treatment with Res significantly suppressed the activation of ERK1/2 and p38 MAPK, but not influenced the JNK activity, suggesting clearly the involvement of ERK1/2 and p38 MAPK proteins in Res conditioning. Meanwhile, Res also blocked the AT1R expression under this condition. These findings suggested that Res possibly ameliorated diabetes-induced cardiac dysfunction through AT1R-ERK/p38 MAPK signaling pathway. Of note, a large body of evidence also suggested that Res could suppress AT1R expression and P38 MAPK signaling pathway [28, 29]. These previous studies strongly supported our opinion. Further research is required to elucidate the mechanisms of Res on AT1R-ERK/p38 MAPK. Recent study indicated that the beneficial effects of SIRT1 activator Res on RAAS involved cardiac remodeling are mediated by blood pressure-dependent pathways [30]. Moreover, Res could improve cardiac function by inhibiting the TGF-β/Smad3, an important activating pathway of p38 MAPK [31]. Taken together, the cardioprotection of Res may be involved in many signaling pathways, and more studies are needed to clarify it.
In summary, the results of the present study showed that chronic oral Res might protect against diabetes-induced cardiac dysfunction. Moreover, the results for the first time indicated that the beneficial effects of Res were directly and/or indirectly due to its hypoglycemic, hypolipidemic effects and inhibition of inflammation response associated with AT1R-ERK/p38 MAPK signaling pathway.
References
Wild, S., Roglic, G., Green, A., Sicree, R., & King, H. (2004). Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care, 27, 1047–1053.
Bell, D. S. (2003). Heart failure: The frequent, forgotten, and often fatal complication of diabetes. Diabetes Care, 26, 2433–2441.
Boudina, S., & Abel, E. D. (2007). Diabetic cardiomyopathy revisited. Circulation, 115, 3213–3223.
Chang, S. H., Liu, C. J., Kuo, C. H., Chen, H., Lin, W. Y., Teng, K. Y., et al. (2011). Garlic oil alleviates MAPKs- and IL-6-mediated diabetes-related cardiac hypertrophy in STZ-induced DM rats. Evidence-Based Complementary and Alternative Medicine, 2011, 950150.
Gava, E., Samad-Zadeh, A., Zimpelmann, J., Bahramifarid, N., Kitten, G. T., Santos, R. A., et al. (2009). Angiotensin-(1–7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrology, Dialysis, Transplantation, 24, 1766–1773.
Borriello, A., Cucciolla, V., Della Ragione, F., & Galletti, P. (2010). Dietary polyphenols: Focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutrition, Metabolism and Cardiovascular Diseases, 20, 618–625.
Liu, K., Zhou, R., Wang, B., & Mi, M. T. (2014). Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. American Journal of Clinical Nutrition, 99, 1510–1519.
Tomé-Carneiro, J., Gonzálvez, M., Larrosa, M., Yáñez-Gascón, M. J., García-Almagro, F. J., Ruiz-Ros, J. A., et al. (2012). One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. American Journal of Cardiology, 110, 356–363.
Olson, E. R., Naugle, J. E., Zhang, X., Bomser, J. A., & Meszaros, J. G. (2005). Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. American Journal of Physiology Heart and Circulatory Physiology, 288, H1131–H1138.
Bidasee, K. R., Zhang, Y., Shao, C. H., Wang, M., Patel, K. P., Dincer, U. D., & Besch, H. R, Jr. (2004). Diabetes increases formation of advanced glycation end products on Sarco(endo) plasmic reticulum Ca2+-ATPase. Diabetes, 53, 463–473.
Li, C., Gao, Y., Tian, J., Xing, Y., Zhu, H., & Shen, J. (2012). Long-term oral Asperosaponin VI attenuates cardiac dysfunction, myocardial fibrosis in a rat model of chronic myocardial infarction. Food and Chemical Toxicology, 50, 1432–1438.
Venkatachalam, K., Mummidi, S., Cortez, D. M., Prabhu, S. D., Valente, A. J., & Chandrasekar, B. (2008). Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. American Journal of Physiology Heart and Circulatory Physiology, 294(5), H2078–H2087.
Novaes, R. D., Peluzio, Mdo C., & Maldonado, I. R. (2012). Resveratrol causes antiatherogenic effects in an animal model of atherosclerosis. Arquivos Brasileiros de Cardiologia, 98(6), 571.
