Abstract
Leafy plants are commonly consumed as vegetables in India due to their high nutrient and vitamin content. This study, conducted in Ambagarh Chowki (India), investigated the accumulation potential of 52 elements (including Al, As, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, Ho, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, P, Pb, Pr, Rb, Sb, Sc, Se, Sm, Sn, Sr, Tb, Te, Th, Ti, Tl, Tm, U, V, W, Y, Yb, and Zn) in seven leafy vegetable species, namely Amaranthus tricolor L., Corchorus olitorius L., Cordia myxa L., Hibiscus sabdariffa L., Ipomoea batatas (L.) Lam., Moringa oleifera Lam., and Spinacia oleracea L. Technique: Inductively coupled plasma mass spectrometry (ICP-MS) was employed for analysis. The maximum concentrations of elements such as Al, Ba, Be, Bi, Cd, Co, Cr, Fe, Ga, Ge, Li, Mn, Ni, Pb, Sb, Th, Tl, U, V, W, and REEs were observed in S. oleracea leaves, indicating their highest accumulation potential. In contrast, the maximum concentrations of As were found in H. sabdariffa leaves; Ca and Si in M. oleifera leaves; Mg, Sr, and Mo in A. tricolor leaves; and P, K, Cu, and Zn in C. myxa leaves, respectively. Twenty-one elements (Cr, Cd, Pb, Ni, Co, V, Cu, Zn, Fe, Mn, Th, Sb, Ba, Be, Li, Sr, Tl, U, Se, Sn, and REEs) exceeded permissible limits set by the WHO. The elevated hazard index values indicated significant non-carcinogenic effects. The sources of these elements could be attributed to a combination of geological factors and agricultural practices. This study highlights the need for further investigation into the potential health implications of consuming these vegetables in the aforementioned region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pedologically, arsenic and other heavy metals in the soil of Ambagarh Chowki demonstrate an affinity for ferruginous material derived from granite [1,2,3]. Hence, analytical studies of fifty-two trace elements (i.e., Al, As, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, Ho, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, P, Pb, Pr, Rb, Sb, Sc, Se, Sm, Sn, Sr, Tb, Te, Th, Ti, Tl, Tm, U, V, W, Y, Yb, and Zn) in the field soils of this region was chosen.
These elements are classified into three categories: beneficial (P, Na, K, Mg, Ca, Co, Fe, Se, Mo, Mn, Zn and REEs), non-beneficial (Al, Ba, Ga, Ge, Nb, Sn, Sc,Te, Ti, Sc, Rb and Tb), and harmful elements (As, Be, Bi, Li, V, Cr, Ni, Cu, Pb, Sb, Tl, Th and U) [4]. Trace elements are highly accumulated with leafy vegetables from the contaminated soils [5].
Green leafy vegetables from diverse families like Amaranthaceae and Brassicaceae are widely consumed globally. These plants possess significant nutritional value due to their abundance of minerals, antioxidants, and vitamins. Research by Morris et al. [6] emphasizes their contribution to improved cognitive function and reduced risk of chronic diseases. Additionally, they are a rich source of essential minerals like phosphorus, potassium, calcium and iron, offering substantial health benefits [7, 8].
However, a growing concern exists regarding the potential accumulation of heavy metals, toxic elements, and rare earth elements within these vegetables, potentially negating their health advantages [9,10,11,12,13,14,15,16,17,18]. Studies suggest a higher tendency for leafy vegetables to accumulate heavy metals compared to other types of vegetables [19, 20]. The potential health risks associated with these pollutants have been explored in various studies [21, 22].
Previous research in Ambagarh Chowki district (Chhattisgarh, India) indicates elevated levels of elements like arsenic and lead in the soil due to natural and anthropogenic factors [1,2,3]. Consequently, investigating the elemental composition of vegetables grown in this region is crucial to understanding potential health impacts and guiding agricultural and environmental regulations.
This study analyzed the multi-element composition of seven commonly consumed leafy vegetables from the Ambagarh Chouki district: Amaranthus tricolor L. (edible amaranth), Corchorus olitorius L. (jute mallow), Cordia myxa L. (Assyrian plum), Hibiscus sabdariffa L. (roselle), Ipomoea batatas (L.) Lam. (sweet potato), Moringa oleifera Lam. (moringa), and Spinacia oleracea L. (spinach).
Among these vegetables, amaranth, a short-lived perennial wild plant, offers a rich source of fiber, minerals, and protein, and possesses anti-inflammatory properties [23]. Jute mallow is recognized for its analgesic, anti-inflammatory, antioxidant, and potential anti-cancer properties [24]. Leaves of the Assyrian plum are traditionally used for treating various ailments [25]. Roselle is rich in beneficial compounds such as polyphenols, anthocyanins, polysaccharides, and organic acids, with diverse therapeutic applications [26]. Sweet potato is considered a functional food with anti-inflammatory and immune-boosting properties [27]. Moringa is valued for its immune-supporting properties due to its richness in essential minerals and vitamins [28]. Finally, spinach is renowned for its exceptional vitamin and nutrient content [29].
The investigation aimed to elucidate the origin and transfer mechanisms of elements (i.e., Al, As, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, Ho, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, P, Pb, Pr, Rb, Sb, Sc, Se, Sm, Sn, Sr, Tb, Te, Th, Ti, Tl, Tm, U, V, W, Y, Yb, and Zn)| within the aforementioned vegetables, their accumulation patterns, potential toxicity, and their overall implications for human health.
