Abstract
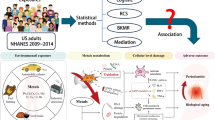
Periodontal disease, one of the most prevalent diseases worldwide, is a chronic inflammatory disease caused by dysbiotic dental biofilms that trigger the host’s immune response. Periodontitis is a type of periodontal disease characterized by the destruction of tissues that support the teeth. The disease is influenced by various systemic and environmental risk factors. As heavy metals have been associated with the development of chronic inflammatory diseases, the present scoping review aimed to determine the coverage of the literature on whether human contamination by heavy metals affects periodontitis, as well as their mechanisms of action. Eight studies were selected, and two reviewers evaluated them. Most studies were cross-sectional studies involving humans and one study was performed on rats. Our review revealed a significant correlation between periodontitis and bioaccumulation of lead and cadmium. Oxidative stress generated by trace metals, characterized by elevated levels of reactive oxygen species, causes tissue damage through lipid peroxidation, enzymatic oxidation, and stimulation of proinflammatory cytokines. In conclusion, heavy metals contamination may be a risk factor for the development of periodontitis. Oxidative stress factors seem to increase the extent of the inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is a chronic multifactorial inflammatory disease triggered by dysbiotic biofilms that is characterized by progressive degradation of the tooth-supporting structures [1]. It is one of the most prevalent diseases worldwide, affecting approximately 45–50% of the global population, with severe forms affecting 11.2% of adults, making it the sixth most common disease [2, 3]. The disease is influenced by various systemic and environmental risk factors, including genetic predisposition, systemic conditions such as diabetes mellitus, smoking, stress, poor oral hygiene, and socioeconomic status [4]. In the chronic inflammatory context of periodontitis, the activation of neutrophil oxidative metabolism and various other reactions contribute to an upsurge in reactive oxygen species that exceeds the host’s antioxidant capacity, thus exacerbating the condition [5, 6]. These mechanisms can be further fostered by contamination with heavy metals.
Heavy metals are metallic elements characterized by high atomic weights and densities. This group includes lead (Pb), mercury (Hg), cadmium (Cd), arsenic (As), and chromium (Cr), among other elements [7]. While heavy metals are naturally occurring chemical elements distributed throughout the Earth’s crust, the primary sources of environmental contamination and human exposure are industrial production and disposal [8], by present and former mining activities, foundries and smelters. Although the unique properties of heavy metals render them invaluable for industrial applications and technological advancement, they pose a significant threat to human health. Certain metals are highly toxic even at minimal concentrations because of their tendency to bioaccumulate in soft and hard tissues. Additionally, when stored within the body, these metals can mimic essential elements, disrupting normal functions [5, 9,10,11].
Human exposure to heavy metals occurs through dermal contact, inhalation, and ingestion [9]. If not properly metabolized, these elements can cause disorders by interacting with specific compounds such as oxygen and chloride, triggering non-specific toxic effects at the cellular and molecular level [12]. The mechanisms of metal intoxication can be classified as direct or indirect. Elements such as Pb, As, copper (Cu), and Cr directly affect the generation of reactive oxygen species, increasing the levels of reactive hydroxyl radicals. Regarding indirect mechanisms of action, Hg, Ag, Cu, Cd, Pb, Cr, and nickel (Ni) increase the concentrations of free radicals by binding to iron (Fe), manganese (Mn), selenium (Se), calcium (Ca), and zinc (Zn), which are involved in antioxidant activities [8]. The persistence of oxidative stress within the cellular milieu is associated with free radical formation and can precipitate DNA alterations, protein degradation, and lipid peroxidation, culminating in irreparable damage and even cell death [13, 14]. Free radicals have been linked to the development of chronic and inflammatory conditions, including periodontal diseases [5, 11].
To the best of our knowledge, only eight studies have investigated the influence of heavy metals on periodontitis and their underlying mechanisms of action in periodontal tissues [15,16,17,18,19,20,21,22]. The present review aims to comprehensively overview the existing literature on the relationship between heavy metal contamination and periodontitis and to elucidate the possible mechanisms behind this relationship. The central hypothesis of this review is that heavy metal contamination in humans is associated with the development of periodontitis.
