Abstract
This experiment aimed to evaluate the beneficial and toxic properties of synthetic zinc oxide nanoparticles (ZnO NPs) on the liver of normal and high-fat diet (HFD) fed-rats. The ZnO NPs were synthesized and, its characterizations were determined by different techniques. Effect of ZnO NP on cell viability, liver enzymes and lipid accumulation were measured in HepG2 cells after 24 h. After that, rats orally received various dosages of ZnO NPs for period of 4 weeks. Toxicity tests were done to determine the appropriate dose. In the subsequent step, the hepatoprotective effects of 5 mg/kg ZnO NPs were determined in HFD-fed rats (experiment 2). The oxidative stress, NLRP3 inflammasome, inflammatory, and apoptosis pathways were measured. Additionally, the activity of caspase 3, nitric oxide levels, antioxidant capacity, and various biochemical factors were measured. Morphological changes in the rat livers were also evaluated by hematoxylin and eosin (H & E) and Masson trichrome. Liver apoptosis rate was also approved by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay. Treatment of animals with 5 mg/ZnO NPs revealed potential hepatoprotective properties, while ZnO NPs at the doses of above 10 mg/kg showed toxic effects. Antioxidant enzyme gene expression and activity were significantly augmented, while apoptosis, NLRP3 inflammasome, and inflammation pathways were significantly reduced by 5 mg/kg ZnO NPs. Liver histopathological alterations were restored by 5 mg/kg ZnO NPs in HFD. Our study highlights the hepatoprotective effects of ZnO NPs against the HFD-induced liver damage, involving antioxidant, anti-inflammatory, and anti-apoptotic pathways, indicating their promising therapeutic potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is known as a predominant form of chronic liver disorders throughout the world [1], affecting approximately 24% of the worldwide population with the highest estimates reaching 32% in the Middle East and 31% in South America [2]. The pathogenesis of NAFLD is defined by the “two hits” model, including lipid accumulation (first hit) and increased oxidative stress, inflammation, mitochondrial dysfunction, and lipid peroxidation (second hit). These factors are mainly held responsible for the progression of onset NAFLD to nonalcoholic steatohepatitis (NASH) and cirrhosis diseases [3]. Oxidative stress itself has been confirmed to mediate various cellular responses, leading to diverse outcomes such as cell growth and apoptosis [4].
In addition to adenosine monophosphate-activated protein kinase (AMPK) stress, the activation of inflammatory pathways, such as tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), and NLR family pyrin domain containing 3 (NLRP3) inflammasome, is associated with the pathogenesis of NAFLD [5]. The activation of inflammasomes leads to caspase-1 activation and interleukin 1 beta (IL-1β) secretion. High levels of IL-1β have been reported in animal models of NASH, induced by HFD [3]. Furthermore, in the IL-1β knockout animal model, there was a significant decrease in liver damage, a reduced progression from simple steatosis to NASH, and lowered liver fibrosis in HFD-induced NASH model [3, 5]. Recently, the importance of these pathways in the context of metabolic syndrome and different type of liver disorders, especially NASH, is receiving much attention [5]. At present, no approved medicines are available for the treatment of NAFLD.
During the last decade, antioxidant agents, such as vitamins E and C, resveratrol, and eugenol, have garnered increasing interest as potential therapeutic option for the occurrence of NAFLD. Nevertheless, many well-recognized substances have not been used in clinical trials for treatment or prevention of NAFLD mostly due to issues of lack of specificity, and subsequent side effects [1].
Previous studies have established the effectiveness of zinc in alleviating oxidative stress, apoptosis, and inflammation [6, 7]. Zinc acts as a cofactor for about 300 enzymes in the body [7]. It has been proved that zinc plays a critical role in the inhibition of lipid peroxidation and protects the liver from free radical damage [7]. Zinc oxide nanoparticles (ZnO NPs), a zinc product with a diameter between 1 and 100 nm, have been considered a useful agent in both in vivo and in vitro experiments [8, 9]. It seems that nanoparticles can be absorbed easily through body barriers, thereby effectively targeting cells and molecules in various disorders [8]. Nanoparticles enhance the safety and efficiency of drugs, promoting their bioavailability and stability, providing targeted delivery, and increasing their effectiveness in the target tissue. They can be used to deliver a wide range of drugs to various organs, including liver, brain, spleen, lungs, and lymphatic system [10]. However, certain nanoparticles come into close contact with humans, and previous studies showed the mechanisms of action of these nanoparticles [11].
ZnO NP administration in NAFLD animal model led to significant reduction in fasting blood glucose, normalization of glucose tolerance, and improvement in insulin resistance [12]. As a result, previous studies provide substantial evidence for the involvement of ZnO NPs in reducing the development of hepatic steatosis through the activation of AMPK signaling [12]. While studies have reported that ZnO NPs can interact with various organs in the body, the liver is a primary target of ZnO NPs [8]. Several studies have reported beneficial effects of ZnO NPs, while others proved their toxic effects [8, 9, 13,14,15]. The antioxidant properties are dependent on and influenced by various factors, including surface properties, concentration, and size of ZnO NPs. Previous studies have demonstrated that ZnO NPs can mimic the activity of the catalase enzyme, which is responsible for the removal of hydrogen peroxide [16].