Materials and Methods
Study Area and Sample Collection
Study Area
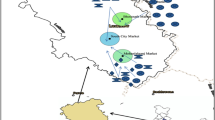
The study was conducted in Ambagarh Chouki, Chhattisgarh, India (20.78209°N, 80.74117°E) (Fig. 1). This region experiences a temperate climate with hot summers, reaching temperatures up to 47 °C in May. Annual rainfall exceeding 100 cm occurs during the summer months (May to September). As reported by Acharyya et al. [30], the soil is rich in minerals like biotite, chlorite, illite, kaolinite, and goethite clay. Previous research indicates high levels of arsenic and other element contamination in both the soil and plants [1, 2].
Soil Sampling
Seven composite soil samples (S1–S7) were collected from the topmost layer (0–10 cm) of the fields where the vegetables were grown. After collection, the soil was air-dried for a week, crushed into a fine powder, and sieved to particles less than 0.1 mm. The pulverized samples were then oven-dried at 50 °C overnight and stored at − 4 °C for further analysis.
Plant Sampling
Seven leaf samples in triplicate were obtained from the seven locations (Fig. 1). They were carefully washed twice with deionized water to remove surface contaminants. The prepared leaf samples were subjected to the same storage and preservation procedures as the soil samples to maintain their integrity for subsequent analysis.
Sample Preparation
Microwave-assisted digestion (Multiwave 3000, Anton Paar, Austria) was employed to analyze the total element content of both plant and soil samples. Plant sub-samples (approximately 0.07 g) underwent a single-step digestion using a mixture of HNO3 (6 mL; 65%, supra pur, Fluka, Steinheim, Switzerland) and HF (0.1 mL; 48%, pro analysi, Kemika, Zagreb, Croatia).
Soil sub-samples (approximately 0.05 g) were subjected to a two-step digestion process. The first step involved utilizing a mixture of HNO3 (4 mL, 65%, pro analysi, Kemika, Zagreb, Croatia), HCl (1 mL; 37%, pro analysi, Fluka, Steinheim, Switzerland), and HF (1 mL, 48%; pro analysi, Kemika, Zagreb, Croatia). Subsequently, 6 mL of H3BO3 (40 g L⁻1, pro analysi, Fluka, Steinheim, Switzerland) were added.
Following digestion, the preparation for analysis differed slightly for the two sample types. In the case of soil digests, a ten-fold dilution with 2% (v/v) HNO3 was performed, followed by the introduction of an internal standard (In, 1 μg L⁻1). For plant digests, samples were solely acidified with 2% (v/v) HNO3 without further dilution. The internal standard (In, 1 μg L⁻1) was also added.
Multielement Analysis
An inductively coupled plasma triple quadrupole mass spectrometer (ICP-MS/MS, 8900. Agilent, USA) was used for quantification of 52 elements (Al, As, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, Ho, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, P, Pb, Pr, Rb, Sb, Sc, Se, Sm, Sn, Sr, Tb, Te, Th, Ti, Tl, Tm, U, V, W, Y, Yb, and Zn) within soil, leaf, and standard samples.
Mass calibration was performed using a multi-element solution from Merck KGaA (Germany), containing B, Ba, Co, Fe, Ga, In, K, Li, Lu, Rh, Sc, Tl, U, and Y. Calibration curves were generated through external standardization with a series prepared from standard solutions, including a blank sample.
For trace elements, quantification employed diluted multi-element reference solutions (100 ± 0.2 mg L⁻1, Analytika, Prague, Czech Republic) containing aluminum Al, As, Ba, Be, Bi, Cd, Co, Cr, Cs, Cu, Fe, Li, Mn, Mo, Ni, Pb, Sr, Ti, Tl, V, and Zn. These solutions were further supplemented by single-element standards of Ga, Ge, Nb, Rb, Sb, Sn, Te, Th, U, and W (1.000 ± 0.002 g L⁻1; Analytika). Concerning major elements, a standard solution (1.000 ± 0.002 g L⁻1, Analytika) facilitated the measurement of Ca, K, Mg, and Na. Phosphorus determination utilized individual reference solutions (1.000 ± 0.002 g L⁻1, Analytika). In relation to rare earth elements, a dedicated multi-element standard (Analytika) containing Ce, La, Nd, and Pm (100 ± 0.2 mg L⁻1) and Dy, Er, Eu, Gd, Ho, Lu, Sc, Sm, Tb, Tm, Y, Yb (20 ± 0.4 mg L⁻1) was employed.
To ensure the accuracy and reliability of the data, blank samples were analyzed alongside the actual samples to identify any potential contamination introduced during sample preparation or analysis. Certified reference materials (CRMs) were also incorporated into the analysis. These CRMs, specifically NCS DC 77302 (soil) and NCS ZC73018 (citrus leaves) obtained from the China National Analysis Center for Iron and Steel (Beijing, China), are certified for most of the elements being measured. The analysis results demonstrated good agreement between the measured concentrations in both the samples and CRMs, which fell within the acceptable range of analytical uncertainties, typically around 10%.
Enrichment and Health Assessment
This study employed various enrichment and health assessment indices to evaluate potential environmental and human health risks associated with element accumulation in the studied vegetables [21, 31,32,33].
Contamination factor (CF): the ratio between the element concentration in the sample (Cn) and the corresponding background concentration (Bn).
Enrichment factor (EF): compares the enrichment of an element in the sample relative to a reference element, considering both sample and background concentrations.
Transfer factor (TF): represents the ability of a plant to uptake an element from the soil, calculated as the ratio between the element concentration (Cn) in the plant and the soil.