Materials and Methods
Inclusion and Exclusion Criteria
The following criteria were employed for inclusion of the studies in this review:1. studies involving periodontal assessment and heavy metals contamination detection; 2. only studies published in English; and 3. studies that met predefined scientific standards, including rigorous methodology, appropriate study design, adequate sample size, clear data analysis, and peer-reviewed publication. The following studies were excluded from our analysis: (1) conference abstracts, reviews, editorials, and letters; (2) non-English language articles; (3) studies that did not provide data that could be extracted or accessed to ensure the robustness of our analysis; (4) studies that did not use validated methods for measuring periodontal effects; and 5.studies reporting incomplete or unclear results.
Database Search
Between May 2023 and May 2024, we conducted comprehensive searches in the following databases: PubMed, Google Scholar, SciELO, and Web of Science. The following keywords and Boolean operators were used to identify eligible articles: (“biological contamination”) AND (“heavy metals”) AND (“periodontal disease” OR “oral manifestation”). These search terms were validated to ensure that they retrieved a representative sample of relevant articles. Furthermore, pertinent references and associated articles were hand searched. No publication date restrictions were imposed and the searches were exclusively conducted in English.
Data extraction and quality assessment
To maintain the integrity of our review, two independent reviewers (CNC and PRC) extracted the data and evaluated the quality of the studies. In the case of disagreement, a final decision was reached through discussion and consensus, ensuring the quality of the selected studies. This process consisted of:
-
1
Initial Screening: Titles and abstracts of all identified studies were initially screened to assess their relevance to the review topic. Studies that met the inclusion criteria were then subjected to full-text review.
-
2
Full-Text Review: Both reviewers independently evaluated each study against the predefined inclusion and exclusion criteria. This step ensured that only studies of sufficient quality and relevance were included.
-
3
Data Extraction: Data extracted from each study included essential information such as authorship, publication year, study location, study design, the specific chemical elements investigated, the source and dose or concentration of heavy metals, and the resulting periodontal outcomes. This data was recorded and organized using Excel (Microsoft, Redmond, WA, US).
-
4
Quality Evaluation: methodological rigor, the adequacy of sample size, the appropriateness of statistical analysis, and the consideration of confounding factors were assessed.
-
5
Confounding Factors: Special attention was given to how each study accounted for potential confounding factors, such as smoking, age, and pre-existing health conditions, which could influence the relationship between heavy metal contamination and periodontitis.
The data extracted for this review included essential information such as authors, publication year, study location, study design, the specific chemical elements investigated, the source and dose or concentration used, and the resulting outcomes.
Results
General Characteristics of Included Studies
Table 1 summarizes the main characteristics of the selected studies. Eight studies were included in the analysis. The studies were conducted in different geographic regions, including five in the United States, two in Korea, and one in Turkey. The publication period of the selected articles ranged from 2009 to 2023, reflecting recent developments in this field of research.
This review included findings from six cross-sectional studies using NHANES data [15,16,17,18,19, 22], one pilot cohort study [20], and one study on rats [21]. The main cofactors of the studies were age, sex, and smoking habits. All selected studies shared a common focus, i.e., to investigate the relationship between exposure to heavy metals and periodontal conditions.
Assessment of Heavy Metal Concentration Levels
Inductively coupled plasma-mass spectrometry (ICP-MS) and atomic absorption spectrophotometry (AAS) were the most widely used analytical techniques for measuring heavy metals in the biological samples (urine, blood, and dental calculus). ICP-MS was used in studies analyzing multiple elements [16, 19, 20, 22]. Conversely, in studies analyzing smoking exposure as the source of specific heavy metals, AAS was used [15, 17, 18]. The established coefficients of variation were 0.95–4.82% (Cd) and 2.65–6.50% (Pb), with detection limits of 0.056 µg/L for Cd and of 0.12 µg/L for Pb.
Sample preparation for analysis was standardized for solid and fluid samples. Urine and blood samples were collected and stored at freezing temperatures (-20 °C) before being sent to the laboratory. Blood samples were transported on dry ice and then submitted to ICP-MS [16] and AAS [17]. Urine samples were double distilled and standardized with concentrated nitric acid before evaluation by ICP-MS [15, 18, 19, 21]. For dental calculus analysis, samples were dried, weighed, and subjected to acid digestion with HNO3 (6 mL, 68%) and HCl (2 mL, 37%), followed by thermal digestion (180°-200°) [20].
