Abstract
Heavy metal bioaccumulation in organisms is primarily a result of dietary uptake. The current study examines the concentrations of heavy metals (Pb, Cd, Cr, and Cu) in fish feed, water, sediment, and three fish species (Catla catla, Labeo rohita, and Cyprinus carpio) from different feeding zones in a polyculture pond system. Furthermore, associated human health risks were also evaluated. The fish samples (n = 25 for each species) were collected from 10 different fish ponds in the Kohat district, Pakistan. Heavy metals were determined using an atomic absorption spectrometer. Results revealed higher concentrations of heavy metals in sediment, followed by water. However, the concentration of heavy metals in fish feed was lower than the standard limits. In the case of fish, the bottom feeder (C. carpio) notably exhibited higher (P < 0.05) levels of heavy metals than the column feeder (L. rohita) and surface feeder (C. catla) fish. Moreover, in the liver of all fish species, the bioaccumulation of heavy metals was higher, followed by the gills. Principal component analysis (PCA) demonstrated a strong correlation of heavy metals in C. carpio gills, flesh, feed, and pond water, while the heavy metals in the liver correlated with the detected metals in sediment. The human health risk analysis shows that bottom feeder fish had higher estimated daily intake (EDI), target hazard quotient (THQ), and hazard index (HI) values (> 1). Consequently, the exposed population may experience adverse health effects. The findings of this study suggest that the bottom feeder (C. carpio) bioaccumulates a higher concentration of heavy metals than column (L. rohita) and surface feeder (C. catla) in the polyculture system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture is a major source of animal protein [1]. Countries with overpopulation problems, such as Pakistan, need to produce more protein from fish [2]. Thus, it is imperative to maximize the yield of all resources that are usable for food production. Increased aquaculture facilities and the expansion of cultured fish ponds can facilitate these efforts [3].
The rapid development of the industrial sector has led to a significant amount of heavy metal pollution in the air, water, sediment, and feed, which constitute major environmental concerns globally [4, 5]. Furthermore, heavy metal pollutants may enter the ecosystem through anthropogenic activities, such as the disposal of sewage sludge and the application of inorganic fertilizers and pesticides [6]. In recent decades, there has been a growing concern about the contamination of freshwater and commercial feeds with several pollutants like heavy metals because they not only pose a threat to public water supplies but also harm aquatic life [7, 8]. As the level of heavy metal contamination increases, the growth of aquatic organisms is reduced, and the ecological balance of the aquatic environment is disrupted [9].
Aquatic ecosystems are widely evaluated using fish because pollutants accumulate in the food chain and cause adverse effects and loss [10]. Studies on diverse fishes have revealed that heavy metals alter their physiology, tissue, and blood biochemical parameters [11, 12]. Several studies have been conducted on the toxic effects of heavy metals, including the bioaccumulation of these metals [13, 14].
The habitats of aquatic organisms and their feeding zones determine the extent of heavy metal accumulation in them [15]. Soil organic carbon, total nitrogen, and total phosphorus play interconnected roles in water pollution and the transfer of heavy metals [11, 16]. Their dynamics are influenced by various environmental factors and human activities [9, 17]. High metal concentrations in the environment enhance the accumulation of metals in aquatic organisms [15]. The food chain can contribute to transferring heavy metals to organisms of high trophic levels, which may threaten human health [9, 18]. Metal-contaminated fish can adversely affect human health, and the toxic effects of metal-contaminated fish can extend to other organs or systems, making them even more dangerous for humans [19].
Fish are particularly vulnerable to pollution in the aquatic environment because they feed and live in an environment polluted by contaminants [20]. Various metals (cadmium, chromium, lead, nickel, copper, mercury, zinc, and arsenic) accumulate in fish bodies, depending on the species, location, temperature, and size [21, 22]. In response to exposures, such as diet or exposure to a higher metal level in their surrounding environment, their tissues and organs accumulate metals [23]. The quality of fish feed is a crucial aspect of aquaculture practice. Aquaculture systems that are intensive or semi-intensive require nutritionally balanced fish feeds [24]. A well-balanced commercial feed is essential for successful aquaculture production [25]. Different types of fish feed and water sources are used to produce fish. Certain heavy metals are a significant source of pollution from these sources [26]. Studies have been limited to evaluating heavy metal levels in fish species based on their feeding zones in polyculture systems. In the current study, we hypothesized that heavy metals bioaccumulation in fish depends on different feeding zones and habits. Therefore, we measured the contents of heavy metals (Pb, Cd, Cr, and Cu) in water, fish feed, sediment, and tissues of fish species (C. carpio, L. rohita, and C. catla) fed in different zones in a polyculture system. Then, the concentration and bioaccumulation of heavy metals in the different fish tissues were compared, and the human health risks were assessed.
