Abstract
Preeclampsia (PE), caused by multiple factors, is one of the most serious complications of pregnancy. Cadmium (Cd) is a heavy metal environmental pollutant, reproductive toxicant, and endocrine disruptor, which can increase the risk of PE. Cd toxicity due to occupational, diet, and environmental factors has worsened the risk. Studies showed elevated Cd concentration in maternal blood and placenta of PE women. However, the implicit association between Cd associated PE is still not highlighted. We systematically reviewed Cd-associated PE and its effect on pregnancy and birth outcomes. Based on “Preferred reporting items for systematic reviews and meta-analyses (PRISMA)” guidelines, eighty-six studies were identified by PubMed, Web of Science (WOS), and Scopus databases. Publications were included until October 2023 and articles screened based on our inclusion criteria. Our study identified that the exposure of controlled and uncontrolled Cd induces PE, which negatively affects pregnancy and birth outcomes. Given the serious nature of this finding, Cd is a potential adverse agent that impacts pregnancy and future neonatal health. Further comprehensive studies covering the whole trimesters of pregnancy and neonatal developments are warranted. Data on the molecular mechanisms behind Cd-induced PE is also essential for potential preventive, diagnostic, or therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is one of the leading causes of preterm birth, direct maternal morbidity, and mortality [1], affecting 8 to 10% of pregnancies around the world [2]. This overwhelming percentage of preeclamptic pregnancies has significantly increased the burden on the economy, clinicians, and healthcare systems [3, 4]. There exist disparities in preeclampsia outcomes between low- and high-income countries. These differences can be attributed to various factors, including inadequate knowledge [5], financial constraints, laboratory result delays, insufficient medical supplies and equipment, psychosocial stress, societal beliefs, inadequate patient counseling, and healthcare resources [6, 7]. Meanwhile, the inducer of PE is multifactorial and may include nutritional, immunological, environmental, or genetic factors [8, 9].
Focusing on environmental element, cadmium (Cd) is a non-essential heavy metal with primary exposure potentially that comes from food such as shellfish, rice, and leafy vegetables. It is also among the abundant compounds found in cigarette smoke. It accumulates in the liver, kidney, and bones [10], making urine Cd representative of Cd accumulation in the renal cortex.
Cadmium is also identified as an endocrine and reproductive system disruptor with an effect on the synthesis and regulation of hormones, as well as vascular functions [11, 12]. Cd contributes to immune regulation related to pregnancy-specific hypertension and is associated with PE. Placental Cd was recorded elevated in PE women, although the association and the mechanism behind this are still poorly understood [13].
Cadmium has a long biological half-life (up to 38 years for the human kidney and 19 years for the human liver) [14]. The common route of exposure is via inhalation (approximately 25%) and oral (5%) [15]. Both inhalational and oral Cd absorption increase during pregnancy [16] due to increased respiratory rate, decreased gastrointestinal motility, or decreased gastric emptying [17]. Overexpression of gut receptors and transporters due to high nutrient demand [18] may also promote Cd absorption. Cd accumulates in the lung and gut before being distributed to the liver, kidneys, placenta, mammary glands, uterus, and fetus [19, 20] and can be excreted into the milk [21, 22]. The presence of Cd in breast milk was detected [23, 24] and transferred to mouse pups via lactation [25]. The intestinal absorption of Cd is facilitated by various transporters, such as the divalent metal transporter-1 (DMT-1), calcium channels, amino acid transporters, and via endocytosis of the cadmium-metallothionein (CdMT) complex [26, 27].
Pregnancy outcomes are negatively impacted by PE [28, 29]. Among the consequences on the offspring from pregnancies complicated with PE include perinatal, neonatal, or infant morbidity/mortality [30]. Often, the only means to halt the progression of PE is preterm delivery of the fetus. The serious outcomes of preterm birth include respiratory complications, intravascular hemorrhage, necrotizing enterocolitis, and retinopathy of prematurity [31]. PE also causes thrombosis towards the placenta vasculature, leading to poor blood supply which subsequently affects the fetal growth potential [30].
A multifaceted approach to the management and therapy of preeclampsia is imperative [32, 33]. Screening for hypertensive disorders in the third trimester using a combination of maternal factors and angiogenic markers significantly improves prediction accuracy compared to biomarkers alone [32]. This indicates the importance of integrating comprehensive maternal health profiles in screening protocols to identify women at high risk earlier and more reliably. New advanced biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) could enhance early detection and intervention strategies [33].
To date, existing systematic literature reviews focused on the effects and management of PE and pregnancy and did not specifically highlight the impact of Cd-induced PE. This review aimed at evaluating the existing literature of Cd-induced PE and its effect on pregnancy and birth outcomes. Our review will provide vital information for the adverse impact of maternal Cd in escalating PE through maternal and fetal parameter alterations.