NPs in the commercial formulations or those used by some of the previous studies tend to agglomerate and accumulate in the target organs, inducing oxidative stress. Moreover, these types of NPs exhibit poor tissue clearance, thereby reducing their biological activity [17]. Indeed, synthetic NPs with appropriate physicochemical properties can increase their positive effects. In this experiment, we aimed to assess the toxicity of ZnO NP, its hepatoprotective effects, and underlying mechanism of action in normal and HFD-fed rats with regard to liver fibrosis, generation of oxidative stress, inflammasome activation, as well as apoptosis pathways.
Methods
Synthesis of ZnO NPs
To obtain pure nanoparticles, ZnO NPs were synthesized based on solvothermal method with minor modifications [18]. Briefly, zinc acetate (50 mM) and urea (150 mM) were dissolved in 160 mL of deionized water and ethylene glycol with a volume ratio of 1:1 and stirred for 30 min. The obtained solution was transferred to a 200-mL Teflon-lined autoclave and kept for 14 h at 180 °C. The produced ZnO NPs were allowed to cool naturally to room temperature and were then collected and washed several times with deionized water. After that, the ZnO NPs were separated by centrifugation, dried at 60 °C, and annealed at 450 °C for 2 h in air atmosphere.
Characterization of ZnO NPs
The morphology of synthesized ZnO NPs particles was evaluated using a field emission scanning electron microscopy (Nova NanoSEM 630, FEI Company, USA) equipped with energy-dispersive spectroscopy (EDS). Transmission electron microscopy (TEM) images were taken by a Tecnai F20 S/TEM operated at 200 kV and equipped with energy-dispersive X-ray spectroscopy. The X-ray diffraction (XRD) pattern was recorded by a Bruker D8 using Cu/Kα radiation to identify the crystal phase. An Autosorb-1, Quantachrome, USA, was used to measure the specific area of the ZnO NPs.
Surface Charge and Energy Band Gap
Zeta potential measurements and the point of zero charge (pHpzc) of catalysts were determined by using a ZetaPlus (Brookhaven Instrument Corp.) In this test, the surface charge and colloidal stability of ZnO NP in Milli-Q water were determined [19]. For calculation of ZnO band gap, the UV–vis diffuse reflectance absorption spectra (DRS) were recorded by a UV–vis spectrophotometer (UV3600, Shimadzu).The energy band gap (Ebg) of synthesized ZnO NPs was calculated using the formula, Ebg:1240/λ (eV), where Ebg and λ represent energy band gap in eV and cutoff wavelength in nanometer, respectively [19].
ZnO Dissolution
At different ZnO dosage, we assessed the equilibrium zinc concentrations in water by dissolving ZnO NPs in 25-mL volumes of water for 4 days at room temperature. After that, 2 mL aliquots were centrifuged, and the zinc concentration was determined according previous paper by using an atomic absorption spectrophotometer (PinAAcle 900 F, PerkinElmer, USA) [20].
Cell Culture
The human hepatoma cell line (HepG2) is usually used in vitro as an alternative to human hepatocytes for various investigations of liver action, drug toxicity, and metabolism. This cell line shows many of the biochemical, physiological, and structural features of normal hepatocytes [15]. Since HepG2 cells retain several of the features of normal liver cells, they were used in this experiment to evaluate whether ZnO NPs have hepatoprotective or toxic properties [15]. HepG2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 1% penicillin/streptomycin and 10% fetal bovine serum in a humidified incubator at 37 °C with 5% CO2. Cells were subcultured at 80% confluence and treated with various concentrations (1–60 µg/mL) for 24 h. After receiving ZnO NPs treatments, the HepG2 cells were lysed in the lysis buffer for the determination of lipid levels. The medium was also used for enzyme activity.
Cytotoxicity and Cell Viability
Cell viability was measured using the thiazolyl blue tetrazolium bromide (MTT) method. The viability was stated as a percentage relative to the control. The cytotoxicity properties of ZnO NPs on HepG2 cells were determined by measuring the lactate dehydrogenase (LDH) activity according to the commercial kit [21].
Lipid Accumulation in HepG2 Cells
The cells were seeded in 24-well plates at a density of 1.5 × 105 cells per well for 24 h and then treated with 250 µM palmitic acid (PA) and ZnO NPs at different concentrations. After treatment for 24 h, the cells were rinsed with PBS, fixed in 4% paraformaldehyde for 30 min, and then stained with 300 µL Oil Red O (0.7 g in 200 mL isopropanol) for 15 min and then 300 µL hematoxylin for 60 s. The cell images were observed under a light microscope at a magnification of ×400 following three rinses with 60% isopropanol (n=3). To take the quantitative measurements of lipids, 500 µL of isopropanol was added to every well of the stained culture plate. The extracted dye was instantly removed, and its absorbance was determined at 510 nm according to the previous published report [22].
Determination of Biochemical Factors
HepG2 cells at about 80% confluence were incubated for 24 h with 250 µM PA and ZnO NPs at various concentrations. After washed with PBS, the intracellular triglyceride (TG) and total cholesterol (TC) were measured using the colorimetric method according to previous published reports [22, 23]. To determine liver enzyme levels, the cells were seeded in a 24-well plate (1.5×105 cells/well) and incubated with different concentrations of ZnO NPs for 24 h at 37 °C. After that, the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using commercial kits (Pars Azmoon, Iran).