Estimated daily intake (EDI, mg kg−1 day−1): estimates the average daily intake of an element by an individual through vegetable consumption. Factors considered include exposure frequency (Ef, 365 days/year), duration (Ed, 77 and 81 years for males and females, respectively), vegetable consumption rate (Fir, 240 g/(person day)), metal concentration (Cm, expressed in mg kg−1), and body weight (BW, 70 and 60 kg for males and females, respectively). Cf is the concentration conversion factor for fresh vegetable weight to dry weight (0.085) [34] and AT the average time (365 days year−1 × Ed). Average vegetable consumption rate and body weight values were adopted as per established guidelines [35].
Target hazard quotient (THQ): assesses the potential non-carcinogenic health risks associated with element exposure. It is calculated by dividing the estimated daily intake (EDI) by the reference oral dosage (RfD, mg day−1 kg−1) for a specific element.
Hazard index (HI): represents the cumulative effect of exposure to multiple elements by summing the individual hazard quotients (THQs) for each element of concern.
Carcinogenic risk (CR): Estimates the potential lifetime cancer risk associated with exposure to carcinogenic elements through vegetable consumption. It is calculated by multiplying the estimated daily intake (EDI) by the cancer slope factor (CSF) for the element.
Reference dosage (RfD = 0.007, 0.003, 0.02, 0.04, 0.0003, 0.001, and 0.0035 mg kg−1 day−1 for V, Cr, Ni, Cu, As, Cd, and Pb, respectively) and cancer slope factor (CSF = Cr, Ni, As, Pb, and Cd were 0.5, 1.7, 1.5, 0.38, and 0.01 mg/kg/day) values were obtained from the literature [36, 37].
Statistical Analysis
A three-factor analysis (FA) was conducted to discern the sources of measured trace metals in the collected leafy vegetable samples using IBM SPSS 20.0 USA [38]. The FA was applied to the leafy vegetable dataset comprising 21 cases by 32 metals, employing Varimax rotation with Kaiser normalization.
Results and Discussion
Distribution of Elements in Surface Soil
The soil of Ambagarh Chowki is alluvium rich with minerals [30], and details of the concentrations of the 52 analyzed elements within the soil samples are shown in Table S1. The soil exhibited characteristics of alkalinity and sodicity, reflected by a sodium adsorption ratio (SAR) of 7.0 ± 2.2. All samples displayed a magnesium hazard, ranging from 46.8% to 70.3%, with an average value of 65.0 ± 7.7%.
Total element concentrations varied significantly, spanning a range of 113 to 150 g kg⁻1. Iron had the highest average concentration (48.4 g kg⁻1), followed by a sequential decrease in abundance for Al, K, Ca, Ti, Mg, Na, Mn, Ba, and P. The remaining elements had progressively lower concentrations, with Te having the lowest (0.05 mg kg⁻1).
Several factors likely contribute to the observed variations in element concentrations and ratios, including complexation reactions (provided that interactions between elements can influence their mobility and availability in the soil), relative solubilities (given that elements like Na and Mg have differing solubilities, affecting their behavior in the soil matrix), and volatility (e.g., P exhibits volatile properties, potentially impacting its concentration in the soil).
The concentration of Rare Earth Elements (REEs) displayed considerable variation within the samples. The total REE content ranged from 210 to 311 mg kg⁻1, with an average of 251 mg kg⁻1. La and Ce were the most abundant REEs, while Tm and Lu were the least abundant. Light REEs (LREEs) constituted a larger proportion (42–53%) of the total REE content compared to Heavy REEs (HREEs). This resulted in LREE/HREE ratios ranging from 12.3 to 17.3. The average LREE to HREE ratio (ΣLREE/ΣHREE) was 14.5 ± 1.8 (Table S1). The leaching behavior of REEs varies, with LREEs and HREEs displaying distinct leaching behaviors, as reported in previous research [39].
Contamination and Enrichment Factors
The analysis of contamination factor (CF) values revealed varying degrees of enrichment for elements in the soil compared to their background concentrations. Sodium exhibited very low contamination (CF = 0.12), while elements like Sc, Ge, Co, Cd, Mn, Se, and Bi displayed moderate contamination (CF ranging from 2.0 to 3.3). Notably, Sb, Te, and As demonstrated extremely high contamination (CF exceeding 10). Figure 2 provides a visual representation of the EF values for further clarity.
Enrichment factor (EF) values were higher than the corresponding CF values. This difference can be attributed to the lower aluminum content in the soil samples compared to the reference values used for calculating EF.
Concerning the assessment of contamination, the combined evaluation of contamination and enrichment factors indicates moderate to significant contamination within the soil. Arsenic, Sb, and Te were identified as the primary contributors to this contamination, originating from both natural processes and human influences such as industrial and agricultural activities.
A detailed discussion of the geochemical characteristics and element origins within the Ambagarh Chouki district's soil can be found in Verma et al. [3]. Their study suggests that the soil in this region exhibits a diverse geochemical composition shaped by natural processes (weathering, soil formation) and anthropogenic activities. This is reflected in the elevated levels observed for CF and EF, particularly evident for Sb, As, and Te.
Distribution of Elements in Leaves
Leafy vegetables are an important part of the diet as they are rich in minerals, fibers, phytochemicals, and other nutrients, which help reduce the risk of many diseases [40]. However, they are seriously contaminated with toxic elements [41]. Hence, Table S2 provides a detailed analysis of the element distribution within leaves collected from the seven leafy vegetable species. Table 1 shows the range and mean values of concentration and transfer factor of elements in the vegetables.
The total element concentrations exhibited a wide range, varying from 110 to 147 g kg⁻1 with an average value of 126 ± 13 g kg⁻1. In terms of elemental abundance, C. olitorius leaves displayed the highest overall element concentration. Key elements and their corresponding concentrations include K (37,679 mg kg⁻1), Ca (20,329 mg kg⁻1), Mg (8,181 mg kg⁻1), P (4,829 mg kg⁻1), and Na (4,423 mg kg⁻1). The remaining elements had progressively lower concentrations, with Te being the least abundant (0.01 mg kg⁻1).