Methods
The research question (RQ) of this study is based on the PICO framework. The aspects are detailed below:
-
Patient/population: pregnant rats and women (JEG-3 cells)
-
Intervention/indicator: Cd-associated PE pregnancy
-
Comparison: pregnancy and birth outcomes
-
Outcomes: adverse effects of Cd exposure on maternal and fetal parameters
-
Research question: what are the adverse effects of Cd-associated PE on pregnancy and birth outcomes in terms of maternal and fetal parameters alteration?
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [34] was employed for this systematic review. PubMed, Web of Science (WOS), and SCOPUS were the search tools used to identify studies focusing on the effects of Cd-induced PE on pregnancy and birth outcomes. The keywords (combinations are presented in Appendix) used for mining the databases were “cadmium,” “preeclampsia,” “pregnancy,” “birth,” “prenatal,” and “maternal.” Initially, the studies were searched without language restriction, type of study (original research, review article, case report, etc.), and full-text availability. The studies published from inception until October 2023 were searched from the databases and included following the inclusion criteria. The original research articles focusing on the effect of Cd-induced PE during pregnancy in human and animal models were included, whereas review articles, case reports, editorials, studies lacking Cd exposure during pregnancy, or studies lacking Cd-induced PE during pregnancy were excluded. For studies with more than one publication from the same sample, the publication with the largest data or the latest publication was considered.
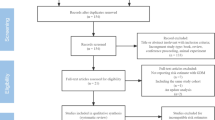
The study quality was evaluated by assessing risk of bias in each study using Cochrane review criteria [35]. The authors FS and RS evaluated each study separately. Based on seven domains (mentioned in Fig. 2), the studies were categorized into “low risk,” “unclear,” and “high risk.” The categories made by FS and RS were discussed with other authors (YSK, FR, and NAMK) to resolve classification discrepancies.
Results
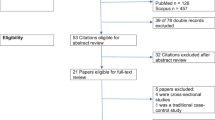
The details of the study selection process are shown in Fig. 1. Out of eighty-six (86) records identified from PubMed, WOS, and SCOPUS databases, only thirteen (13) studies met predefined inclusion/exclusion criteria. The number of studies excluded along with the reasons is detailed in Fig. 1.
Table 1 presents factors discussed by the studies included in this review.
The characteristics and findings of the studies discussing the effects of Cd-induced PE on JEG-3 (clonal human cell line) [47], women, and rats to determine the effects on pregnancy and birth outcomes are summarized in Table 2.
Risk of bias was evaluated in each of the thirteen included studies (Fig. 2). Six studies [28, 29, 36, 37, 39, 45] involving women and JEG-3 cells were unclear in terms of randomized sequence generation, while seven others published on rats/rodents model stated the method. None of the studies clearly mentioned allocation concealment. Studies on women [29, 37, 42] involved blinding of the study personnel, although it was unclear for the remaining 10 studies. All studies were categorized as “low risk” in the remaining four biases (detection, attrition, reporting, other).
Assessment of multiple systematic reviews (AMSTAR 2) was employed to further categorize the studies as poor, moderate, or good. Based on assessment criteria of type of study, publication status, effectiveness of methodology, quality of conclusions, risk of bias, and category of findings pool, all included studies were regarded as of high quality.
Discussion
The results presented in Table 2 highlight Cd as an inducer of preeclampsia and its effects on pregnancy and birth outcomes. Detailed discussion on the effects of Cd-induced PE in the light of existing literature on hypertension, proteinuria, maternal weight, endothelial dysfunction, placental growth and antiangiogenic factors, oxidative stress, fetal growth, spontaneous abortion/miscarriage, preterm birth and gestational age, and birth weight is presented in the following sections.
Preeclampsia: Cadmium as an Inducer
Evidence of contaminant Cd exposure is emerging [49, 50]. Cd is a known heavy metal, toxicant, and endocrine disruptor involved in the regulation of several hormones [11, 12]. It enters the systems through inhalation, ingestion, or dermal contact [29]. Contaminated food (plant-derived, rice), water, and tobacco are the major sources of Cd exposure [51]. Cadmium concentration level is associated with PE. High maternal blood and placental Cd levels were reported in PE patients [28] with a 1.15 times higher risk of PE for each standard deviation increase in Cd level [37].
By geographical locations, Cd concentrations were reported higher in Asian than Swedish or South African women. On clinical data, the incidence of PE shows wide variation between regions and is highest among developing countries [52, 53]. In developed countries, dietary Cd intake has decreased due to stricter environmental controls, although it has increased in some developing countries [11]. For instance, the average Cd intake in 2016–2019 in China was 17.3 lg/day, a 47% decrease over 2009–2013 [13]. On the other hand, in Vietnam, rice consumption caused the highest exposure to Cd, while metal recycling communities consumed higher amounts of Cd in their diets [15].
Dietary intake of Cd, use of consumer goods (electric batteries, paints, etc.), industrial wastes, soil fertilizers, and tobacco smoke were reported as the sources of Cd [54]. High blood Cd in preeclamptic women and animal models following Cd exposure were documented [12, 55].