Materials and Methods
Study Site
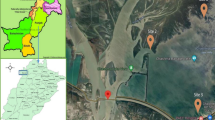
The study site was located in Kohat district in the southern region of the province (Khyber Pakhtunkhwa), Pakistan (Fig. 1). The total area of the district is 2973 km2 with geographical coordinates 33.4973° N, 71.5249° E. In Pakistan, it ranks 35th in terms of population and fourth in Khyber Pakhtunkhwa. It is estimated that the district has a population of more than 562,644 residents, according to the Khyber Pakhtunkhwa survey report. Three major water reservoirs (Tanda, Gandiali, and Kandat) can be found in the area. The climate at the site is hot and semi-arid. May to September are the hottest months in Kohat and the surrounding area. The site has a harsh and cold winter. Guava is the most popular fruit in the country and a major crop of the site. Rainfall occurs throughout the year in the area. Monsoon rains occur from May to October. In August, the area receives the most rain, averaging 114 mm. There are three main crops grown in the district: wheat (Triticum aestivum), maize (Zea mays), and barley (Hordeum vulgare); the area is also known for the production of several vegetables. Tube wells are used to irrigate most agricultural lands. Warm-water fish farming is a common practice on the site. Mostly, the fish are cultured in earthen ponds in polyculture form. The commonly cultured fish species are carp (major Indian carp), Nile tilapia, and catfish.
Modified map of District Kohat Khyber Pakhtunkhwa, Pakistan (adopted from Tasleem et al. [20])
Sample Collection and Preparation
All the fish (C. catla, L. rohita, and C. carpio) samples (n = 25 for each species) were collected from 10 different earthen ponds in district Kohat where the fishes were cultured in polyculture form having different feeding zones (C. catla: surface feeder, L. rohita: column feeder, and C. carpio: bottom feeder). Fish species were caught by using a cast net. The weight and length of sampled fish were measured using an electronic weighing balance (model name: Newtech) and measuring tape made up of fiberglass. The mean weight (g) and length (cm) of C. catla were approximately 423.13 g and 19.89 cm, respectively. For L. rohita, these values were approximately 430.09 g and 20.03 cm, while for C. carpio, they were around 433.17 g and 20.02 cm, respectively. After that, all the samples were correctly labeled and transported to the laboratory in ice-filled containers and stored at − 20 °C for further analysis.
The next morning, the samples were thawed and washed with distilled water. Using a sharp knife, the scales were carefully removed from the fish and paper towels were used to dry the samples. A plastic tray was used to separate the tissue and organ samples for the dissection. The edible parts (flesh) from each sample were separated, and a sterilized stainless-steel knife was used to slice them into smaller pieces. Following the method of Habib et al. [15], the gills and liver were dissected. They were subsequently dried in a plastic petri dish at 80 °C to achieve constant weight, then cooled in a desiccator before being ground in a porcelain mortar and pestle [24].
Fish feed samples from Supreme Company Lahore, Pakistan, were used to feed the fish species. About 0.5 kg of feed samples were collected from fish farmers in clean plastic bags. The samples were ground using a grinding machine and stored in airtight containers to avoid contamination. They were subsequently used for proximate [27] and heavy metals analyses [15]. Table 1 presents the proximate composition of fish feed.
Fish pond water samples were collected in clean (already autoclaved) glass-made bottles (1000 ml) from each pond in triplicates at 15–30 cm underwater. Water samples were filtered with a 0.45-µm membrane and then treated with 2% nitric acid before analysis [20].
For sediment samples, a stainless-steel grab sampler was used to collect sediment samples in clean polyethylene bags, which were then freeze-dried for 60 h. After grounding in a mortar, the sediments were sieved through a 0.125-mm mesh screen.
Water Quality Parameters
During sampling, water temperature (°C), dissolved oxygen (mg/l), pH (log H +), and total dissolved solids (ppm) were measured on the spot. Water temperature was measured using digital thermometer “model = HI-991002,” dissolved oxygen (DO) through DO-meter “HI98198-Hanna,” pH by using a pH meter “Beckman model-72,” and total dissolved solids (TDS) was examined through a TDS meter “HM-COM-80”. Other parameters, including ammonia (NH3), nitrite (NO-2), and nitrate (NO-3), were observed through the Freshwater Master Test Kit “API, USA” from the sampled water. The details of water quality parameters are presented in Table 2.