Regarding species-specific accumulation, S. oleracea leaves exhibited the highest concentrations for over 73% of the analyzed elements (viz. Al, Ba, Be, Bi, Cd, Cr, Fe, Ga, Ge, Li, Mn, Na, Nb, Ni, Pb, Sb, Sc, Th, Ti, Tl, U, V, W, and REEs), Fig. 3. In contrast, leaves from M. oleifera, A. tricolor, H. sabdariffa, and C. myxa displayed peak concentrations of elements like Ca, Se, and Te; Mg, Sr, and Mo; As, Co, and Sn; and P, Cu, K, Rb, and Zn, respectively.
Concentration variation of metals in the plant leaves of Amaranthus tricolor (AT), Cordia myxa (CM), Corchorus olitorius (CO), Hibiscus sabdariffa (HS), Ipomoea batatas (IB), Moringa oleifera (MO), and Spinacia oleracea (SO). Subfigure (A) shows the nutrient concentration (NC), while subfigures (B) and (C) display the heavy metal concentration (HMC)
As for the element ratios, the mass ratios of specific elements (Ca/Mg = 3.1 ± 1.1, K/Na = 63.6 ± 44.8, K/P = 7.8 ± 2.6, Th/U = 3.8 ± 0.7) in the leaf samples deviated from those observed in the soil (1.7 ± 0.4, 4.1 ± 0.6, 207 ± 317, 5.3 ± 0.6). This variation primarily stems from the significant accumulation of K and P within the leaves. Additionally, the average LREEs to HREEs ratio (ΣLREE/ΣHREE) was 10.4 ± 0.6 (Table S2).
As noted above, leafy vegetables may pose a higher risk for human consumption due to their greater capacity to accumulate potentially harmful substances compared to root and fruiting vegetables [42, 43]. Leafy vegetables from the Ambagarh Chouki district exhibited higher element contents compared to those reported in studies from other regions [18, 42, 44,45,46].
For instance, concerning the heavy metal content, Sobukola et al. [46], analyzed the composition of various leafy vegetables in Nigerian markets. They found that the Cd level ranged from 0.03 to 0.09 mg kg⁻1, Co from 0.02 to 0.36 mg kg⁻1, Cu from 0.02 to 0.07 mg kg⁻1, Ni from 0.05 to 0.24 mg kg⁻1, Pb from 0.09 to 0.21 mg kg⁻1, and Zn up to 0.01 mg kg⁻1 across these vegetables. Similarly, Ali and Qahtani [43] investigated vegetables grown in industrial and urban areas. They identified the presence of various heavy metals like Cd, Cu, Fe, Mn, Pb, and Zn, with the highest concentrations observed in leafy vegetables. Interestingly, C. olitorius displayed significantly higher levels of Mn (94.1 mg kg⁻1) and Zn (33.2 mg kg⁻1) compared to this study (188 mg kg⁻1 and 54.3 mg kg⁻1, respectively). However, the opposite trend was observed for Cd in S. oleracea: while Ali and Qahtani [43] reported a concentration of 4.13 mg kg⁻1, the current study revealed a significantly lower value of 0.73 mg kg⁻1.
As for REEs, Zhuang et al. [18] reported the highest REE concentrations in leafy vegetables from mining areas in China (0.98 mg kg⁻1) compared to control areas (0.08 mg kg⁻1). Fiket and Medunić [45] documented slightly higher REE levels in vegetables from Croatia (from a naturally REE-enriched soil). The reported REE concentrations ranged from 0.003 mg kg⁻1 (Tm) to 0.999 mg kg⁻1 (Ce), with an average total REE (ΣREE) content of 2.42 mg kg⁻1. These values remain considerably lower than those observed in the current study.
Transfer Factor
To explore the uptake and accumulation of various elements by the studied leafy vegetable species, transfer factors —which represent the ratio of element concentration in plants compared to the surrounding soil— were calculated (Table 1 and Table S3).
TF values varied significantly across the studied leafy vegetables. Essential nutrients generally exhibited the highest TFs, indicating their efficient absorption by these plants. Phosphorus displayed significant hyperaccumulation in all studied leaves, with values ranging from 24.0 to 133 and the highest concentration in A. tricolor leaves. Similarly, hyperaccumulation of both K and Ca was observed in all leaves, with TFs ranging from 1.65 to 6.32 for K and 1.42 to 3.72 for Ca. Cordia myxa leaves showed the highest K accumulation, while A. tricolor leaves had the highest Ca concentration.
Other elements such as Na, Mg, Mo, and Sr exhibited varying degrees of hyperaccumulation in some vegetables, with values ranging from 0.11 to 9.34. Spinacea oleracea and A. tricolor leaves displayed the highest accumulation of these elements.
These findings align with previous research on heavy metal uptake in leafy vegetables [41, 47]. Additionally, the observed TF values are comparable to or exceed those reported by Petrović et al. [48] for vegetables grown in areas impacted by anthropogenic activities like mining and coal combustion.