Information about the molecular mechanisms behind Cd-induced PE is essential not just for detection but also for effective PE control. Brooks et al. [39] demonstrated that Cd-induced PE led to miRNA dysregulation. Furthermore, the changes in miRNA expression were robust in relation to Cd level in the preeclamptic group compared to the normotensive group. Hence, the role of miRNAs in predicting the regulation of angiogenesis-associated transforming growth factor-beta (TGF-β) pathway will be an area of future targets for PE treatment.
Hypertension
Cd exposure leads to hypertension [56, 57]. There exists a possibility of dysfunction and damage of the renal tubule due to Cd exposure and high blood pressure [58]. The association between blood pressure and blood Cd level is positive, and Cd level was significantly higher among Korean hypertension subjects [56]. Aramjoo et al. [59] conducted a systematic review and documented a positive relationship between hypertension and hair Cd levels. The relationship between urinary Cd and blood pressure was also positive [60].
Experimental evidence has also suggested an association between Cd and cardiovascular diseases [61]. In animal model of Cd induction, increased systolic blood pressure in pregnant rats was evident [38, 46].
Proteinuria
Proteinuria, an elevated protein level in urine, is another feature of PE [40]. Cd exposure induces renal and proximal tubules damage, resulting in urinary abnormalities, including filtered urinary protein [38, 62]. Furthermore, increased urinary albumin was also evident in pregnant rats following Cd administration [46]. Renal intoxication and nephrotoxicity induced by Cd were reported due to the activation of inflammatory responses [63].
Maternal Weight
Preeclampsia due to Cd exposure was reported to reduce maternal and fetal weights [38, 40, 43, 64]. However, inconsistency occurs as the effect of Cd on maternal body mass index (BMI) was reported high in another study [37].
Endothelial Dysfunction
The mechanism of endothelial dysfunction behind Cd exposure is closely related to oxidative stress (OS) [65]. The deposition of Cd on endothelium signifies abnormal lipid metabolism and endothelial dysfunction [66]. Cd accelerates triglyceride decomposition and accumulation of free fatty acids, leading to cell death and endothelial dysfunction due to cytotoxicity and induced abundance of reactive oxygen species [48].
On the other hand, preeclampsia is also associated with early and late pro-inflammatory states which contributes to maternal endothelial dysfunction [67], also with OS as the principal mechanism [48, 65]. Increased contraction of capillary vessels, endothelial swelling, and hyperplasia [43] reduced capillary space and thickened renal walls [38, 40], ultimately leading to endothelial dysfunction [38, 40, 43].
Placental Growth and Antiangiogenic Factors
Cadmium-induced PE poses risks for placental growth [68]. Cd placental accumulation reduces membrane protein expression, leading to poor placental transport [69]. Increased lipid hydro-peroxides and activation of lipid peroxidation were previously proposed as the mechanism to explain placental Cd accumulation [44].
Increased placental and maternal blood Cd in preeclamptic women decreased angiogenesis by down-regulating thyroid hormone receptor signaling [28]. Environmental Cd exposure causes GCN-2-mediated mitochondrial stress leading to poor placental angiogenesis and fetal growth [70]. On the other hand, gestational Cd exposure impairs placental angiogenesis via downregulating vascular endothelial growth factor A (VEGF-A) and dysregulation in the hormone receptor signaling pathway [71]. On the other hand, decreased CD34 staining in the placenta was consistent with anti-angiogenesis in the Cd-treated rats, with poor placentation caused by angiogenic factors and aberrant growth signaling was postulated to be the casual reason [39].
The decidual natural killer (dNK) cells secrete VEGF and placental growth factor (PGF), as well as cytokine interferon-γ (IFN-γ) [30]. VEGF and PGF promote angiogenesis beginning during the early pregnancy, while dNK cell-derived IFN-γ modify and promote vasodilation of decidual spiral arteries. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1), a tyrosine kinase protein with antiangiogenic properties, in the placenta of PE women diminished the circulating free VEGF and placental growth factor (PLGF), promotes endothelial dysfunction in vitro, and induces hypertension, proteinuria, and also glomerular endotheliosis [28]. In addition, dysregulation in the hormone receptor signaling [71] and decreased maternal circulating CD34 were also correlated with PE [38, 39].
Oxidative Stress (OS)
Cadmium-induced PE causes overabundance of reactive oxygen species leading to oxidative toxicity [73]. The effects of Cd-induced OS on DNA [41, 74], molecular metabolism, and immune responses were reported previously [37, 75]. When naturally produced free radicals in humans may pose a positive impact on the immune system, their negative effects on proteins, lipids, and DNA oxidation have been largely illustrated [72, 76]. Normally functioned cellular machinery will ensure non-pathological embryonic as well as placental growth.