Heavy Metals Analysis
For heavy metals (Pb, Cd, Cr, and Cu) analysis, 0.5 g of each sample, such as fish feed, sediment, fish tissues, and 50 ml water, was digested with three concentrated acids (nitric acid: HNO3, sulfuric acid: H2SO4, and perchloric acid: HClO4 (37%); Sigma–Aldrich) at a temperature of 80 °C [20]. Once the sample had been digested, it was cooled to room temperature and filtered using Whatman’s filter paper of 0.45-μm pore size. Then, the sample was diluted with deionized water to a volume of 50 ml before further analysis. Estimated concentrations of heavy metals were determined. Flame conditions for atomic absorption have been optimized in order to achieve a higher absorption rate and a linear response. Blank, working standard, and actual biological samples were directly aspirated into the flame and with the help of atomic absorption spectrometer “model AA240FS, Varian Atomic Absorption Spectrophotometer,” absorption was recorded. HNO3 was added to 100 ml of filtered water and digested at 100 °C for 10 min. After cooling the samples to room temperature, they were diluted and filtered using the Whatman-42 filter paper. HNO3 (0.01 N) was used to prepare 50 ml of the filtrate; samples were then analyzed. To ensure that the process was safe and effective, good quality chemicals and reagents were used during the process, as well as thorough cleaning and deionization of the glassware.
Bioaccumulation Factor
Based on the equation below, we calculated the bioaccumulation factor (BAF):
Estimated Daily Intake
In order to estimate the possibility of metal poisoning in humans, the estimated daily intake (EDI) of metals from fish muscles is used. Based on the following formula, an estimated daily intake (EDI) can be determined [28].
where metal concentrations in fish muscles were converted into dry weight using an average concentration factor of 4.8 [29]. Pakistani men consume, on average, 70 g of fish daily, while their average weight was taken as 60 kg [30, 31].
Human Health Risk Assessment
To assess the risk associated with the consumption of metal-loaded fish, the estimated daily intake (EDI) was calculated. It is possible to measure the potential health risks associated with consuming heavy metal-contaminated food through the use of a target hazard quotient (THQ) and hazard index (HI) [32]. Calculations were made using the following formula.
Statistical Analysis
A one-way ANOVA was performed in GraphPad Prism (version 10.1.1) to separate the means (significance level: P < 0.05) of heavy metals. In addition, Duncan’s multiple range test was used to determine whether there were significant differences between the detected heavy metals in fish species. Furthermore, to assess the association between the analyzed metals, principal component analysis (PCA) was applied.
Results
Heavy Metal Concentration inFish Feed, Water, and Sediment
The concentration of heavy metals in fish feed, pond water, and sediment is shown in Figs. 2, 3, and 4, respectively. According to the results, the Pb, Cu, and Cd concentrations were higher in sediment than in feed and pond water. The Cd level was almost similar in feed and pond water. The Cr level in feed was below the detection limit, while a slightly higher concentration was observed in the sediment than in pond water. In most cases, the mean concentration of heavy metals was greater in sediment, followed by water. However, the least amount was recorded in fish feed. The concentration of heavy metals in fish feed was lower than the FAO limit. However, the Pb and Cd levels were above the standard limit in water. In the case of sediment, the Cd, Cr, and Cu concentrations were recorded above the permissible limit (Table 3).
Comparative Analysis of Heavy Metals Concentration in Body Tissues of Fishes
The comparison of the heavy metals in the gills, liver, and flesh of different fish species based on their feeding zones is illustrated in Figs. 5, 6, and 7. After data analysis, it was found that the level of Pb and Cu concentration in gills, liver, and flesh were significantly higher (P < 0.05) in C. carpio (bottom feeder). The amount of Cd in body tissues of C. catla (surface feeder) and L. rohita (column feeder) revealed no significant difference (P > 0.05), while notably higher (P < 0.05) in the case of C. carpio. The concentration of Cr in fish species of different feeding zones showed no significant difference (P > 0.05). The mean concentration of heavy metals recorded was significantly higher (P < 0.05) in the liver, followed by gills in fish species. However, the least amount was found in the flesh of fishes. Overall, the bottom feeder fish showed significantly higher (P < 0.05) concentrations of heavy metals in their body tissues than the other fish species. The Pb and Cr levels in gills, liver, and flesh were higher in L. rohita and C. carpio while lower in C. catla flesh than the standard limit of WHO. The amount of Cd was higher in the liver of all fish species compared to the WHO standard. However, the Cu level was lower than the standard limit (Table 3).
Bioaccumulation of Heavy Metals
Table 4 presents the bioaccumulation factor of heavy metals for different fish species. The results showed that the higher bioaccumulation of heavy metals was recorded in the liver, followed by gills in all experimental fish species of different feeding zones. However, the lower bioaccumulation factor was found in fish flesh. Comparatively, the bioaccumulation of metals was higher in bottom feeder fish (C. carpio) followed by column feeder (L. rohita), while lower bioaccumulation was found in surface feeder fish (C. catla).