Periodontal Assessment
The clinical parameters used for periodontal assessment varied among studies. Probing depth (PD) and clinical attachment level (CAL) were the most commonly used parameters. In NHANES-based studies, periodontitis was defined as CAL > 4 mm at more than 10% of the evaluated sites in index teeth (11, 16, 17, 26, 27, 31, 36, 37, 46, and 47) [15, 16, 19]. The KNHANES database used the WHO Community Periodontal Index (CPI), in which periodontitis was defined PD > 3.5 mm in index teeth [17, 18]. Huang et al. (2022) and Li et al. (2023) categorized periodontitis according to severity based on the assessment of CAL and PD [16, 22]. Another study used a self-reported questionnaire for periodontitis [19]. The plaque index was also evaluated, with a supragingival calculus sample being collected from the mesial, palatal/lingual, and distal surfaces of index teeth [20]. In the study on rats, periodontal bone loss was quantified by measuring the distance from the cementoenamel junction to the alveolar bone crest for each molar teeth using the Image Pro Plus software [21].
Main Findings
All studies evaluated one or more heavy metals, including Pb, Hg, Cd, and As, among others. Pb and Cd were the primary heavy metals associated with periodontitis [15,16,17,18,19,20,21,22].
One study categorized blood plasma concentrations of Pb and Cd into low, medium and high in a population of 1,966 adult Koreans [18]. Multivariate logistic regression analysis revealed that individuals with high Cd levels had a 1.57 times (95% confidence interval [CI], 1.03–2.38) higher odds ratio (OR) for periodontitis than those with low Cd. High Cd levels were associated with periodontitis in females and current smokers, and medium Pb levels were associated with periodontitis in females and non-smokers [18].
In a survey of 11,412 Americans, the age-adjusted mean urine Cd concentration was significantly higher among participants with periodontitis (OR = 0.50; 95% CI, 0.45–0.56) compared to those without periodontitis. Multivariable-adjusted analyses, which included adjustments for tobacco exposure, showed that a three-fold increase in urinary Cd concentrations [corresponding to an increment from the 25th (0.18 µg/g) to the 75th (0.63 µg/g) percentile] was associated with 54% higher odds of prevalent periodontitis (OR = 1.54; 95% CI, 1.26–1.87) [15].
In a study of 4,566 American adults considering all heavy metals analyzed in urine (Cd, antimony, thallium, Pb, and uranium), only Cd (OR = 1.43; 95% CI, 1.05–1.94; p = 0.02) was associated with self-reported periodontitis. Cd was also associated with self-reported bone loss around teeth (OR = 1.64; 95% CI, 1.15–2.34; p = 0.009) [19].
Han et al. (2013) studied 6,352 Koreans and found significantly higher Cd and Pb levels in the periodontitis group, defined by the WHO CPI, compared to the control population. Periodontitis was significantly associated with serum Cd (OR = 1.37; 95% CI, 1.00–1.87) and Pb (OR = 1.60; 95% CI, 1.15–2.21) levels [17]. There was a dose-dependent association between increasing number of teeth with periodontitis and increasing serum Cd and Pb concentrations, particularly among active smokers.
Browar et al. (2018) examined the relationship between Cd exposure and periodontitis in experimental animals. Male Sprague/Dawley rats were given daily subcutaneous injections of Cd (0.6 mg/kg/day) for up to 12 weeks. The animals were euthanized, and their mandibles and maxillae were evaluated for levels of alveolar bone. After 12 weeks of Cd exposure, there was a significantly greater bone loss in exposed animals when compared to controls (p < 0.0001). This study shows that Cd has significant, time-dependent effects on periodontal bone in an animal model of Cd exposure [21]. In the study conducted by Huang et al. (2022) involving 4,964 participants, CAL was positively associated with blood Pb and Cd levels, while blood Hg showed no association. The positive association between Pb and CAL was significant in older adults and patients with diabetes. The association between blood Cd levels and CAL was significant in males, in Mexican-Americans, in older adults, and in the diabetic group [16].