Göçmen, A. Y., Burgucu, D., Karadoğan, I., Timurağaoğlu, A., & Gümüşlü, S. (2013). The effect of trans-resveratrol on platelet-neutrophil complex formation and neutrophil burst in hypercholesterolemic rats. Experimental & Clinical Cardiology, 18(2), e111–e114.
Farb, M. G., Bigornia, S., Mott, M., Tanriverdi, K., Morin, K. M., Freedman, J. E., et al. (2011). Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. Journal of the American College of Cardiology, 58, 232–237.
Movahed, A., Nabipour, I., Lieben, L. X., Thandapilly, S. J., Yu, L., Kalantarhormozi, M., et al. (2013). Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evidence-Based Complementary and Alternative Medicine, 2013, 851267.
Adeghate, E., Kalasz, H., Veress, G., & Teke, K. (2010). Medicinal chemistry of drugs used in diabetic cardiomyopathy. Current Medicinal Chemistry, 17, 517–551.
Luo, B., Li, B., Wang, W., Liu, X., Xia, Y., Zhang, C., et al. (2014). An FNLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One, 9(8), e104771.
Cong, X., Li, Y., Lu, N., Dai, Y., Zhang, H., Zhao, X., & Liu, Y. (2014). Resveratrol attenuates the inflammatory reaction induced by ischemia/reperfusion in the rat heart. Molecular Medicine Reports, 9, 2528–2532.
Thomas, C. M., Yong, Q. C., Seqqat, R., Chandel, N., Feldman, D. L., Baker, K. M., & Kumar, R. (2013). Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: Comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor. Clinical Science (Lond), 124, 529–541.
Yong, Q. C., Thomas, C. M., Seqqat, R., Chandel, N., Baker, K. M., & Kumar, R. (2013). Angiotensin type 1a receptor-deficient mice develop diabetes-induced cardiac dysfunction, which is prevented by renin-angiotensin system inhibitors. Cardiovascular Diabetol, 12, 169.
Rosenkranz, S. (2004). TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovascular Research, 63, 423–432.
Sugden, P. H., & Clerk, A. (1998). Stress-responsive mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circulation Research, 83, 345–352.
Tibbles, L. A., & Woodgett, J. R. (1999). The stress-activated protein kinase pathways. Cellular and Molecular Life Sciences, 55, 1230–1254.
Lakshmanan, A. P., Harima, M., Sukumaran, V., Soetikno, V., Thandavarayan, R. A., Suzuki, K., et al. (2012). Modulation of AT-1R/AMPK-MAPK cascade plays crucial role for the pathogenesis of diabetic cardiomyopathy in transgenic type 2 diabetic (Spontaneous Diabetic Torii) rats. Biochemical Pharmacology, 83, 653–660.
Erdogan, D., Yucel, H., Uysal, B. A., Ersoy, I. H., Icli, A., Akcay, S., et al. (2013). Effects of prediabetes and diabetes on left ventricular and coronary microvascular functions. Metabolism, 62, 1123–1130.
Li, J., Peng, L., Du, H., Wang, Y., Lu, B., Xu, Y., et al. (2014). The protective effect of beraprost sodium on diabetic cardiomyopathy through the inhibition of the p38 MAPK signaling pathway in high-fat-induced SD rats. International Journal of Endocrinology, 2014, 901437.
Xu, H., Xiong, C., He, L., Wu, B., Peng, L., Cheng, Y., et al. (2013). Trans- resveratrol attenuates high fatty acid-induced P2X7 receptor expression and IL-6 release in PC12 cells: Possible role of P38 MAPK pathway. Inflammation, 94, 63–70.
Miyazaki, R., Ichiki, T., Hashimoto, T., Inanaga, K., Imayama, I., Sadoshima, J., & Sunagawa, K. (2008). SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1263–1269.
Biala, A., Tauriainen, E., Siltanen, A., Shi, J., Merasto, S., Louhelainen, M., et al. (2010). Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Pressure, 19, 196–205.
Chen, T., Li, J., Liu, J., Li, N., Wang, S., Liu, H., et al. (2014). Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via TGF-β/Smad3 pathway. American Journal of Physiology Heart and Circulatory Physiology. doi:10.1152/ajpheart.00454.2014.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2014JL048) and National Natural Science Foundation of China (No. 20973149).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, Y., Kang, L., Li, C. et al. Resveratrol Ameliorates Diabetes-Induced Cardiac Dysfunction Through AT1R-ERK/p38 MAPK Signaling Pathway. Cardiovasc Toxicol 16, 130–137 (2016). https://doi.org/10.1007/s12012-015-9321-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-015-9321-3