Fetal Growth
Fetal growth is a useful marker for in utero fetal well-being. PE and fetal growth restriction (FGR) occur in 3 to 5% of pregnancies. It has been reported that Cd-induced PE restricts fetal growth [68, 70, 77] causing decreased birth weight [78] and head-tail length [38, 43] in a dose-dependent manner [29, 41]. The prevalence of PE (caused due to hypertensive disorders of pregnancy) is up to 15% in the developing countries with a mortality rate of 5–15%, leading to a risk factor for the health of fetal due to preterm birth [78].
Spontaneous Abortion/ Miscarriage
Placental accumulation of Cd leads to PE, growth restrictions, and miscarriage [42, 79, 80]. However, none of the studies included in this systematic review focused on miscarriage. This could be probably due to the criteria set in this study, which was dependent on the development of hypertension 20 weeks into pregnancy and the major focus being after viable pregnancy (more than 22 weeks). However, to the best of our knowledge, the complications pertaining to those 2-week intervals (between 20 and 22 weeks) have not been described in literature.
Hence, this study highlights the need for a comprehensive study to provide data on spontaneous abortion following PE diagnosis. It is also essential to establish how Cd exposure affects early fetal and post-natal development.
Preterm Birth and Gestational Age
Preterm birth is one of the leading causes of prenatal mortality and morbidity [81, 82]. Cd exposure might be associated with preterm birth [61] and originates from maternal pathophysiology [83].
Placental dysfunction in preeclamptic women of reproductive age poses a higher risk of preterm birth, lower mean birth weight percentile, and shorter gestational age [84]. The risk is higher among preeclamptic women with age less than 25 years [85]. The pathology behind gestational age, low birth weight, and PE is similar in exposure to toxicities [2]. However, in contrast, the role of Cd (induced by smoking) in causing placental abruption [44] and preterm birth [29] was insignificant as opposed to other reports [28, 37].
Birth Weight (BW)
Low BW is defined as birth weight less than 2500 g [86]. It is associated with increased neonatal and infant mortality [87]. Elevated maternal blood Cd has been associated with decreased fetal BW [29, 88, 89] and influences postnatal survival [90]. Accumulation of reactive oxygen and dysfunction of placental mitochondria resulting from Cd exposure were thought to be among the mechanisms [91]. In addition, low placental and fetal BW in preeclamptic women may also be associated with shorter gestational age [28, 29, 37, 41].
Strength and Limitations
The study has summarized the effects of PE induced by the environmental pollutant Cd on pregnancy and birth outcomes, providing useful insights for clinicians and the overall healthcare system. Another strength is the holistic review of the search question by including studies published until October 2023.
The use of terminologies/synonyms in keywords such as “gestation” or “pregnancy-induced hypertension” in place for “pregnancy” or “preeclampsia” might affect the search outcomes and number of articles captured by the employed databases. The exclusion of articles in a language other than English may also create bias in the search results.
With regards to the absence of protocol registration, this review was initiated prior to the awareness of the importance of protocol registration; hence, this aspect is unavailable here. However, despite the absence of protocol registration, the established PRISMA guidelines strictly followed here helped maintain the transparency and methodological rigor. The predefined search strategy, inclusion/exclusion criteria, and data extraction process helped ensure a systematic approach. The importance of protocol registration, although not mandatory, is recommended to enhance the credibility and reproducibility of systematic reviews and will be considered in our future reviews.
Conclusion and Recommendations
The findings of this study demonstrated the adverse effects of Cd-induced PE on pregnancy and birth outcomes in both human and animal models. Our review has linked Cd-induced PE to adversities related to the symptoms, altered biochemical markers as well as maternal-fetal changes.
Further works covering all pregnancy trimesters and postnatal development are warranted to acquire definitive results on the sequelae of Cd-induced PE during pregnancy. Data on the molecular mechanisms behind Cd-induced PE is also essential for potential preventive, diagnostic, or therapeutic targets. In addition, biomonitoring of maternal exposure to Cd will help reduce future adverse pregnancy and birth outcomes associated with PE.
Data Availability
Data sharing does not apply.