Animals
Experimental Design
Male Wistar rats (8 weeks old) weighing about 200 ± 10 g were purchased from Hamadan University of Medical Sciences (Hamadan, Iran). They were kept in standard condition at a temperature of 23 ± 2 °C, relative humidity of 65 ± 2%, and 12 h light/12 h dark cycle. After 10 days of acclimatization in the animal house, the rats were randomly divided into eight groups (n = 6–8) as follows:
-
Experiment 1(toxicity assessment): group 1: normal diet (ND), 2: ND + 5 mg/kg ZnO NPs, 3: ND + 10 mg/kg ZnO NPs, 4: ND + 25 mg/kg ZnO NPs, 5: ND + 50 mg/kg ZnO NPs, 6: ND + 100 mg/kg ZnO NPs. Rats received ZnO NPs every day for 4 weeks by oral gavage.
-
Experiment 2 (hepatoprotective assessment): Group 1: normal diet (ND), 2: high-fat diet (HFD), 3: HFD + 5 mg/kg ZnO NPs. Animals were treated with high fat diet (with 60 kcal% fat) for 8 weeks to induce nonalcoholic fatty liver disease model, then received ZnO NPs every day for 4 weeks by oral gavage according to previously published paper [24]. The normal chow diet (21% protein, 32.5% carbohydrate, 3.69% fat, and 5.5% crude fiber) and HFD (D12492, Research Diet, 20% protein, 20% carbohydrate, and 60% fat) were purchased from Royan Laboratory Animal Feeds (Isfahan, Iran).
Samples Preparation
At the end of the experiment, rats were anesthetized with ether after overnight fasting, and blood samples were collected from the hearts; serum was prepared by centrifugation at 2000 rpm for 15 min. A piece of liver, kidney, testis, brain, intestine, heart, and pancreas tissues were fixed in 10% formalin for 48 h and stained and were studied under a light microscope. A small portion of tissues also was rapidly frozen in liquid nitrogen and stored at −70 °C for biochemical analysis.
Determination of Toxicity
About 500 mg of different tissues was homogenized with lysis buffer (Sigma-Aldrich), and the supernatant was used to measure chemical factors. For the total antioxidant assay, 10 µL of BHT (0.5M in acetonitrile) was added to all samples to prevent the oxidative reaction. Total antioxidant capacity (TAC) and total oxidant status (TOS) were measured using the xylenol orange and ferric ion reducing antioxidant potential (FRAP) method, respectively [25]. The glutathione (GSH) levels and catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) activity were measured using commercially available ELISA kits (ZellBio, Ulm, Germany). Malondialdehyde (MDA) levels were also determined by thiobarbituric acid reactive substances (TBARS) [25].
Reactive oxygen species (ROS) levels in the liver tissues were measured by fluorometric assay using 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Briefly, liver was homogenized in saline solution and incubated in the presence of DCFH-DA at 37 °C for 60 min. The intensity of fluorescence was evaluated with excitation and emission wavelengths set at 485 and 530 nm, respectively [26].
Determination of Biochemical Factors
Serum levels of alanine aminotransferase (ALT), aspartate amino-transferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), urea, creatinine and uric acid, triglycerides (TG), and total cholesterol (TC) were determined with a commercial colorimetric assay kit (Pars Azmoon, Iran). The zinc levels were determined by an atomic absorption spectrophotometer (Varian, Spectron 220, Brunn amGebirge, Australia) according to previous paper [24].
Real-time qRT-PCR Assay
Total RNA was isolated from liver tissue using RNX-plus solution (Cinnagen, Tehran, Iran). The purity and concentration of extracted RNA were evaluated by agarose gel electrophoresis and Nano-Drop spectrophotometry, respectively. After that, cDNA synthesis was done using a Reverse Transcription Kit (Fermentas Life Sciences, Vilnius, Lithuania). Gene expression levels were determined by Real-Time PCR using a QuantiTect® SYBR® Green PCR Kit, as previously reported. Primers used in this experiment were used according to the previous papers [14, 27]. The calculated formulas were as follows: ΔCt = Target gene (Ct) − Internal control (Ct), ΔΔCt = Test gene ΔCt − Control ΔCt, expression= 2−(ΔΔCt).
TNF-α, IL-1β, and Nitric Oxide (NO) Levels
The TNF-α and IL-1β protein levels were determined using an ELISA kit according to the manufacturer’s protocols (Biolegend, UK). The level of NO was measured according to the Griess method with small modifications [28].
TUNEL Assay and Caspase 3 Activity
Cell deaths in the livers of different treatment groups were examined in the paraffin-embedded sections by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay using an in situ cell death detection kit (Roche Diagnostics, Germany). Caspase 3 activity in rat livers was determined using the ELISA kit according to the manufacturer’s instructions (Beijing, PR China).
Histological Procedures
To identify morphological changes, a small slice of different tissues (n = 4 rats per group) was removed and fixed in 10% formalin. After dehydration, samples were embedded in paraffin, and sections of 5-µm thickness were prepared. Brain and liver slides were stained with Nissl and Masson trichrome staining, respectively. All tissue slides also were stained with hematoxylin and eosin (H & E) and photographed by using a light microscopy. The quality of spermatogenesis was scored based on Johnson’s scoring method [29].
Statistical Analysis
Data were presented as mean ± SD. Statistical analysis and comparisons among various groups were done using one-way analysis of variance (ANOVA) and Tukey test, respectively. The significance levels were considered when p value was less than 0.05.