Li et al. (2023) showed that individuals with periodontitis, corresponding to 40.9% in a sample of 2,269 participants, had elevated concentration levels of heavy metals. Pb was the most pronounced, followed by Ba [22].
One study did not find any association between heavy metals in dental calculus and the diagnosis of periodontitis [20]. This study analyzed 29 supragingival dental calculus samples obtained from non-smokers (n = 14) and smokers (n = 15). Using ICP-MS to assess 26 metals and metalloids, the study was unable to establish an association between the accumulation of heavy metals in supragingival dental calculus and the presence of gingivitis or periodontitis.
Discussion
Exposure to heavy metals poses challenges to human health and longevity, with effects that are not fully understood. The present scoping review aimed to overview the existing literature on whether heavy metals affect periodontal tissues, as well as their mechanisms of action. According to the reviewed studies, heavy metals may be a risk factor for the development of the periodontal inflammatory response. Oxidative stress factors seem to increase the amplitude of this response.
All included studies highlighted the potential influence of the cofactors age and sex on the relationship between heavy metals and periodontal conditions. Two studies accounted for these factors by adjusting them in their statistical analyses [17, 19]. Older age and male sex may be associated with an increased risk of heavy metal accumulation and a greater likelihood of developing periodontal disease [15, 16]. Conversely, in a Korean population, female sex was found to predispose to the impact of Cd accumulation on the development of periodontitis [18]. On the other hand, Yaprak et al. (2016) observed no correlation between age or sex and heavy metal accumulation in dental calculus [20]. The ubiquity of heavy metal exposure from various sources in daily life (underscores the notion that older individuals may experience greater exposure, potentially elevating their risk of heavy metal accumulation [23]. A wide range of biological samples, including urine, blood [16,17,18] and dental calculus [20] was subjected to analysis and revealed the presence of heavy metals. These findings reinforce the pervasive capacity of heavy metals to diffuse, accumulate, and elicit responses in the body. Differences in endocrine and hormone functions, as well as diverse social factors, may contribute to sex differences in the interplay between heavy metals and their biological consequences [24].
The consequences of heavy metal accumulation and inflammatory response in the periodontium were mainly evaluated using clinical parameters, particularly CAL and PD. However, the varied diagnostic criteria applied to define periodontitis compromised the comparison of study results and led to discrepancies in data interpretation. Notably, only one study categorized periodontitis in humans according to severity and found a significant positive association between blood Pb and Cd levels and the severity of periodontitis [16]. The positive association agrees with the findings of Browar et al. (2018) who reported increased bone loss due to continuous heavy metal exposure in laboratory rats [21]. These findings suggest that heavy metals influence the progression of periodontitis.
The included studies predominantly relied on [16,17,18] and urine [15, 19, 22] samples for assessing heavy metal accumulation. One exception was the study by Yaprak et al. (2016), which analyzed dental calculus [20]. Urinary and blood samples are widely recognized as primary sources for quantifying concentrations of toxic substances [25]. Urinary concentrations particularly reflect the overall body burden of heavy metals, while blood is considered the most reliable indicator of recent exposure, with concentrations typically being evaluated in whole blood [26]. The inability to establish the link between heavy metals in dental calculus and periodontal health suggests that heavy metals influence may be more closely related to the systemic inflammatory responses rather than exerting a direct local effect.
This study reviewing the relationship between heavy metals and periodontitis had several limitations. First, the majority of the analyzed studies employed a cross-sectional design, which restricted their ability to establish a definitive causal relationship between heavy metals and periodontitis [15,16,17,18,19,20,21,22]. Furthermore, these studies primarily focused on examining the association between heavy metals, mainly Pb and Cd, and periodontitis but failed to address the precise mechanisms of how these metals induce pathological changes in periodontal diseases. Some studies also assessed individuals who smoke [15, 18, 20]. Given the well-known detrimental effects of smoking on the periodontium, caution should be exercised in interpreting the findings of these studies. However, it is crucial to acknowledge that cigarettes serve as a significant source of heavy metals, particularly Cd, thereby rendering smokers more vulnerable to the combined consequences. Additionally, the lack of methodological consensus among the studies is a significant limitation since it reduces the comparability and robustness of the study outcomes.