Toxicity
The analysis of leafy green vegetables revealed that concentrations of most detected elements surpassed established safety thresholds. Spinacea oleracea leaves demonstrated the highest propensity for element accumulation. Twenty-one elements, including Cr, Cd, Pb, Ni, Co, V, Cu, Zn, Fe, Mn, Th, Sb, Ba, Be, Li, Sr, Tl, U, Se, Sn, and REEs, were found in concentrations exceeding established safety limits (2.30, 0.20, 0.30, 0.1, 0.01, 0.03, 10, 50, 450, 500, 0.27, 0.02, 0.002, 0.002, 0.004, 0.01, 0.001, 0.0004, 0.01, and 0.01 mg kg−1, respectively) [49,50,51,52,53]. Seventeen elements, including Be, Ba, Cd, Co, Cu, Fe, Li, Ni, Pb, Sb, Se, Sn, Sr, Tl, U, V, and REEs, consistently surpassed these permissible limits across all seven types of leafy vegetables analyzed (refer to Table 1 and Table S2 for details). Two additional elements, Cr and Th, exceeded their limits in all except the M. oleifera leaves. Zinc exceeded the allowable limit only in three types of leaves (S. oleracea, A. tricolor, and C. myxa), while Mn surpassed its limit solely in S. oleracea leaves.
The calculated EDI values for seven elements (V, Cr, Ni, Cu, As, Pb, and Cd) in both men and women ranged from 0.223 to 0.578 mg kg−1 and 0.262 to 0.675 mg kg−1, respectively (refer to Fig. 4 and Table S4 for details). Notably, three elements (Cr, Cu, and V) contributed most significantly to the total intake. The presence of Cr(VI) in edible vegetables poses a health risk to living organisms. A method could be developed for the detection of Cr(VI) in aqueous extracts using hydroxyamidines as a reagent [54].
Figure 5 and Table S5 present the THQ values for the mentioned elements. Chromium exhibited the most concerning THQ, ranging from 18 to 103 for men and 21 to 120 for women, highlighting the severity of potential health risks associated with this element. Arsenic and Pb also displayed high THQs, indicating serious risks for both sexes. Vanadium and Cd posed significant health risks as well, with THQ values ranging from 4.4 to 32.7 and 0.7 to 16.4 for men, respectively. Copper, while essential, showed THQ values suggesting potential toxicity at high concentrations. Nickel had comparatively lower THQ values but still raised concerns. It is crucial to note that THQ values are generally higher in women due to their lower average body weight compared to men. This weight difference translates to a higher relative dose of pollutants absorbed, consequently elevating their potential health risk. This gender-specific vulnerability necessitates careful consideration in risk assessments and the development of protective measures.
Similar work on Cr-contaminated soil reported that leafy vegetables had higher Cr (125.20–168.99 mg kg−1) and Cd (0.19–0.83 mg kg−1) values than those observed in this study, but had lower contents of other metals such as Ni, Cu, and Zn [41].
The HI for the analyzed elements varied between 98 and 274 for men and 192 and 645 for women across the seven types of leaves. The highest HI values were observed in S. oleracea leaves, followed by other vegetables (H. sabdariffa > A. tricolor > C. olitorius > C. myxa > I. batatas > M. oleifera).
Regarding carcinogenic risk, Fig. 6 and Table S6 present the CR associated with exposure to five elements (As, Cd, Cr, Ni, and Pb) across the leafy vegetables. CR values ranged from 0.223 to 0.675 for men and 0.262 to 0.578 for women. Spinach leaves again exhibited the highest CR values. Among these elements, Ni, Cr, and As were the primary contributors to the total CR, exceeding the acceptable limit of 1 × 10−4 [55]. Notably, the Hazard Quotient (HQ) for all vegetables surpassed 1, indicating a potential non-carcinogenic health risk.
Correlation and Sources
Thirty-four elements, namely Al, Ba, Be, Bi, Co, Fe, Ga, Ge, Li, Nb, Ni, Pb, Sc, Th, Ti, Tl, U, V, W, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu, exhibited strong correlations (ranging from 0.70 to 1.00) within the studied leafy vegetables. This suggests similar uptake patterns and potential links to the elemental composition of the soil. Additionally, noteworthy correlations (ranging from 0.70 to 0.92) were observed between specific elements: As, Co, and Sb; Ca and Sr; Cd, Cr, Mn, Na, and Ni; Ca and Sr; Cr and Ni; Mg and Mo; Na, Ti, and Eu; and Sb and Ce.
Factor analysis (FA) was applied to the leafy vegetable composition data, revealing three key factors contributing to the overall elemental profile (refer to Table S7 for details).
Factor 1, explaining 52.96% of the total variance, is primarily characterized by REEs alongside Al, Be, Bi, Fe, Li, Ni, and Pb. These elements likely serve as indicators of the soil composition [56, 57]. Notably, Pb and Ni are additionally associated with fossil fuel combustion and vehicle emissions, accumulating on leaves through atmospheric deposition [58]. Therefore, this factor represents a combination of influences from the soil's origin and vehicular pollution.
Factor 2, accounting for 14.88% of the variance, exhibits high loadings for Mg, Cd, Ca, Ba, and Mn. Calcium and Mg are classified as lithogenic elements [58]. Barium and Mn primarily stem from anthropogenic sources such as mining, refining, and fossil fuel burning [59]. Consequently, this factor also signifies the combined contributions of human activities and natural geological sources.
Factor 3 is defined by Cu, K, Rb, Zn, and P. The primary sources of Cu and Zn in agricultural settings are fertilizers and sewage irrigation [60,61,62]. Therefore, this factor is tentatively attributed to the influence of agricultural practices.
Conclusions
An extensive analysis of nutrients, trace, toxic and rare earth elements in seven leafy vegetables grown in the Ambagarh Chouki district revealed concerning accumulation patterns. While most elements surpassed established safety limits, S. oleracea displayed the highest overall accumulation. Notably, M. oleifera exhibited elevated levels of Ca, Se, and Te, while A. tricolor showed enrichment in Mg, Sr, and Mo. Hibiscus sabdariffa had heightened concentrations of As, Co, and Sn, and C. myxa had higher levels of P, Cu, K, Rb, and Zn. This diversity highlights the varying degrees of element uptake among these vegetables.