Results
In Vitro Tests
Nanoparticle Characterization
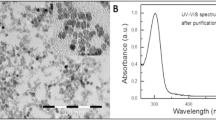
Fig. 1A shows the scanning electron microscope (SEM) images of pure ZnO NP. As illustrated, the synthesized ZnO NP consist of some irregular shapes with different sizes which were agglomerated in some parts. The X-ray spectroscopy (EDS) spectrum of the sample presented in Fig. 1B confirms the presence of Zn and O elements in the structure without impurities. The transmission electron microscope (TEM) images of ZnO NP are provided in Fig. 1C, indicating that the nanoparticles are about 30–35 nm in size and exhibiting spherical or polygonal morphology. The N2 adsorption-desorption isotherm of ZnO NP is presented in Fig. 1D. The XRD pattern of the ZnO NP shows narrow and sharp peaks, which confirms that the prepared sample was well crystallized. The peaks of ZnO NP are compatible with the hexagonal phase of ZnO (JCPDS 65-3411) [30]. The sharp peaks at 31.6°, 34.2°, and 36.3° correspond with the (100), (002), and (101) faces of ZnO, respectively (Fig. 1E). The isotherm was a typical IV-type, and the H3 hysteretic loop in the isotherms indicates the presence of mesoporous in the structure. The specific surface area of the ZnO sample was found to be 27.32 m2/g when measured by the BET method.
Characterization of synthetic ZnO NP. A Scanning electron microscopy (SEM) image of synthesized ZnO. B Energy-dispersive spectroscopy spectrum (EDS) of ZnO NP. C Transmission electron microscopy (TEM) images of ZnO NP. D N2 adsorption–desorption isotherms of ZnO NP.
E X-ray powder diffraction (XRD) patterns. F ZnO NP dissolution. Abbreviation: SEM scanning electron microscopy, EDS energy-dispersive spectroscopy spectrum, TEM transmission electron microscopy, XRD X-ray powder diffraction
The pH of zero point charge of ZnO NPs (pHZPC) is calculated as 8.7, and the energy bandgap ( Ebg) of ZnO NPs was found to be 3.1 eV. Our finding established that dissolution of ZnO NPs increased in dose-dependent manner (Fig. 1F).
Effect of ZnO NPs on the Viability of HepG2 Cells
The effect of different doses of ZnO NPs on HepG2 cells viability was assessed in this study. As depicted in Fig. 2A, only the doses of 20–60 µg/mL ZnO NPs reduced the cells’ viability 24 h after treatment.
Effect of ZnO NP on cell viability, liver enzymes, and lipid accumulation in HepG2 cells after 24 h. A ZnO NP at the concentration of 20–60 significantly reduced cell viability. B Treatment of HepG2 by ZnO NP at low doses normalized aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) after 24 h in HepG2 cells. C ZnO NP significantly reduced lipid accumulation in HepG2 cells after 24 h in a dose-dependent manner, as determined by Oil Red O staining. D Quantitative analysis of lipid accumulation in HepG2 cells. Values are expressed as mean ± SD (n = 3). ###p < 0.001 as compared to control. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to high fat model (PA-treated cells). Abbreviation: AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, PA palmitic acid, C control
Biochemical Factors in Animals and HepG2 Cells
The liver enzymes (AST, ALT) and lactate dehydrogenase (LDH) levels (as a marker of cell integrity and toxicity), were markedly elevated in the palmitate-treated HepG2 cells (Fig. 2B). Treatment by ZnO NPs at doses of 2.5, 5, 7.5, 10, 12.5, and 15 normalized these enzyme levels in HepG2 cells after 24 h.
Lipid Accumulation in HepG2 Cells
At a concentration of 250 µM, palmitic acid induced cellular lipid accumulation in HepG2 cells. Oil Red O staining revealed that the ZnO NPs treatment at the concentrations ranging from 5 to 15 µg/mL led to a dose-dependent reduction in intracellular lipid droplets within HepG2 cells, as compared to the palmitate-treated cells (p < 0.05, Fig. 2C, D).
Experiment 1
Toxicity Assessment
Oxidative Stress and Biochemical Factors
Administration of NPs at doses of 25, 50, and 100 mg/kg significantly elevated AST, ALT, ALP, and GGT levels. A dose of 10 mg/kg ZnO NP also increased ALT levels (Fig. 3A). Furthermore, MDA and TOS levels were markedly higher in the liver of ZnO NP-treated rats (10, 25, 50, and 100 mg/kg) compared to untreated animals, while GSH and TAC were significantly lower (p < 0.05).The activity of antioxidant enzyme, including SOD, catalase, and GPx, also significantly reduced in an animal treated with ZnO NPs except at the dosage of 5 mg/kg (p < 0.05) (Fig. 3B, C).