PCA Analysis for Heavy Metals
According to the PCA analysis for surface feeder (C. catla), the heavy metals observed in fish flesh and feed are strongly correlated, which means that they are positively correlated. On the other hand, the heavy metals in the liver are closer to the sediment and gills. Furthermore, the Cd level is not significantly correlated to other heavy metals, as the vector direction indicates (Fig. 8).
Similar results for the column feeder (L. rohita) were found in which the feed, flesh, gills, and pond water correlate (Fig. 9).
In the case of the bottom feeder (C. carpio), the detected heavy metals in fish feed, flesh, gills, and pond water are correlated, as shown in Fig. 10. The heavy metals Cr, Pb, and Cu are also strongly associated with each other, while Cd is slightly positively correlated.
Health Risk Analysis
The association of heavy metals with human health risks from the flesh of different fish species is depicted in Table 5. The results revealed that for all the heavy metals investigated (Pb, Cd, Cr, and Cu), the values of EDI and THQ were higher in the flesh of C. carpio than in L. rohita and C. catla reared in polyculture form. Fish consumption by children and adults was associated with a higher risk of exposure to harmful elements when the EDI value was high. Furthermore, an elevated HI value greater than 1 indicates a greater threat to human health. In our study, the HI value for the C. carpio was higher than one compared to other fish species. According to the current research, bottom feeder fish such as C. carpio pose a higher risk to consumers than column and surface feeders. The value of HI was the least recorded in surface feeder fish.
Discussion
Various factors influence fish’s heavy metal bioaccumulation, such as feeding habits, water temperature, pH, salinity, metal interaction, sediment, feed, sex, and age [15, 37]. However, little literature exists that compares heavy metal bioaccumulation in fish species with different feeding zones in a similar habitat. Due to this, our study investigated and compared the heavy metal levels in three fish species (C. catla, L. rohita, and C. carpio) reared in a polyculture system fed in different feeding zones. Furthermore, this study analyzed the heavy metals in fish feed, water, sediment, and related human health risks.
Results revealed that bottom feeder fish, C. carpio, tend to have higher concentrations of heavy metals in their gills, liver, and flesh compared to fish species in different feeding zones. This suggests that bottom feeders are likely more exposed to polluted sediments and absorb more heavy metals from earthen pond sediments. The sediments play a crucial role in the uptake of heavy metals by fishes, and the foraging behavior and swimming of bottom-dwelling fishes may contribute to a transfer of heavy metals from the sediments to the fish [38].
Other studies also revealed similar findings, such as Kumar et al. [39] found that the bottom feeder fish (Channa striata) has the highest concentration of heavy metals than the column (L. rohita) and surface feeder fish (C. catla). Jiang et al. [40] reported that the bioaccumulation of heavy metals (Pb, Cd, Zn, and Cu) in demersal fishes was higher compared to pelagic and benthopelagic fishes in Dongting Lake, China, which is similar to this study. Asante et al. [34] observed notably significantly higher levels of heavy metals (Mn, Cs, Co, Cr, Se, and As) in demersal fishes. Similar findings were also reported by Jitar et al. [35] about the higher amount of heavy metals bioaccumulation in demersal fishes from the Black Sea. Heavy metals are absorbed from sediment by demersal fish as the primary environment is closely related to them. Furthermore, heavy metals are transferred to fish via both dietary and dissolved routes during predation and swimming [41, 42]. Inconsistencies in heavy metal concentrations in surface and bottom feeder fish may be due to geographic factors, living environment factors, and the specific toxicity of the metal [43].
High concentrations of heavy metals (Pb, Cd, and Cr) observed in the liver, exceeding WHO [44, 45] standards, may be linked to natural protein binding, including metallothioneins [46]. Moreover, in the liver, metals are stored, detoxified, and re-distributed [47]. Metabolic function may be affected when heavy metals are concentrated in the liver. In addition, fish liver is considered an important indicator of water pollution [48]. Other studies also show a higher concentration of heavy metals in fish liver [14]. In this study, the higher concentrations of heavy metals recorded in the gills than in the liver may be because metals are mostly transferred in water through the gill pores, which have a vast surface area that permits the metals to spread more quickly [15, 49]. Several investigations have shown that the high quantity of heavy metals in fish gills is mainly concentrated in the water [50, 51]. High levels of lead (Pb) in fish tissues can potentially harm human health if ingested through food. Pb-contaminated fish can negatively affect a person’s liver, brain, neurological, kidneys, and reproductive systems [36]. When fish are exposed to toxicants, bioaccumulation is the primary mechanism for Pb-induced toxicity [52]. However, the means by which the chemicals accumulate differ, depending on the environmental conditions, for example, freshwater or seawater, as well as the route of exposure (dietary or waterborne) of the chemicals [53]. The concentration of Pb in the water and fish was higher than the WHO limit but lower in the flesh of the surface feeder.