References
Kahramanoglu Ö, Schiattarella A, Demirci O, Sisti G, Ammaturo FP, Trotta C, Ferrari F, Rapisarda AMC (2022) Preeclampsia: state of art and future perspectives. A special focus on possible preventions. J Obstet Gynaecol 42:766–777
Stone J, Sutrave P, Gascoigne E, Givens MB, Fry RC, Manuck TA (2021) Exposure to toxic metals and per-and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: a review. Am J Obstet Gynecol MFM 3:100308
Hao J, Hassen D, Hao Q, Graham J, Paglia MJ, Brown J, Cooper M, Schlieder V, Snyder SR (2019) Maternal and infant health care costs related to preeclampsia. Obstet Gynecol 134:1227–1233
Shih T, Peneva D, Xu X, Sutton A, Triche E, Ehrenkranz RA, Paidas M, Stevens W (2016) The rising burden of preeclampsia in the United States impacts both maternal and child health. Am J Perinatol 33:329–338
Hackelöer M, Schmidt L, Verlohren S (2023) New advances in prediction and surveillance of preeclampsia: role of machine learning approaches and remote monitoring. Arch Gynecol Obstet 308:1663–1677
Robbins T, Hanlon C, Kelly AH, Gidiri MF, Musiyiwa M, Silverio SA, Shennan AH, Sandall J (2021) Pills and prayers: a comparative qualitative study of community conceptualisations of pre-eclampsia and pluralistic care in Ethiopia, Haiti and Zimbabwe. BMC Pregnancy Childbirth 21:1–14
Atluri N, Beyuo TK, Oppong SA, Moyer CA, Lawrence ER (2023) Challenges to diagnosing and managing preeclampsia in a low-resource setting: a qualitative study of obstetric provider perspectives from Ghana. PLOS Glob Public Health 3:e0001790
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P (2004) The importance of genetic and environmental effects for pre-eclampsia and gestational hypertension: a family study. BJOG: Int J Obstet Gynaecol 111:200–206
Williams PJ, Pipkin FB (2011) The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 25:405–417
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24:378–399
Henson MC, Chedrese PJ (2004) Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med 229:383–392
Knazicka Z, Forgacs Z, Lukacova J, Roychoudhury S, Massanyi P, Lukac N (2015) Endocrine disruptive effects of cadmium on steroidogenesis: human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing. J Environ Sci Health Part A 50:348–356
Zhang X, Chen K, Meng Z, Jia R, Lian F, Lin F (2022) Cadmium-induced preeclampsia-like phenotype in the rat is related to decreased progesterone synthesis in the placenta. Xenobiotica 52:625–632
Kjellström T, Nordberg GF (1978) A kinetic model of cadmium metabolism in the human being. Environ Res 16:248–269
Jacobo-Estrada T, Santoyo-Sánchez M, Thévenod F, Barbier O (2017) Cadmium handling, toxicity and molecular targets involved during pregnancy: lessons from experimental models. Int J Mol Sci 18:1590
Mikolić A, Schönwald N, Piasek M (2016) Cadmium, iron and zinc interaction and hematological parameters in rat dams and their offspring. J Trace Elem Med Biol 38:108–116
Moya J, Phillips L, Sanford J, Wooton M, Gregg A, Schuda L (2014) A review of physiological and behavioral changes during pregnancy and lactation: potential exposure factors and data gaps. J Eposure Sci Environ Epidemiol 24:449–458
Astbury S, Mostyn A, Symonds ME, Bell RC (2015) Nutrient availability, the microbiome, and intestinal transport during pregnancy. Appl Physiol Nutr Metab 40:1100–1106
Mikolić A, Piasek M, Sulimanec Grgec A, Varnai VM, Stasenko S, Kralik Oguić S (2015) Oral cadmium exposure during rat pregnancy: assessment of transplacental micronutrient transport and steroidogenesis at term. J Appl Toxicol 35:508–519
Blum JL, Xiong JQ, Hoffman C, Zelikoff JT (2012) Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol Sci 126:478–486
Petersson Grawé K, Oskarsson A (2000) Cadmium in milk and mammary gland in rats and mice. Arch Toxicol 73:519–527
Brako EE, Wilson AK, Jonah MM, Blum CA, Cerny EA, Williams KL, Bhattacharyya MH (2003) Cadmium pathways during gestation and lactation in control versus metallothoinein 1, 2-knockout mice. Toxicol Sci 71:154–163
Rebelo FM, Caldas ED (2016) Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants. Environ Res 151:671–688
Jacquillet G, Barbier O, Rubera I, Tauc M, Borderie A, Namorado M, Martin D, Sierra G, Reyes JL, Poujeol P (2007) Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am J Physiol-Ren Physiol 293:F1450–F1460
Blum JL, Edwards JR, Prozialeck WC, Xiong JQ, Zelikoff JT (2015) Effects of maternal exposure to cadmium oxide nanoparticles during pregnancy on maternal and offspring kidney injury markers using a murine model. J Toxicol Environ Health A 78:711–724
Thévenod F (2010) Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 23:857–875