Effect of ZnO NP on liver function and structure in normal rats. A Effects of ZnO NP on serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT). B Effects of ZnO NP on liver total antioxidant capacity (TAC), total oxidant status (TOS) and malondialdehyde (MDA), and glutathione (GSH) levels. C Effects of ZnO NP on liver on superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase activity in animal models. D Hematoxylin and eosin (H & E) stained liver sections (original magnification 100 × and 400 ×). E Liver injury score. Values are presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to control or normal diet (ND) group (n = 6). 0: No damage; 1: Mild injury; 2: Moderate injury; 3: Severe injury. Scale bar: 20 µm. Abbreviation: AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, GGT gamma-glutamyl transferase, TAC total antioxidant capacity, TOS total oxidant status, MDA malondialdehyde, GSH glutathione, H & E hematoxylin and eosin. In histology picture: A: Control; B: C + 5 mg/kg NPs; C: C + 10 mg/kg NPs; D: C + 25 mg/kg NPs; E: C + 50 mg/kg NPs; F: C + 100 mg/kg NPs
Livers Histological Changes
Analysis of liver histopathological alterations (H & E) within the control group revealed normal structure. Conversely, the livers of the animals treated with ZnO NP at the doses of 25, 50, and 100 mg/kg showed hepatocellular necrosis, portal inflammation, ballooning degeneration, and leukocyte infiltration. However, treatment with 10 mg/kg ZnO NP induced mild changes in the liver, and administration of 5 mg/kg ZnO NP did not elicit any detrimental effects (Fig. 3D, E).
Experiment 2
Biochemical Factors and Lipid Accumulation in Animals
The administration of ZnO NP significantly normalized the levels of GGT, ALP, AST, and ALT in HFD-fed rats (Fig. 4A).
Effect of 5 mg/kg ZnO NP on chemical factors. A Effect of 5 mg/kg ZnO NP on serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT). B Serum levels of triglyceride (TG) and total cholesterol (TC). C Effects of ZnO NP on liver zinc levels in animal models. Treatment with ZnO NP significantly reduced liver enzymes and blood lipid in HFD-treated rats. Values are presented as mean ± SD. ##p < 0.01 and ###p < 0.001 as compared to control. *p < 0.05 and ***p < 0.001 as compared to HFD group. Abbreviation: AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, GGT gamma-glutamyl transferase, TG triglyceride, TC total cholesterol, ND normal diet, HFD high-fat diet, TG triglyceride, TC total cholesterol
Lipid and Zinc Levels in the Serum and Liver
Rats fed with HFD significantly exhibit a significant increase in TC and TG levels in comparison to control rats. Conversely, ZnO NP reduced TC and TG levels compared to HFD group (Fig. 4B). Zinc concentrations in the liver of various treated groups are presented in Fig. 4C.
Antioxidant Pathways
Antioxidant Genes Expression
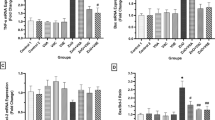
The expression of antioxidant genes, including SOD, catalase, and GPx, exhibited a significant decrease in HFD animals (p < 0.05). However, in animals treated with 5 mg/kg ZnO NPs, the expression of antioxidant genes was notably increased compared to the HFD group (p < 0.05, Fig. 5A).
Effect of ZnO NP on antioxidant enzyme gene expression and activity. A, B Treatment with 5 mg/kg ZnO NP significantly was increased superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase gene expression and activity in NAFLD animal models. C Effects of 5 mg/kg ZnO NP on liver total antioxidant capacity (TAC), total oxidant status (TOS), and malondialdehyde (MDA) levels. D Effects of 5 mg/kg ZnO NP on glutathione (GSH) levels. E Effects of 5 mg/kg ZnO NP on reactive oxygen species (ROS) levels. Values are presented as mean ± SD (n = 6). #p < 0.05 and ###p < 0.001 as compared to control. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to HFD group. Abbreviation: TAC total antioxidant capacity, TOS total oxidant status, MDA malondialdehyde, GSH glutathione, SOD superoxide dismutase, GPx glutathione peroxidase, GSH glutathione, ROS reactive oxygen species, ND normal diet, HFD high-fat diet
Antioxidant Activity and Oxidative Stress Markers
Based on our data, the activities of SOD, GPx, and CAT were significantly reduced (p < 0.001) in the liver of HFD rats compared to control rats. However, the administration of ZnO NP improved the activity of these antioxidant enzymes compared to the HFD group (p < 0.05, Fig. 5B).
TAC and GSH levels in the liver and serum were observed to be lower in HFD rats compared to normal animals, while MDA and TOS levels were higher (p < 0.001). In ZnO NP-treated rats, the levels of TAC and GSH increased, while MDA and TOS levels decreased compared to the HFD rats (p < 0.001, Fig. 5C, D).
The ROS levels significantly increased in HFD group when compared to controls (p < 0.001). However, in ZnO NP-treated rats, ROS levels were found to be reduced compared to untreated animals (p < 0.05, Fig. 5E).
Inflammatory and Inflammasome Pathway
NLRP3 Inflammasome
The expression of genes related to NLRP3 inflammasome pathway, including NLRP3, caspase-1, and IL-1β, exhibits a significant increase in HFD animals (p < 0.001). However, the administration of ZnO NP markedly normalized inflammasome pathways compared with the HFD animals (p < 0.05, Fig. 6A).