A study by Okocha and Adedeji [54] reported a higher concentration of Cd in fish liver and gills, which is according to the current research. Fish exposure to Cd for a longer duration slows bone development, induces tumors, and elevates blood pressure [11, 55]. According to Fazio et al. [11], the growth and hematological parameters of fish (Mystus seenghala) were lowered by the sub-lethal effect of Cd. The Cd recorded in this study was higher in fish species’ liver, water, and sediment than the WHO [44] and USEPA [56] limits. The Cr level in sediment, gills, liver, and flesh of column and bottom feeder fish was higher than the USEPA and WHO standards. The presence of high levels of Cr in fish can alter blood parameters as well as damage to the liver and kidneys [57]. Overconsumption of Cr has been associated with serious human health risks [33].
The current study findings revealed a lower level of heavy metals in fish feed than the standard limit [44]. This is consistent with the findings of other studies, such as Habib et al. [13, 15], who reported the lower level of heavy metals (Cd, Cr, Pb, Cu, and Zn) in commercial feed used for the culturing of Oreochromis niloticus and Cyprinus carpio in biofloc technology. Other studies from Bangladesh also revealed lower levels of heavy metals (Cr, Cu, Zn, Pb, and Cu) in different commercial feeds [25].
In the current sediment study, heavy metals concentration was higher than in water and feed. Moreover, compared to the permissible limits [56, 58], the Cd, Cr, and Cu amounts were higher. This is according to the results reported by Weber et al. [59], who recorded higher concentrations of heavy metals (Cd, Cr, Zn, Fe, Cu, Mn, As, and AI) in sediment followed by water in the Brazilian River. The sediment was the primary source of metal pollution in this polyculture pond. Thus, the bottom feeder fish accumulate more metals in their liver than the column and surface feeder. The suspended sediments act as adsorbents, thus reducing the concentration of pollutants in the water column [37].
The reference dose is the maximum acceptable daily consumption of a particular metal with no harmful effects on health [60]. The human health risk assessment showed that the reference dose for bottom feeder fish was higher, indicating a potentially greater risk to human health associated with their consumption. Thus, there is a chance of health risks in children and adults consuming bottom-dwelling fish. Similar results were reported by Kumar et al. [39], who revealed a higher HI value (> 1) for the bottom feeder than the surface feeder. The current study is contrary to the results of Wu et al. [61], who revealed a lower reference dose for heavy metals in pelagic, benthopelagic, and demersal fish. When exposed to high metal concentrations, fish can absorb and accumulate the metals through their gills and skin or ingest contaminated water and food [15, 62]. As a result, the metal concentration in fish tissues might be many times greater than that of the surrounding medium [24].
Conclusion
This study suggests that the concentration of heavy metals in fish feed, water, and sediment samples from polyculture ponds varies. They were found to be higher for water (Pb and Cd) and sediment (Cd, Cr, and Cu) and lower for fish feed than the permissible limit proposed by several agencies. A higher concentration was found in sediment than in water and feed. Thus, the sediments are the major sink of heavy metals and play a significant role in the uptake of heavy metals by bottom-feeding fish. This bottom feeder fish might be useful as bioindicators to trace metal contamination. Long-term consumption of fish, particularly the bottom feeders from this region, is not advisable, as evidenced by risk assessments in the form of THQ and HI. This may cause adverse health consequences to humans.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Fazio F, Habib SS, Naz S, Filiciotto F, Cicero N, Rehman HU, Saddozai S, Rind KH, Rind NA, Shar AH (2022) Effect of fortified feed with olive leaves extracts on the haematological and biochemical parameters of Oreochromis niloticus (Nile tilapia). Nat Prod Res 36(6):1575–1580
Habib SS, Batool AI, Rehman MFU, Naz S (2023) Comparative analysis of hemato-biochemical profile, growth performance, and body composition of Common Carp cultured under different feed and habitats (biofloc technology and earthen pond systems). N Am J Aquac 85(3):277–290