Vesey DA (2010) Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol Lett 198:13–19
Li X, Yu T, Zhai M, Wu Y, Zhao B, Duan C, Cheng H, Li H, Wei Z, Yang Y (2022) Maternal cadmium exposure impairs placental angiogenesis in preeclampsia through disturbing thyroid hormone receptor signaling. Ecotoxicol Environ Saf 244:114055
Wang F, Fan F, Wang L, Ye W, Zhang Q, Xie S (2018) Maternal cadmium levels during pregnancy and the relationship with preeclampsia and fetal biometric parameters. Biol Trace Elem Res 186:322–329
Sones JL, Davisson RL (2016) Preeclampsia, of mice and women. Physiol Genomics 48:565–572
McCormick MC, Litt JS, Smith VC, Zupancic JA (2011) Prematurity: an overview and public health implications. Annu Rev Public Health 32:367–379
Schiattarella A, Magee L, Wright A, Syngelaki A, Akolekar R, Von Dadelszen P, Nicolaides K (2023) Prediction of hypertensive disorders after screening at 36 weeks’ gestation: comparison of angiogenic markers with competing-risks model. Ultrasound Obstet Gynecol 62:345–352
Sisti G, Fochesato C, Elkafrawi D, Marcus B, Schiattarella A (2023) Is blood pressure 120–139/80–89 mmHg before 20 weeks a risk factor for hypertensive disorders of pregnancy? A meta-analysis. Eur J Obstet Gynecol Reprod Biol 284:66–75
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1–9
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343. https://doi.org/10.1136/bmj.d5928
Paniagua L, Diaz-Cueto L, Huerta-Reyes M, Arechavaleta-Velasco F (2019) Cadmium exposure induces interleukin-6 production via ROS-dependent activation of the ERK1/2 but independent of JNK signaling pathway in human placental JEG-3 trophoblast cells. Reprod Toxicol 89:28–34
Liu T, Zhang M, Guallar E, Wang G, Hong X, Wang X, Mueller NT (2019) Trace minerals, heavy metals, and preeclampsia: findings from the Boston Birth Cohort. J Am Heart Assoc 8:e012436
Zhang Q, Huang Y, Zhang K, Yan Y, Wu J, Wang F, Zhao Y, Xu H, Jiang W, Yu D (2018) Progesterone attenuates hypertension and autoantibody levels to the angiotensin II type 1 receptor in response to elevated cadmium during pregnancy. Placenta 62:16–24
Brooks SA, Martin E, Smeester L, Grace MR, Boggess K, Fry RC (2016) miRNAs as common regulators of the transforming growth factor (TGF)-β pathway in the preeclamptic placenta and cadmium-treated trophoblasts: links between the environment, the epigenome and preeclampsia. Food Chem Toxicol 98:50–57
Zhang Q, Huang Y, Zhang K, Huang Y, Yan Y, Wang F, Wu J, Wang X, Xu Z, Chen Y (2016) Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ Pollut 218:770–782
Zhang X, Xu Z, Lin F, Wang F, Ye D, Huang Y (2016) Increased oxidative DNA damage in placenta contributes to cadmium-induced preeclamptic conditions in rat. Biol Trace Elem Res 170:119–127
Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, Fry RC (2015) Placental cadmium levels are associated with increased preeclampsia risk. PLoS ONE 10:e0139341
Shen W, Huang Y, Zhang Q, Lin F, Wang X, Ye D, Huang Y (2020) SCH58261, the antagonist of adenosine A2A receptor, alleviates cadmium-induced preeclampsia via sirtiun-1/hypoxia-inducible factor-1α pathway in rats. Eur Rev Med Pharmacol Sci 24:10941–10953
Eisenmann CJ, Miller RK (1995) Cadmium and glutathione: effect on human placental thromboxane and prostacyclin production. Reprod Toxicol 9:41–48
Brooks SA, Fry RC (2017) Cadmium inhibits placental trophoblast cell migration via miRNA regulation of the transforming growth factor beta (TGF-β) pathway. Food Chem Toxicol 109:721–726
Wang F, Zhang Q, Zhang X, Luo S, Ye D, Guo Y, Chen S, Huang Y (2014) Preeclampsia induced by cadmium in rats is related to abnormal local glucocorticoid synthesis in placenta. Reprod Biol Endocrinol 12:1–9
Serrano M, Macias R, Briz O, Monte M, Blazquez A, Williamson C, Kubitz R, Marin J (2007) Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta 28:107–117
McCann Haworth SM, Zhuge Z, Nihlén C, Von Rosen MF, Weitzberg E, Lundberg JO, Krmar RT, Nasiell J, Carlström M (2021) Red blood cells from patients with pre-eclampsia induce endothelial dysfunction. J Hypertens 39:1628–1641
Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, Vahter M (2012) Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect 120:284–289
Kahn LG, Trasande L (2018) Environmental toxicant exposure and hypertensive disorders of pregnancy: recent findings. Curr Hypertens Rep 20(10):87
Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, Rudisill C (2012) Agency for toxic substances and disease registry (ATSDR) toxicological profiles. Toxicol Profile Cadmium
Abalos E, Cuesta C, Grosso AL, Chou D, Say L (2013) Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 170:1–7