Effect of 5 mg/kg ZnO NP on NLRP3 inflammasome pathway. A Treatment with 5 mg/kg ZnO NP significantly reduced NLR Family Pyrin Domain Containing 3 (NLRP3), interleukin 1beta (IL-1β), and caspase-1 genes expression. B Effects of 5 mg/kg ZnO NP on IL-1β levels. C Treatment with 5 mg/kg ZnO NP significantly reduced iNOS and TNF-α genes expression in HFD rats. D NO and TNF-α levels reduced by ZnO NP in HFD rats. Values are presented as mean ± SD (n = 6). #p < 0.05, ##p < 0.01, and ###p < 0.001 as compared to control. *p < 0.05 and **p < 0.01 as compared to HFD group. Abbreviation: NLRP3 NLR Family Pyrin Domain Containing 3, IL-1β interleukin 1beta, ND normal diet, HFD high-fat diet, NLRP3 NLR Family Pyrin Domain Containing 3, IL-1β interleukin 1beta, TNFα tumor necrosis factor alpha, iNOS inducible nitric oxide synthase, NO nitric oxide
The level of IL-1β was markedly increased in HFD rats compared to normal rats (p < 0.001). Nevertheless, the administration of ZnO NP led to a significant reduction in IL-1β levels when compared to the HFD group (p < 0.001, Fig. 6B).
Inflammation
The gene expression of the inflammatory markers, including TNF-α, iNOS were significantly increased in HFD animals (p < 0.001). Nevertheless, the administration of ZnO NP notably normalized inflammatory genes expression compared with the HFD rats (p < 0.05, Fig. 6C). Both TNF-α and NO levels were significantly elevated in HFD animals in comparison to normal rats (p < 0.001). Nevertheless, treatment with ZnO NPs led to a significant reduction in both TNF-α and NO levels when compared to the HFD group (p < 0.001, Fig. 6D).
Apoptosis Pathways
Apoptosis Pathways Gene Expression
The expression of P53, Bax, and Bcl2 was significantly augmented in the liver of the HFD groups (p < 0.001). However, animals treated with ZnO NP showed a notable decrease in apoptosis gene expression compared with the HFD group (p < 0.05, Fig. 7A). These findings were further supported by the high cleaved caspase-3 levels in the livers of HFD-fed animals as compared to normal rats. Treatment with ZnO NP also led to reduction in cleaved caspase-3 activity (p < 0.05, Fig. 7B).
Effect of 5 mg/kg ZnO NP on liver apoptosis. Administration of ZnO NP significantly reduced apoptosis in HFD group. A Effect of 5 mg/kg ZnO NP on apoptosis genes expression (P53, Bax, Bcl2). B Effect of 5 mg/kg ZnO NP on caspase 3 activity in the liver. C Effect of 5 mg/kg ZnO NP on liver apoptosis determined by terminal deoxynucleotidyl nick-end labeling (TUNEL) staining. Counter staining was done using DAPI nuclear staining, and pictures were taken using DAPI (i, left column), fluorescein isothiocyanate (ii, apoptotic cells, middle column), and Merged (iii, right column) filters of liver (magnification, × 400). D Percentage of TUNEL-positive nuclei in liver. Values are presented as mean ± SD (n = 6). #p < 0.05 and ###p < 0.001 as compared to control. *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to HFD group (n = 6). Abbreviation: ND normal diet, HFD high-fat diet, TUNEL terminal deoxynucleotidyl transferase dUTP nick end labeling, DAPI 4′,6-diamidino-2-phenylindole, ND normal diet, HFD high-fat diet
TUNEL Assay
A number of TUNEL-positive apoptotic cells were significantly detected in the livers of the HFD group compared to the control rats (Fig. 7C, D). The administration of 5 mg/kg ZnO NP significantly reduced TUNEL-positive apoptotic cells in hepatocytes.
Livers Histological Changes
The livers of rats in the control group exhibited a normal structure, whereas the livers of the HFD group revealed hepatocellular necrosis, fatty changes, ballooning degeneration, portal inflammation, necrosis, and leukocyte infiltration (Fig. 8A, B). However, liver injury significantly normalized in animals that received ZnO NPs.
Histological changes of rat liver treated with 5 mg/kg ZnO NP. A Hematoxylin and eosin (H & E) stained and Masson trichrome stained (collagen and reticulum appear blue) liver sections (original magnification 400 ×). B Liver injury score. In both staining, liver showed potential injury (fatty changes, ballooning degeneration, portal inflammation, focal necrosis, and confluent necrosis), while treatment with ZnO NP normalized histological alteration. Values are presented as mean ± SD. ###p < 0.001 as compared to control. ***p < 0.001 as compared to HFD group. 0: No damage; 1: Mild injury; 2: Moderate injury; 3: Severe injury. Scale bar: 20 µm. Abbreviation: H & E hematoxylin and eosin, ND normal diet, HFD high fat diet
Discussion
The synthesized ZnO NPs in this study were found to be very pure with good scale homogeneity, 30–35 nm, and uniform morphology which prompt us to evaluate the ZnO NPs effects on the livers of normal and HFD-fed rats.
In general, zinc function as an antioxidant, preventing the activating of oxidative stress pathways through the protection of proteins sulfhydryl groups against oxidative damage and decreasing the formation of free radicals through protective mechanisms [7]. However, ZnO NPs with smaller sizes can be more easily absorbed by the body. ZnO NP is usually applied as a food additive and has shown potential antioxidant, anticancer, anti-inflammatory, and antidiabetic activities [31]. Furthermore, ZnO is graded as a generally recognized as safe (GRAS) agent by the US Food and Drug Administration (FDA) [31].