The estimated daily intake of elements like As, Cd, Cu, Cr, Ni, Pb, and V differed significantly across the vegetables. This variation resulted in particularly high target hazard quotient values for Cr, Cu, and V, indicating severe potential health risks. These risks were especially concerning for women due to their lower body weight, leading to a higher relative element intake. The hazard index further emphasizes significant health risks associated with consuming these vegetables, particularly S. oleracea. Additionally, the calculated carcinogenic risk underscores the need for vigilant monitoring due to the presence of elements potentially linked to cancer.
These findings highlight the critical importance of regularly monitoring the elemental content of leafy vegetables, especially in areas prone to contamination. Further research and regulatory actions are essential to ensure the safety and nutritional quality of this widely consumed food source.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Patel KS, Pandey PK, Martín-Ramos P, Corns WT, Varol S, Bhattacharya P, Zhu Y (2023) A review on arsenic in the environment: contamination, mobility, sources, and exposure. RSC Adv 13:8803–8821. https://doi.org/10.1039/D3RA00789H
Patel KS, Pandey PK, Martín-Ramos P, Corns WT, Varol S, Bhattacharya P, Zhu Y (2023) A review on arsenic in the environment: bioaccumulation, remediation, and disposal. RSC Adv 13:14914–14929. https://doi.org/10.1039/d3ra02018e
Verma D, Dewangan A, Patel KS, Sharma PK, Pandey PK, Wakhle B, Sharma S, Petrović M, Fiket Z, Yurdakul S, Varol S, Martín-Ramos P (2024) Environmental and health implications of land pollution in Ambagarh Chowki, Chhattisgarh. Environ Monit Assess Submitted, India
Hossain M, Karmakar D, Begum SN, Ali SY, Patra PK (2021) Recent trends in the analysis of trace elements in the field of environmental research: A review. Microchem J 165:106086. https://doi.org/10.1016/j.microc.2021.106086
Kerketta A, Kumar H, Powell MA, Sahoo PK, Kapoor HS, Mittal S (2023) Trace Element Occurrence in Vegetable and Cereal Crops from Parts of Asia: A Meta-data Analysis of Crop-Wise Differences. Curr Pollution Rep 9:1–21. https://doi.org/10.1007/s40726-023-00248-9
Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL (2018) Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 90(3):e214–e222. https://doi.org/10.1212/WNL.0000000000004815
Baj J, Flieger W, Teresiński G, Buszewicz G, Sitarz R, Forma A, Karakuła K, Maciejewski R (2020) Magnesium, calcium, potassium, sodium, phosphorus, selenium, zinc, and chromium levels in alcohol use disorder: A review. J Clin Med 9(6):1901. https://doi.org/10.3390/jcm9061901
Heaney RP (2015) Sodium, potassium, phosphorus, and magnesium. In: Holick, M., Nieves, J. (eds) Nutrition and bone health. nutrition and health. Humana Press, New York, NY, 2015. https://doi.org/10.1007/978-1-4939-2001-3_24
Bai J, Xu X, Duan Y, Zhang G, Wang Z, Wang L, Zheng C (2022) Evaluation of resource and environmental carrying capacity in rare earth mining areas in China. Sci Rep 12:6105. https://doi.org/10.1038/s41598-022-10105-2
Clair-Caliot G, Marks SJ, Hug SJ, Bretzler A, N’guessan ND, Tihe SFK, Lalanne F (2021) Lalanne, Uptake of arsenic by irrigated vegetables and cooked food products in Burkina Faso. Front Water 3:667308. https://doi.org/10.3389/frwa.2021.667308
Gao J, Zhang D, Proshad R, Uwiringiyimana E, Wang Z (2021) Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci Rep 11:22824. https://doi.org/10.1038/s41598-021-02069-6
Kumar S, Prasad S, Yadav KK, Shrivastava M, Gupta N, Nagar S, Bach Q, Kamyab H, Khan SA, Yadav S, Malav LC (2019) Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches - A review. Environ Res Part A 179:108792. https://doi.org/10.1016/j.envres.2019.108792
Manzoor J, Sharma M, Khursheed W (2018) Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J Plant Nutr 41:1–20. https://doi.org/10.1080/01904167.2018.1462382
Nur R, Azura A (2020) Heavy metal contamination in vegetables and its detection: A review. Sci Herit J 4:1–5. https://doi.org/10.26480/gws.01.2020.01.05
Rusin M, Domagalska J, Rogala D, Razzaghi M, Szymala I (2021) Concentration of cadmium and lead in vegetables and fruits. Sci Rep 11:11913. https://doi.org/10.1038/s41598-021-91554-z
Ruzaidy N, Amid A (2020) Heavy metal contamination in vegetables and its detection: A review. Sci Herit J 4:01–05. https://doi.org/10.26480/gws.01.2020.01.05
Shi Z, Yong L, Liu Z, Wang Y, Sui H, Mao W, Zhang L, Li Y, Liu J, Wei S, Song Y (2022) Risk assessment of rare earth elements in fruits and vegetables from mining areas in China. Environ Sci Pollut Res 29(32):48694–48703. https://doi.org/10.1007/s11356-022-19080-7
Zhuang M, Zhao J, Li S, Liu D, Wang K, Xiao P, Yu L, Jiang Y, Song J, Zhou J, Wang L, Chu Z (2017) Concentrations and health risk assessment of rare earth elements in vegetables from mining area in Shandong, China. Chemosphere 168:578–582. https://doi.org/10.1016/j.chemosphere.2016.11.023