Habib SS, Batool AI, Rehman MFU, Naz S (2022) Comparative analysis of the haemato-biochemical parameters and growth characteristics of Oreochromis niloticus (Nile tilapia) cultured under different feed and habitats (biofloc technology and earthen pond system). Aquac Res 53(17):6184–6192
Sankhla MS, Kumari M, Nandan M, Kumar R, Agrawal P (2016) Heavy metals contamination in water and their hazardous effect on human health-a review. Int J Curr Microbiol App Sci 5(10):759–766
Bai B, Xu T, Nie Q, Li P (2020) Temperature-driven migration of heavy metal Pb2+ along with moisture movement in unsaturated soils. Int J Heat Mass Transf 153:119573
Zorpas AA, Pedreño JN, Candel MBA (2021) Heavy metal treatment and removal using natural zeolites from sewage sludge, compost, and agricultural soils: a review. Arab J Geosci 14(12):1098
Okereafor U, Makhatha M, Mekuto L, Uche-Okereafor N, Sebola T, Mavumengwana V (2020) Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int J Environ Res Public Health 17(7):2204
Han X, Wu H, Li Q, Cai W, Hu S (2024) Assessment of heavy metal accumulation and potential risks in surface sediment of estuary area: a case study of Dagu river. Mar Environ Res 196:106416. https://doi.org/10.1016/j.marenvres.2024.106416
Zhang Y, Lu X, Wang N, Xin M, Geng S, Jia J, Meng Q (2016) Heavy metals in aquatic organisms of different trophic levels and their potential human health risk in Bohai Bay, China. Environ Sci Pollut Res 23:17801–17810
Nilsen E, Smalling KL, Ahrens L, Gros M, Miglioranza KS, Picó Y, Schoenfuss HL (2019) Critical review: grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ Toxicol Chem 38(1):46–60
Fazio F, Habib SS, Naz S, Hashmi MAH, Saoca C, Ullah M (2022) Cadmium sub-lethal concentration effect on growth, haematological and biochemical parameters of Mystus seenghala (Sykes, 1839). Biol Trace Elem Res 200(5):2432–2438
Adeogun AO, Ibor OR, Omiwole R, Chukwuka AV, Adewale AH, Kumuyi O, Arukwe A (2020) Sex-differences in physiological and oxidative stress responses and heavy metals burden in the black jaw tilapia, Sarotherodon melanotheron from a tropical freshwater dam (Nigeria). Comp Biochem Physiol C: Toxicol Pharmacol 229:108676
Habib SS, Batool AI, Rehman MFU, Naz S (2023) Evaluation and association of heavy metals in commonly used fish feed with metals concentration in some tissues of O. niloticus cultured in biofloc technology and earthen pond system. Biol Trace Elem Res 201(6):3006–3016
Naz S, Fazio F, Habib SS, Nawaz G, Attaullah S, Ullah M, Hayat A, Ahmed I (2022) Incidence of heavy metals in the application of fertilizers to crops (wheat and rice), a fish (Common carp) pond and a human health risk assessment. Sustainability 14(20):13441
Habib SS, Batool AI, Rehman MFU, Naz S (2023) Assessment and bioaccumulation of heavy metals in fish feeds, water, and some tissues of Cyprinus carpio cultured in different environments (Biofloc Technology and Earthen Pond System). Biol Trace Elem Res 201(7):3474–3486
Liu J, Wang Y, Li Y, Peñuelas J, Zhao Y, Sardans J, Tetzlaff D, Liu J, Liu X, Yuan H, Li Y (2023) Soil ecological stoichiometry synchronously regulates stream nitrogen and phosphorus concentrations and ratios. Catena 231:107357
Zhang T, Song B, Han G, Zhao H, Hu Q, Zhao Y, Liu H (2023) Effects of coastal wetland reclamation on soil organic carbon, total nitrogen, and total phosphorus in China: a meta-analysis. Land Degrad Dev 34(11):3340–3349
Yi J, Li H, Zhao Y, Zhang H, Liu M (2022) Assessing soil water balance to optimize irrigation schedules of flood-irrigated maize fields with different cultivation histories in the arid region. Agric Water Manag 265:107543
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2013) Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch Environ Contam Toxicol 65:753–764
Tasleem S, Masud S, Habib SS, Naz S, Fazio F, Aslam M, Ullah M, Attaullah S (2023) Investigation of the incidence of heavy metals contamination in commonly used fertilizers applied to vegetables, fish ponds, and human health risk assessments. Environ Sci Pollut Res Int 30(45):100646–100659. https://doi.org/10.1007/s11356-023-29480-y
Yi YJ, Zhang SH (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19:3989–3996