The results of toxicity assay involving different doses of ZnO NPs showed harmful effects on body for doses of 25 mg/kg, 50 mg/kg, and 100 mg/kg, with the exception of 5 mg/kg which demonstrated beneficial effects. As well documented, the treatment of rats with ZnO NPs at doses ranging from 200 to 600 mg/kg caused significant detrimental effects on inflammatory markers, liver enzymes, oxidative stress, hematologic parameter, and histologic characteristics of liver [32,33,34]. These finding has recently been proved by Tang et al. who reported that ZnO NPs at high doses (above 10 mg/kg) are toxic and have adverse effects on the body [8]. On the other hand, previous study highlighted that oral administration of ZnO NP at 5 mg/kg seems to exert anti-inflammatory, antioxidant, and antidiabetic effects in rats [35]. Similarly, when considering the administration of different doses of ZnO NPs (5, 10, and 15 mg/kg) in fish, the dose of 5 mg/kg possessed the greatest antioxidant activity [36].
Hussein et al. showed that ZnO NPs oral administration in diabetic rats at the doses of 5 and 10 mg/kg for 1 month yields similar antidiabetic effects [37]. Hence, based on our first experiment that showed toxicity of high dosage, it can be argued that high dose of ZnO NPs might reduce its efficiency by increasing its toxicity which leads us to choose ZnO NPs at the dose of 5 mg/kg to carry out experiment 2 (treatment of HFD rats). The higher dose used in this study was considerably lower than what is expected in human body daily ingestion [38]. Indeed, ZnO dissolution within the lysosomes is responsible for injuries of the lysosomal membrane, and it is released into the cell, causing damages to organelles and ultimately leading to cell death.
Our results demonstrated an increase in the dissolution of ZnO NPs in a dose-dependent manner. Previous studies have also reported that ZnO dissolution is heightened by increasing of concentration, under acidic pH condition, and in the existence of biological components such as peptides and amino acids [39, 40]. In fact, the toxicity of Zn depends on the concentration of free ions. There are numerous papers describing the harmful effect of Zn at high concentrations, which include the changes in mitochondrial membrane stability and permeability, oxidative stress generation, and activation of apoptosis [36, 41]. ROS production upon nanoparticle treatment strongly depends on its energy band gap. Reducing the band gap increase the photocatalytic activity, consequently increasing ROS production in biological situation. Therefore, the nanoparticle with increased photocatalytic activity exhibits more ROS-dependent cytotoxicity. Hence, change of the ZnO NP band gap can alter the ROS-mediated cytotoxicity. In this experiment, ZnO NP had wide band gap (indicating low energy donor capacity), resulting in low photocatalytic activity and low ROS-dependent cytotoxicity. Therefore, ZnO NP can act as novel photosensitizers and enhance the effects of conventional photosensitizing drugs in photodynamic therapy for cancer, as reported in various studies [42, 43]. Furthermore, the tendency of nanoparticle to form aggregates depends on the surface charge. Z-potentials results proposed that this particle is relatively stable in an aqueous condition [43]. Accordingly, the oxidative responses mostly depend on factors such as dosage, size, physicochemical properties, and duration of exposure to ZnO NPs [44]. Our study showed that treatment of animals with different doses of ZnO NP did not cause any significant change in body and organs weight (data not shown).
Our results are in agreement with previous reports that demonstrated ZnO NPs significantly reduced TG and TC in animal model [45,46,47,48,49]. Zinc plays a vital role in metabolic diseases by regulating oxidative stress, inflammation, and lipid metabolism. Previous research has indicated that ZnO NPs significantly reduce adipose tissue weight, body mass index (BMI), and food intake in obese rats. Moreover, zinc promotes the biosynthesis of serotonin, inducing satiety and decreasing food intake. Furthermore, ZnO NPs can affect the leptin and adiponectin secretions, which consequently affect lipid levels [45, 50].
It has been reported that ZnO NPs increase the expression of carnitine palmitoyltransferase (CPT1), enhancing fatty acid oxidation. Additionally, zinc reduces the activities of lipogenic enzymes such as 6-phosphogluconate (6PGD), malic enzyme (ME) and isocitrate dehydrogenase (ICDH), and fatty acid synthase (FAS) [51]. Wei reported that zinc reduces hepatic lipid deposition via calcium/calmodulin-dependent protein kinase kinase-β (Ca2+/CaMKKβ)/AMPK pathway [52].
In this experiment, ZnO NP also significantly reduced lipid accumulation in HepG2 cells. HepG2 cells is used for various experiments due to their exhibition of most phenotypic and genotypic characteristics of normal liver cells. This type of cell also present numerous normal functions of human liver cells. HepG2 show various physiological and biochemical and structural features of normal hepatocytes. These cells are generally used as an alternative to human hepatocytes in diverse experiments related to liver function, metabolism, drug studies, and drug toxicity. Additionally, these cells are also used for drug screening to evaluate the safety and efficacy of possible drug candidates. Since these cell lines retain several features of normal liver cells, we used HepG2 to survey the effects of ZnO NPs [53].
In the same trend, previous studies have reported that the use of HFD in rats for a short period (4 weeks) caused liver steatosis and for longer period (8–12 weeks) led to steatosis accompanied by mild inflammation [54]. In agreement with previous study [55], our results show that HFD (60 kcal%) induced liver steatosis, inflammation, oxidative stress, and lipid peroxidation (LPO) after an 8-week duration. Increased liver oxidative stress can lead to the formation of MDA and subsequently result in extensive liver damage. It has been reported that elevated intracellular MDAconcentration and oxidative DNAdamage are associated with the stage of necro-inflammatory alterations seen in steatosis among NASH patients [56]. Supportive evidence of clinical studies has revealed that patients with high levels of MDAand TOS and low TAClevels are prone to experiencing liver damage, type 2 diabetes (T2D), atherosclerosis, and other metabolic disorders [57]. Increased levels of enzymes may contribute to hepatic oxidative stress and inflammation in rats treated with high dose ZnO NP [54]. Regarding these findings, administration of 5 mg/kg ZnO NPs appears to normalize TAC, TOS, MDAlevels and an improvement of antioxidant capacity in various tissues, all without inducing side effects on biochemical markers and histological alterations. Conversely, treatment with the high doses of ZnO NPs increased liver enzymes, biochemical markers and induced oxidative stress.