Technological advances and industrial growth expose humans to environmental agents that can affect health. Among these agents, heavy metals are poorly understood in terms of their mechanism of action and reference values. Exposure to these metals leads to bioaccumulation in tissues and can trigger an inflammatory response. Although a causal relationship between periodontitis and heavy metals has not been established, studies have shown an association between elevated concentration levels and clinical effects on periodontal tissues. This association suggests a possible environmental modifying factor in the etiopathogenesis of periodontitis. Therefore, further research can contribute to developing preventive measures and strategies designed to minimize damage to health and environmental exposure.
Conclusion
In summary, the present findings highlight the association between Cd and Pb human contamination and periodontitis. Nonetheless, the cross-sectional analysis of metal toxicity does not permit to conclusively establish a causal relationship. Additionally, interactions with other exposure factors may influence the inflammatory response across different levels of periodontitis. This fact underscores the need for further prospective or interventional human studies in order to establish a definitive causal link and to define reference values for heavy metals. Moreover, research exploring the mechanisms of action of heavy metals, whether alone or in combination, is essential.
Data Availability
No datasets were generated or analysed during the current study.
References
Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Tonetti MS (2018) A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Clin Periondol 89(S1):S1–S8. https://doi.org/10.1002/JPER.18-0157
Kassebaum NJ, Barnabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 93(11):1045–1053. https://doi.org/10.1177/0022034514552491
Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J (2017) Impact of the global burden of periodontal diseases on health, nutrition, and well-being of mankind: a call for global action. J Clin Periodontol 44(5):456–462. https://doi.org/10.1111/jcpe.12732
Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal Disease. Lancet 366(9499):1809–1820. https://doi.org/10.1016/s0140-6736(05)67728-8
Silva MR, Matono D, Bosco AM, Baptistiolli L, Torrecilha RBP, Ciarlini PC (2018) Estresse oxidativo em cães com doença periodontal: comparação dos biomarcadores plasmáticos e salivares. Arq Bras Med Vet Zootec 70(05):1369–1377. https://doi.org/10.1590/1678-4162-9246
Sosa V, Aicardo A, Valez V (2022) Estrés oxidativo en saliva generado por el humo de tabaco: impacto en la periodontitis y perspectivas hacia el uso de farmacología redox. Odontoestomatología 24(39):e307. https://doi.org/10.22592/ode2022n39e307
Obiri S, Yeboah PO, Osae S, Adu-Kumi S, Cobbina SJ, Armah FA, Ason B, Antwi E, Quansah R (2016) Human Health Risk Assessment of Artisanal Miners Exposed to Toxic Chemicals in Water and Sediments in the Prestea Huni Valley District of Ghana. Int J Environ Res Public Health 13(1):139. https://doi.org/10.3390/ijerph13010139
Alfadaly RA, Elsayed A, Hassan RYA, Noureldeen A, Darwish H, Gebreil AS (2021) Microbial sensing and removal of heavy metals: bioelectrochemical detection and removal of chromium(VI) and cadmium(II). Molecules 26(9):2549. https://doi.org/10.3390/molecules26092549
Purewal R, Christley R, Kordas K, Joinson C, Meints K, Gee N, Westgarth C (2017) Companion Animals and Child/Adolescent development: a systematic review of the evidence. Int J Environ Res Public Health 14(3):234. https://doi.org/10.3390/ijerph14030234
Brown CJ, Chenery SRN, Smith B, Mason C, Tomkins A, Roberts GJ, Ssrunjogi L, Tiberindwa JV (2004) Environmental influences on the trace element content of teeth—implications for disease and nutritional status. Arch Oral Biol 49(9):705–717. https://doi.org/10.1016/j.archoralbio.2004.04.008
Mitchell E, Frisbie S, Sarkar B (2011) Exposure to multiple metals from groundwater—a global crisis: Geology, climate change, health effects, testing, and mitigation. Metallomics 3(9):874. https://doi.org/10.1039/c1mt00052g