Ling X, Zheng H, Huang J, Sun H, Xu S, Zeng H, Cai A, Wang Q, Deng J (2024) The novel application of polyoxometalates for achieving sludge deep dewatering using low-temperature thermal hydrolysis pretreatment. J Clean Prod 444:141125
Liu JL, Xu XR, Ding ZH, Peng JX, Jin MH, Wang YS, Hong YG, Yue WZ (2015) Heavy metals in wild marine fish from South China Sea: levels, tissue-and species-specific accumulation and potential risk to humans. Ecotoxicology 24:1583–1592
Channa MJ, Ayub H, Ujan JA, Habib SS, Ullah M, Attaullah S, Khayyam K, Khan K (2024) Human health risk assessment due to the incidence of heavy metals in different commercial feeds used for the culturing of biofloc fish (Nile tilapia: oreochromis niloticus). Biol Trace Elem Res 202(4):1741–1751. https://doi.org/10.1007/s12011-023-03767-0
Sarkar MM, Rohani MF, Hossain MAR, Shahjahan M (2022) Evaluation of heavy metal contamination in some selected commercial fish feeds used in Bangladesh. Biol Trace Elem Res 200(2):844–854
Saha B, Mottalib MA, Al-Razee AN (2021) Heavy metals accumulation in different cultivated fish tissues through commercial fish feeds and health risk estimation in consumers in Bangladesh. Chem Rev Lett 4(1):10–20
AOAC (2000) Official methods of analysis, 17th edn. Association of official analytical chemists, Gaithersburg
Bo SONG, Mei LEI, Tongbin CHEN, Zheng Y, Yunfeng XIE, Xiaoyan LI, Ding GAO (2009) Assessing the health risk of heavy metals in vegetables to the general population in Beijing, China. J Environ Sci 21(12):1702–1709
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem 134(4):1847–1854
Baset A (2020) Fish consumption study at a household level in Dir Lower, Khyber Pakhtunkhwa, Pakistan. RJFSN 5:98–104
Qasim M, Qasim S, Nazir N (2020) Factors affecting fish consumption of traditional subsistence Fishers in Khyber Pakhtunkhwa, Pakistan. Mar Sci Technol Bull 9(2):178–187
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69(3):513–524
Pratush A, Kumar A, Hu Z (2018) Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Int Microbiol 21:97–106
Asante KA, Agusa T, Kubota R, Mochizuki H, Ramu K, Nishida S, Ohta S, Yeh HM, Subramanian A, Tanabe S (2010) Trace elements and stable isotope ratios (δ13C and δ15N) in fish from deep-waters of the Sulu Sea and the Celebes Sea. Mar Pollut Bull 60(9):1560–1570
Jitar O, Teodosiu C, Oros A, Plavan G, Nicoara M (2015) Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol 32(3):369–378
Pareja-Carrera J, Mateo R, Rodríguez-Estival J (2014) Lead (Pb) in sheep exposed to mining pollution: implications for animal and human health. Ecotoxicol Environ Saf 108:210–216
Janadeleh H, Kameli MA (2017) Metals contamination in sediment and their bioaccumulation in plants and three fish species from freshwater ecosystem. Toxin Rev 36(4):297–305
Rahman MS, Saha N, Molla AH, Al-Reza SM (2014) Assessment of anthropogenic influence on heavy metals contamination in the aquatic ecosystem components: water, sediment, and fish. Soil Sediment Contam Int J 23(4):353–373
Kumar M, Gupta N, Ratn A, Awasthi Y, Prasad R, Trivedi A, Trivedi SP (2020) Biomonitoring of heavy metals in river Ganga water, sediments, plant, and fishes of different trophic levels. Biol Trace Elem Res 193:536–547
Jiang X, Wang J, Pan B, Li D, Wang Y, Liu X (2022) Assessment of heavy metal accumulation in freshwater fish of Dongting Lake, China: effects of feeding habits, habitat preferences and body size. J Environ Sci 112:355–365
Naccari C, Cicero N, Ferrantelli V, Giangrosso G, Vella A, Macaluso A, Naccari F, Dugo G (2015) Toxic metals in pelagic, benthic and demersal fish species from Mediterranean FAO zone 37. Bull Environ Contam Toxicol 95:567–573
Abdolahpur Monikh F, Safahieh A, Savari A, Doraghi A (2013) Heavy metal concentration in sediment, benthic, benthopelagic, and pelagic fish species from Musa Estuary (Persian Gulf). Environ Monit Assess 185(1):215–222. https://doi.org/10.1007/s10661-012-2545-9
Singh A, Singh D, Yadav H (2017) Impact and assessment of heavy metal toxicity on water quality, edible fishes and sediments in lakes: a review. Trends Biosci 10(8):1551–1560
WHO (1995) Heavy metals environmental aspects, Tech. Rep., Environmental Health criteria No. 85, Geneva, Switzerland 38
WHO/FAO (1990) Food standards programme, Guideline levels for cadmium and lead in food. Codex committee of food additives and contamination, 22nd session, Haugue, the Netherlands