Gan Y, Wang L, Yang G, Dai J, Wang R, Wang W (2017) Multiple factors impact the contents of heavy metals in vegetables in high natural background area of China. Chemosphere 184:1388–1395. https://doi.org/10.1016/j.chemosphere.2017.06.072
Hu W, Huang B, Tian K, Holm PE, Zhang Y (2017) Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: levels, transfer and health risk. Chemosphere 167:82–90. https://doi.org/10.1016/j.chemosphere.2016.09.122
Gruszecka-Kosowska A (2019) Potentially harmful element concentrations in the vegetables cultivated on arable soils, with human health-risk implications. Int J Environ Res Pub Health 16(20):4053. https://doi.org/10.3390/ijerph16204053
Da Silva V, Gabriel G, Botero W, Fernandes A, Carmo J, Oliveira L (2022) Leafy vegetables marketed as organic and conventional: assessment of essential and non-essential elements’ content. Environ Monit Assess 194:758. https://doi.org/10.1007/s10661-022-10439-4
Sarker U, Oba S (2019) Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci Rep 9:20413. https://doi.org/10.1038/s41598-019-50977-5
Tosoc JPS, Nuñeza OM, Sudha T, Darwish NHE, Mousa SA (2021) Anticancer effects of the corchorus olitorius aqueous extract and its bioactive compounds on human cancer cell lines. Molecules 26(19):6033. https://doi.org/10.3390/molecules26196033
Oza MJ, Kulkarni YA (2017) Traditional uses, phytochemistry and pharmacology of the medicinal species of the genus Cordia (Boraginaceae). J Pharm Pharmoco 69(7):755–789. https://doi.org/10.1111/jphp.12715
Riaz G, Chopra RA (2018) review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmaco 102:575–586. https://doi.org/10.1016/j.biopha.2018.03.023
Majid M, Nasir B, Zahra SS, Khan MR, Mirza B, Haq IU (2018) Ipomoea batatas L. Lam. ameliorates acute and chronic inflammations by suppressing inflammatory mediators, a comprehensive exploration using in vitro and in vivo models. BMC Complement Altern Med 18(1):216. https://doi.org/10.1186/s12906-018-2279-5
Xiao X, Wang J, Meng C, Liang W, Wang T, Zhou B, Wang Y, Luo X, Gao L, Zhang L (2020) Moringa oleifera Lam and its therapeutic effects in immune disorders. Front Pharmaco 17(11):566783. https://doi.org/10.3389/fphar.2020.566783
Jiraungkoorskul W (2016) Review of neuro-nutrition used as anti-alzheimer plant, Spinach. Spinacia oleracea Pharmaco Rev 10(20):105–108. https://doi.org/10.4103/0973-7847.194040
Acharyya SK, Shah BA, Ashyiya ID, Pandey Y (2005) Arsenic contamination in groundwater from parts of Ambagarh-Chowki block, Chhattisgarh, India: source and release mechanism. Environ Geol 49:148–158. https://doi.org/10.1007/s00254-005-0074-3
Ahmad W, Alharthy RD, Zubair M, Ahmed M, Hameed A, Rafique S (2021) Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci Rep 11:17006. https://doi.org/10.1038/s41598-021-94616-4
Barbieri M, Nigro A, Sappa G (2015) Soil contamination evaluation by Enrichment Factor (EF) and Geoaccumulation Index (Igeo). Senses Scis 2(3):94–97. https://doi.org/10.14616/sands-2015-3-9497
Rudnick R, Gao S (2003) Composition of the Continental Crust. Treatise on Geochemistry 3:1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4
Arora M, Kiran B, Rani S, Rani A, Kaur B, Mittal N (2009) Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem 111(4):811–815. https://doi.org/10.1016/j.foodchem.2008.04.049
WHO (2002) The World Health report: reducing risks, promoting Healthy life. World Health Organization, Geneva, Switzerland
Alsafran M, Usman K, Rizwan M, Ahmed T, Aljabri H (2021) The Carcinogenic and Non-Carcinogenic Health Risks of Metal(oid)s Bioaccumulation in Leafy Vegetables: A Consumption Advisory. Front Environ Sci 9:742269. https://doi.org/10.3389/fenvs.2021.742269
Gebeyehu HR, Bayissa LD (2020) Levels of heavy metals in soil and vegetables and associated health risks in Mojo area. Ethiopia PLoS ONE 15(1):e0227883. https://doi.org/10.1371/journal.pone.0227883
MacInnes J (2017) An introduction to secondary data analysis with IBM SPSS statistics. SAGE publications Ltd., New Delhi
Patel KS, Sharma S, Maity JP, Martín-Ramos P, Fiket Ž, Bhattacharya P, Zhu Y (2023) Occurrence of uranium, thorium and rare earth elements in the environment: A review. Front Environ Sci 10:1058053. https://doi.org/10.3389/fenvs.2022.1058053
Randhawa M, Khan A, Javed MS, Sajid M (2015) (2015). Green Leafy Vegetables: A Health Promoting Source. In: Watson RR (ed) Handbook of fertility: Nutrition, diet, lifestyle and reproductive health. Academic Press, London, UK, pp 205–220
Ahmed S, Fatema-Tuj-Zohra MMM, Nurnabi M, Alam MZ, Choudhury TR (2022) Health risk assessment for heavy metal accumulation in leafy vegetables grown on tannery effluent contaminated soil. Toxicol Rep 9:346–355. https://doi.org/10.1016/j.toxrep.2022.03.009
Kumar D, Kumar S, Shekhar C (2020) Nutritional components in green leafy vegetables: A review. J Pharmaco Phytochem 9(5):2498–2502
Ali MHH, Al Qahtani KM (2012) Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. Egypt J Aquat Res 38:31–37. https://doi.org/10.1016/j.ejar.2012.08.002