Zhushan F, Shuhua X (2019) The effects of Heavy metals on Human metabolism. Toxicol Mech Methods. https://doi.org/10.1080/15376516.2019.1701594
Bahorun T, Soobrattee MA, Luximon-Ramma V, Aruoma OI (2006) Free radicals and antioxidants in cardiovascular health and disease. Internet J Med Update 1(2):25–41
Lee J-C, Son Y-O, Pratheeshkumar P, Shi X (2012) Oxidative stress and metal carcinogenesis. Free Radic Biol Med 53(4):742–757. https://doi.org/10.1016/j.freeradbiomed.2012.06.002
Arora M, Weuve J, Schwartz J, Wright RO (2009) Association of environmental cadmium exposure with periodontal disease in U.S. adults. Environ Health Perspect 117(5):739–744. https://doi.org/10.1289/ehp.0800312
Huang H, Yao J, Yang N, Yang L, Tao L, Yu J, Gao Y, Liu Z (2022) Association between levels of blood trace minerals and periodontitis among United States adults. Front Nutr 9:999836. https://doi.org/10.3389/fnut.2022.999836
Han DH, Lee HJ, Lim S (2013) Smoking induced heavy metals and periodontitis: findings from the Korea National Health and Nutrition examination surveys 2008–2010. J Clin Periodontol 40(9):850–858. https://doi.org/10.1111/jcpe.12133
Won YS, Kim JH, Kim YS, Bae KH (2013) Association of internal exposure of cadmium and lead with periodontal disease: a study of the Fourth Korean National Health and Nutrition Examination Survey. J Clin Periodontol 40(2):118–124. https://doi.org/10.1111/jcpe.12033
Shiue I (2015) Urinary heavy metals, phthalates, phenols, thiocyanate, parabens, pesticides, polyaromatic hydrocarbons but not arsenic or polyfluorinated compounds are associated with adult oral health: USA NHANES, 2011–2012. Environ Sci Pollut Res Int 22(20):15636–15645. https://doi.org/10.1007/s11356-015-4749-3
Yaprak E, Yolcubal I, Sinanoğlu A, Doğrul-Demiray A, Guzeldemir-Akcakanat E, Marakoğlu I (2017) High levels of heavy metal accumulation in dental calculus of smokers: a pilot inductively coupled plasma mass spectrometry study. J Periodontal Res 52(1):83–88. https://doi.org/10.1111/jre.12371
Browar AW, Koufos EB, Wei Y, Leavitt LL, Prozialeck WC, Edwards JR (2018) Cadmium exposure disrupts periodontal bone in experimental animals: implications for periodontal disease in humans. Toxics 6(2):32. https://doi.org/10.3390/toxics6020032
Li ZH, Li J, Mao YC, Zhao JW, Hu HY, Zhang S, Liu ZY, Liu XJ, Huang K, Hu CY, Zhang XJ (2023) Association of urinary heavy metal combined exposure with periodontitis among US adults from NHANES 2011–2014. Environ Sci Pollut Res Int 30(49):107887–107898. https://doi.org/10.1007/s11356-023-29888-6
Rebelo FM, Caldas ED (2016) Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants. Environ Res 151:671–688. https://doi.org/10.1016/j.envres.2016.08.027
Duan W, Xu C, Liu Q, Xu J, Weng Z, Zhang X, Basnet TB, Dahal M, Gu A (2020) Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: a population-based cohort study. Environ Pollut 263(Pt A):114630. https://doi.org/10.1016/j.envpol.2020.114630
Angerer J, Ewers U, Wilhelm M (2007) Human biomonitoring: state of the art. Int J Hyg Environ Health 210(3–4):201–228. https://doi.org/10.1016/j.ijheh.2007.01.024
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmcol 238(3):201–208. https://doi.org/10.1016/j.taap.2009.04.020
Acknowledgements
The authors are supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico under Grants number 404088/2023-6, 403344/2022-0 and 306780/2022-40; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; and Fundação de Amparo à Pesquisa do Estado da Bahia.
Author information
Authors and Affiliations
Contributions
TC, CNC, PRC: conceptualization, methodology
MJFC, JNS and DAR: conceptualization, writing, resources
PRC: Funding acquisition, Project Administration, Resources, Supervision, Writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coelho, T., Cardoso, C.N., Carvalho, M.J.F. et al. The Impact of Heavy Metal Contamination in Humans and Periodontitis: A Scoping Review. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04357-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04357-4