Teigen SW, Andersen RA, Daae HL, Skaare JU (1999) Heavy metal content in liver and kidneys of grey seals (Halichoerus grypus) in various life stages correlated with metallothionein levels: some metal—binding characteristics of this protein. Environ Toxicol Chem Int J 18(10):2364–2369
Blickley TM, Matson CW, Vreeland WN, Rittschof D, Di Giulio RT, McClellan-Green PD (2014) Dietary CdSe/ZnS quantum dot exposure in estuarine fish: bioavailability, oxidative stress responses, reproduction, and maternal transfer. Aquat Toxicol 148:27–39
Tashla T, Žuža M, Kenjveš T, Prodanović R, Soleša D, Bursić V, Petrović A, Pelić DL, Bošković J, Puvača N (2018) Fish as an important bio-indicator of environmental pollution with persistent organic pollutants and heavy metals. J Agron 28:52–56
Fairbrother A, Wenstel R, Sappington K, Wood W (2007) Framework for metals risk assessment. Ecotoxicol Environ Saf 68(2):145–227
Yilmaz F (2009) The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) inhabiting Koycegiz Lake-Mugla (Turkey). Turk J Sci Technol 4(1)
Bashir FH, Othman MS, Mazlan AG, Rahim SM, Simon KD (2013) Heavy metal concentration in fishes from the coastal waters of Kapar and Mersing, Malaysia. Turk J Fish Aquat Sci 13(2)
Rzymski P, Poniedzialek B, Niedzielski P, Tabaczewski P, Wiktorowicz K (2014) Cadmium and lead toxicity and bioaccumulation in Microcystis aeruginosa. Front Environ Sci Eng 8:427–432
Benedetti M, Giuliani ME, Regoli F (2015) Oxidative metabolism of chemical pollutants in marine organisms: molecular and biochemical biomarkers in environmental toxicology. Ann N Y Acad Sci 1340(1):8–19
Okocha RC, Adedeji OB (2011) Overview of cadmium toxicity in fish. J Appl Sci Res 7(7):1195–1207
Zuluaga Rodríguez J, Gallego Ríos SE, Ramírez Botero CM (2015) Content of Hg, Cd, Pb and As in fish species: a review. Vitae 22(2):148–149
USEPA (1999) US Environmental Protection Agency: screening level ecological risk assessment protocol for hazardous waste combustion facilities. Appendix E Toxicity Ref Values 3
Mohamed AAR, El-Houseiny W, Abd Elhakeem EM, Ebraheim LL, Ahmed AI, Abd El-Hakim YM (2020) Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: role of curcumin supplemented diet. Ecotoxicol Environ Saf 188:109890
Food and Agriculture Organization (FAO) (1985) Irrigation and drainage paper 29. Rev. 1, Italy, p 174
Weber P, Behr ER, Knorr CDL, Vendruscolo DS, Flores EM, Dressler VL, Baldisserotto B (2013) Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem J 106:61–66
Baars AJ, Theelen RMC, Janssen PJCM, Hesse JM, van Apeldoorn MV, Meijerink MV, Verdam L, Zeilmaker MJ (2001) Re-Evaluation of Human-Toxicological Maxi-Mum Permissible Risk Levels; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht
Wu D, Feng H, Zou Y, Xiao J, Zhang P, Ji Y, Lek S, Guo Z, Fu Q (2023) Feeding habit-specific heavy metal bioaccumulation and health risk assessment of fish in a tropical reservoir in Southern China. Fishes 8(4):211
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem. https://doi.org/10.1155/2019/6730305
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project (RSPD2024R758), King Saud University, Riyadh, Saudi Arabia.
Funding
This work was funded by the Researchers Supporting Project (RSPD2024R758), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, Saira Naz and Allah Bachayo Rajar; data curation, Zainab Malik, and Javed Ahmed Ujan; formal analysis, Khalid Hussian Rind, and Raqeeb Ullah; investigation, Saira Naz; methodology, Mujeeb Ullah; resources, Muhammad Zahid, and Khalid Khan; software, Khayyam Khayyam; writing – original draft, Saira Naz and Allah Bachayo Rajar; writing – review and editing, Salim S. Al-Rejaie, Mohamed Mohany, and Zainab Malik.
Corresponding author
Ethics declarations
Ethics Approval
The authors followed all the valid national rules for the use and care of animals, and the study was conducted after the ethical committee approval of the Department of Zoology, University of Lahore, Pakistan.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajar, A.B., Malik, Z., Ujan, J.A. et al. Implications of Heavy Metal Accumulation in Fish Feed, Water, Sediment, and Different Fish Species in a Polyculture System. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04217-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04217-1