Anke M, Seeber O, Müller R, Schäfer U, Zerull J (2009) Uranium transfer in the food chain from soil to plants, animals and man. Geochem 69:75–90. https://doi.org/10.1016/j.chemer.2007.12.001
Fiket Ž, Medunić G (2023) Dataset of rare earth elements distribution in soils and vegetables. Results Earth Sci 1:100004. https://doi.org/10.1016/j.rines.2023.100004
Sobukola P, Adeniran OM, Odedairo AA, Kajihausa OE (2010) Heavy metal levels of some fruits and leafy vegetables from selected markets in Lagos, Nigeria. Afr J Food Sci 4:389–393
Begum S, Rao M, Magham SR (2020) Evaluation of antidiabetic activity of swertia chirayita and panax ginseng. EJPMR 7:516–522
Petrović M, Medunić G, Fiket Ž (2023) Essential role of multi-element data in interpreting elevated element concentrations in areas impacted by both natural and anthropogenic influences. Peer J 11:15904. https://doi.org/10.7717/peerj.15904
Alireza M (2015) The evaluation and determination of heavy metals pollution in edible vegetables, water and soil in the south of Tehran province by GIS. Arch Environ Prot 41(2):64–74. https://doi.org/10.1515/aep-2015-0020
Aquilina G, Bories G, Brantom P, Caloni F, Chesson A, Cocconcelli PS, Knecht J, Dierick NA, Gralak MA, Gropp J, Halle I, Ilbäck N-G, Kroker R, Leng L, Lindgren S, Haldorsen AL, Mantovani A, Mézes M, Renshaw D, Saarela M (2009) Scientific opinion on the use of cobalt compounds as additives in animal nutrition. EFSA J 7(12):1383. https://doi.org/10.2903/j.efsa.2009.1383
FAO/WHO (1995) CXS 193-1995: General standard for contaminants and toxins in food and feed. Codex alimentarius commission. Available at: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf. Accessed 20th June 2024
Food standard (2003) 20th Australian Total Diet Survey - Part B. https://www.foodstandards.gov.au/sites/default/files/2023-11/20th-ATDS.pdf. Accessed 20th June 2024
Pearson J, Ashmore E (2020) Risk assessment of antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium concentrations in the New Zealand diet. FAC: Part A 37(3):451–464. https://doi.org/10.1080/19440049.2019.1704445
Golwelker A, Patel KS, Mishra RK (1990) Extraction-Spectrophotometric Determination of Chromium(VI) with Hydroxyamidines. Bull Chem Soc Japan 63(2):605–608. https://doi.org/10.1246/bcsj.63.605
US EPA (2005) Guidelines for carcinogen risk assessment (EPA/630/P-03/001F). U.S. environmental protection agency, Washington, DC, Risk assessment forum, p 166. https://www.epa.gov/sites/default/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf. Accessed 20th June 2024
Wimolwattanapun W, Hopke PK, Pongkiatkul P (2011) Source apportionment and potential source locations of PM2.5 and PM2.5–10 at residential sites in metropolitan Bangkok. Atmos Pollut Res 2(2):172–181. https://doi.org/10.5094/APR.2011.022
Kovaříková M, Tomášková I, Soudek P (2019) Rare earth elements in plants. Biol Plant 63(1):20–32. https://doi.org/10.1007/s11105-023-01369-7
Wang J, Yu D, Wang Y, Du X, Li G, Li B, Zhao Y, Wei Y, Xu S (2021) Source analysis of heavy metal pollution in agricultural soil irrigated with sewage in Wuqing. Tianjin Sci Rep 11(1):1–12. https://doi.org/10.1038/s41598-021-96367-8
Giri I, Ritika KC, Khadka UR (2022) Water quality status in Bagmati river of Kathmandu valley, Nepal. Ecological Significance of River Ecosystems 481–502. https://doi.org/10.1016/B978-0-323-85045-2.00017-0
Brzezicha-Cirocka J, Grembecka M, Szefer P (2016) Monitoring of essential and heavy metals in green tea from different geographical origins. Environ Monit Assess 188(3):183. https://doi.org/10.1007/s10661-016-5157-y
Li Y, Xu Z, Ren H, Wang D, Wang J, Wu Z, Cai P (2022) Spatial Distribution and source apportionment of heavy metals in the topsoil of Weifang City, East China. Front Environ Sci 469:893938. https://doi.org/10.3389/fenvs.2022.893938
Sulaiman FR, Hamzah HA (2018) Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol Processes 7:28. https://doi.org/10.1186/s13717-018-0139-3
Acknowledgements
We are thankful to Croatian Science Foundation for analysis support through project number FORtIS (IP-2019-04-9354).
Funding
The funding is provided by the King Saud University, Riyadh, Saudi Arabia through the project number (RSP2024R119).
Author information
Authors and Affiliations
Contributions
A. Khageshwar Singh Patel and Piyush Kant Pandey: conceptualization and drafting of manuscript. B. Bhagyashri Wakhle and Saroj Sharma: sampling, processing and data compilation. C. Mavro Lučić and Željka Fiket: ICP-MS data generation. D. Sema Yurdakul, Simge Varol and Pablo Martín-Ramos: statistical and health hazard analysis and source apportionment. E. Hanan Mohamed Al-Yousef and Ramzi Ahmed Mothana: Editing and funding.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wakhle, B., Sharma, S., Patel, K.S. et al. Multi-element Contamination and Health Risks in Green Leafy Vegetables from Ambagarh Chowki, Chhattisgarh, India. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04285-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04285-3