Meazaw MW, Chojenta C, Muluneh MD, Loxton D (2020) Systematic and meta-analysis of factors associated with preeclampsia and eclampsia in sub-Saharan Africa. PLoS ONE 15:e0237600
King KE, Darrah TH, Money E, Meentemeyer R, Maguire RL, Nye MD, Michener L, Murtha AP, Jirtle R, Murphy SK, Mendez MA (2015) Geographic clustering of elevated blood heavy metal levels in pregnant women. BMC public health 15:1–2
Silva CP, Lima DL, Schneider RJ, Otero M, Esteves VI (2013) Development of ELISA methodologies for the direct determination of 17β-estradiol and 17α-ethinylestradiol in complex aqueous matrices. J Environ Manage 124:121–127
Eum K-D, Lee M-S, Paek D (2008) Cadmium in blood and hypertension. Sci Total Environ 407:147–153
Wang Q, Wei S (2018) Cadmium affects blood pressure and negatively interacts with obesity: findings from NHANES 1999–2014. Sci Total Environ 643:270–276
Satarug S, Nishijo M, Ujjin P, Vanavanitkun Y, Moore MR (2005) Cadmium-induced nephropathy in the development of high blood pressure. Toxicol Lett 157:57–68
Aramjoo H, Arab-Zozani M, Feyzi A, Naghizadeh A, Aschner M, Naimabadi A, Farkhondeh T, Samarghandian S (2022) The association between environmental cadmium exposure, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Sci Pollut Res 29:35682–35706
Whittemore AS, DiCiccio Y, Provenzano G (1991) Urinary cadmium and blood pressure: results from the NHANES II survey. Environ Health Perspect 91:133–140
Messner B, Bernhard D (2010) Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals 23:811–822
Bernard A (2004) Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals 17:519–523
Hassanein EH, Mohamed WR, Ahmed OS, Abdel-Daim MM, Sayed AM (2022) The role of inflammation in cadmium nephrotoxicity: NF-κB comes into view. Life Sci 308:120971
Chen N, Tong X, Wu S, Xu X, Chen Q, Wang F (2022) Cadmium induces placental glucocorticoid barrier damage by suppressing the cAMP/PKA/Sp1 pathway and the protective role of taurine. Toxicol Appl Pharmacol 440:115938
Almenara CC, Oliveira TF, Padilha AS (2020) The role of antioxidants in the prevention of cadmium-induced endothelial dysfunction. Curr Pharm Des 26:3667–3675
Liang H, Yue R, Zhou C, Liu M, Yu X, Lu S, Zeng J, Yu Z, Zhou Z, Hu H (2021) Cadmium exposure induces endothelial dysfunction via disturbing lipid metabolism in human microvascular endothelial cells. J Appl Toxicol 41:775–788
Valencia-Ortega J, Zárate A, Saucedo R, Hernández-Valencia M, Cruz JG, Puello E (2019) Placental proinflammatory state and maternal endothelial dysfunction in preeclampsia. Gynecol Obstet Invest 84:12–19
Xu P, Guo H, Wang H, Lee SC, Liu M, Pan Y, Zheng J, Zheng K, Wang H, Xie Y (2019) Downregulations of placental fatty acid transporters during cadmium-induced fetal growth restriction. Toxicology 423:112–122
Geng H-X, Wang L (2019) Cadmium: toxic effects on placental and embryonic development. Environ Toxicol Pharmacol 67:102–107
Xiong Y-W, Xu X-F, Zhu H-L, Cao X-L, Yi S-J, Shi X-T, Zhu K-H, Nan Y, Zhao L-L, Zhang C (2021) Environmental exposure to cadmium impairs fetal growth and placental angiogenesis via GCN-2-mediated mitochondrial stress. J Hazard Mater 401:123438
Shi X-T, Zhu H-L, Xu X-F, Xiong Y-W, Dai L-M, Zhou G-X, Liu W-B, Zhang Y-F, Xu D-X, Wang H (2021) Gestational cadmium exposure impairs placental angiogenesis via activating GC/GR signaling. Ecotoxicol Environ Saf 224:112632
Finaud J, Lac G, Filaire E (2006) Oxidative stress. Sports Med 36:327–358
Park JH, Lee BM, Kim HS (2021) Potential protective roles of curcumin against cadmium-induced toxicity and oxidative stress. J Toxicol Environ Health Part B 24:95–118
Lin A-J, Zhang X-H, Chen M-M, Qing C (2007) Oxidative stress and DNA damages induced by cadmium accumulation. J Environ Sci 19:596–602
Yang H, Wang Z, Wang J, Lv B, Wu Z, Tian J, Yang J (2021) Cadmium-induced oxidative stress and transcriptome changes in the wolf spider Pirata subpiraticus. Sci Total Environ 785:147364
Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748
Zhang H, Liu X, Zheng Y, Zha X, Elsabagh M, Zhang Y, Ma Y, Loor JJ, Wang M, Wang H (2022) Effects of the maternal gut microbiome and gut-placental axis on melatonin efficacy in alleviating cadmium-induced fetal growth restriction. Ecotoxicol Environ Saf 237:113550
Zhang B, Chen X, Yang C, Shi H, Xiu W (2024) Effects of hypertensive disorders of pregnancy on the complications in very low birth weight neonates. Hypertens Pregnancy 43:2314576
Dong F, Xiao P, Li X, Chang P, Zhang W, Wang L (2021) Cadmium triggers oxidative stress and mitochondrial injury mediated apoptosis in human extravillous trophoblast HTR-8/SVneo cells. Reprod Toxicol 101:18–27
Omeljaniuk WJ, Socha K, Soroczynska J, Charkiewicz AE, Laudanski T, Kulikowski M, Kobylec E, Borawska MH (2018) Cadmium and lead in women who miscarried. Clin Lab 64:59–67
Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK (2007) Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol 110:311–317
Asefi Y, Gohari Mahmoudabad A, Habibian Sezavar A, Mirshahvaladi S, Abyadeh M, Abyareh M (2022) Association between maternal cadmium exposure and preterm birth: a meta-analysis. Int J Environ Health Res 32:628–637
Kim S, Richardson L, Radnaa E, Chen Z, Rusyn I, Menon R, Han A (2022) Molecular mechanisms of environmental toxin cadmium at the feto-maternal interface investigated using an organ-on-chip (FMi-OOC) model. J Hazard Mater 422:126759
Cavoretto PI, Farina A, Salmeri N, Syngelaki A, Tan MY, Nicolaides KH (2024) First trimester risk of preeclampsia and rate of spontaneous birth in patients without preeclampsia. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2024.01.008
Li X, Zhang W, Lin J, Liu H, Yang Z, Teng Y, Duan S, Li Y, Xie Y, Lin X (2018) Preterm birth, low birthweight, and small for gestational age among women with preeclampsia: does maternal age matter? Pregnancy hypertension 13:260–266
Wardlaw TM (2004) Low birthweight: country, regional and global estimates. Unicef
Wen Y-H, Yang H-I, Chou H-C, Chen C-Y, Hsieh W-S, Tsou K-I, Tsao P-N (2019) Association of maternal preeclampsia with neonatal respiratory distress syndrome in very-low-birth-weight infants. Sci Rep 9:1–8
Huang S, Kuang J, Zhou F, Jia Q, Lu Q, Feng C, Yang W, Fan G (2019) The association between prenatal cadmium exposure and birth weight: a systematic review and meta-analysis of available evidence. Environ Pollut 251:699–707
Kuhnert B, Kuhnert P, Debanne S, Williams T (1987) The relationship between cadmium zinc and birth weight in pregnant women who smoke. Am J Obstet Gynecol 157:1247–1251
Nakimuli A, Starling JE, Nakubulwa S, Namagembe I, Sekikubo M, Nakabembe E, Scott JG, Moffett A, Aiken CE (2020) Relative impact of pre-eclampsia on birth weight in a low resource setting: a prospective cohort study. Pregnancy Hypertens 21:1–6
Yildirim RM, Ergun Y, Basar M (2022) Mitochondrial dysfunction, mitophagy and their correlation with perinatal complications: preeclampsia and low birth weight. Biomedicines 10:2539
Acknowledgements
This study has been conducted in the framework of the research activities of the projects funded by University Technology Mara (UiTM) under grants: 600-UiTMSEL (PI.5/4) (023/2022) and 600-TNCPI 5/3/DDF (MEDIC) (004/2021). The authors are grateful to University Technology Mara (UiTM) for supporting this research. The authors also appreciate and acknowledge the valuable time of the Editor and the reviewers.
Funding
This study has been conducted in the framework of the research activities of the projects funded by University Technology Mara (UiTM) under grants: 600-UiTMSEL (PI.5/4) (023/2022) and 600-TNCPI 5/3/DDF (MEDIC) (004/2021).
Author information
Authors and Affiliations
Contributions
The authors FS and RS identified and evaluated each study separately. Based on seven domains (mentioned in Fig. 2), the studies were categorized into “low risk”, “unclear”, and “high risk” by FS and RS and then checked by YSK, FR, and AAA. The detailed review evaluation of the selected studies was made by FS and RS. The results were discussed with four other authors (YSK, FR, AAA, and NAMK). These four authors checked and verified the findings.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Keyword combinations used for searching databases
No | Keywords combination |
|---|---|
1 | “cadmium” AND “preeclampsia” AND “pregnancy” |
2 | “cadmium” AND “preeclampsia” AND “pregnancy” AND “birth” |
3 | “cadmium” AND “preeclampsia” AND “pregnancy” AND “prenatal” |
4 | “cadmium” AND “preeclampsia” AND “pregnancy” AND “maternal” |
5 | “cadmium” AND “preeclampsia” AND “birth” |
6 | “cadmium” AND “preeclampsia” AND “birth” AND “prenatal” |
7 | “cadmium” AND “preeclampsia” AND “birth” AND “maternal” |
8 | “cadmium” AND “preeclampsia” AND “pregnancy” AND “birth” AND “prenatal” |
9 | “cadmium” AND “preeclampsia” AND “pregnancy” AND “birth” AND “maternal” |
10 | “cadmium” AND “preeclampsia” AND “pregnancy” AND “birth” AND “prenatal” AND “maternal” |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sardar, F., Kamsani, Y.S., Ramly, F. et al. Cadmium Associated Preeclampsia: A Systematic Literature Review of Pregnancy and Birth Outcomes. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04364-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04364-5