These observations were along with the findings of Atef et al. who showed an increase in oxidative stress and tissue damage following exposure to high doses of ZnO NPs, in contrast to the beneficial effects observed with low doses [58]. Furthermore, Abdel-Daim et al. reported that high doses of ZnO increase in MDA and reduce GSH SOD and catalase activities in fish [59]. Zinc serves as a cofactor for various antioxidant systems and has a pivotal role in stability of protein and biomembranes, aiding in the equilibrium between scavenging and production of reactive oxygen species (ROS) due to its existence in SOD enzyme structure. It appears that significant increases were observed in the SOD gene expression and its activity in the liver tissue of animal treated with only 5 mg/kg ZnO NPs. Zhang et al. reported that high doses of ZnO NPs significantly reduced antioxidant activity in different organs. It has also been reported that SOD activity is reduced in the presence of excess zinc [60].
In our study low-dose ZnO NPs normalized CAT, GPx, and GSHcontents in HFD fed animals. Previous reports have indicated that the treating of diabetic rats with 10 mg/kg ZnO NPs reduced MDAlevels, increased GSHcontents, and enhanced the activity of antioxidant enzymes and gene expression of SOD, CAT, GPx, and GSHin the testis [14]. These findings suggest that high ZnO NPs concentration might not contribute to biological function [9]. The influence of NPs may be related to particle dosage and size. Whereas low concentrations of ZnO NPs have not shown toxic effects in vivo, high doses can lead to cell death [8].
Treatment of rats with 5 mg/kg ZnO NPs normalized blood factors, increased the activity of antioxidant enzymes, and reduced the inflammatory and inflammasome pathways, as well as alleviated liver apoptosis in HFD-fed rats. Moreover, it normalized pathological changes in the liver of HFD group. In agreement with previous reports demonstrating the beneficial effects of ZnO NPs in various diseases, [36, 37], we propose that low dose of ZnO NPs can ameliorate the oxidative stress, inflammation, and metabolic abnormalities in NAFLD model.
Hepatic apoptosis is induced by several signaling pathways and cell membrane death receptor cascades, including ROS production, endoplasmic reticulum (ER) stress, and mitochondria dysfunction. Apoptotic death is a main feature of nonalcoholic steatohepatitis and is often accompanied by fibrosis [54]. Zinc is required for the protection of cells against various pro-apoptotic molecules and can suppress apoptosis [61]. However, zinc deficiency may also be involved in the activation of ROS-induced intrinsic apoptotic pathway. Interestingly, in this experiment zinc nanoparticle significantly inhibits the intrinsic apoptotic pathway in the liver [62].
We observed a significant increase in the expression of pro-apoptotic protein p53 and Bax, alongside a reduction of anti-apoptotic Bcl-2 in the HFD diet compared with the control rats. The administration of 5 mg/kg ZnO NPs notably normalized p53, Bax, and Bcl-2 levels in the HFD group, initiating a cascade of events that led to the inactivation of caspase-3, and finally inhibited hepatocyte death. As a results, treatment with 5 mg/kg ZnO NPs markedly decreased the number of TUNEL-positive cells and caspase-3 activity in the HFD group. Several findings strongly support the notion that increases in oxidative stress and inflammation in NAFLD stimulated p53 and Bax expression while suppressing Bcl-2 [63]. Another investigation suggested that zinc nanoparticle reduced apoptosis, which could be due to a reduction in the number of TUNEL-positive cells and caspase activities [64].
As reported in this study, HFD stimulated liver apoptosis and inflammation which is associated with a notable increase in liver oxidative stress, TNF-α, and iNOS gene expression. This suggests that elevated apoptosis could potentially contribute to liver inflammation and damage [4]. Moreover, lipid accumulation in the liver can sensitize hepatocytes to Fas and TNF-α-induced apoptosis, leading to the development of liver steatosis [65].
TNF-α expression considerably increased in patients with steatosis and plays a significant role in progression of liver steatosis [63]. In animals fed a HFD diet, TNF-α stimulated the release of cytochrome c, activates caspase 3, and triggers ROS production [65]. Furthermore, the expression of iNOS was increased in hepatic cells in pathological situations. The induction iNOS is associated with the development of liver fibrosis, making it a therapeutic target for this condition [66]. The current study showed that the administration of 5 mg/kg ZnO NPs normalized the gene expression of TNF-α and iNOS, along with the levels of TNF-α and NO in the liver of HFD-fed rats. In agreement with these results, Mohammed et al. showed that ZnO NPs has anti-inflammatory activity by reducing the expression of nuclear factor kappa B (NF-κB) and TNF-α. They also reported that ZnO NPs have potential antioxidant activity[67].
It has been documented that that inflammasome activation is involved in the development of NAFLD and NASH [3, 68]. According to our results, the inflammasome pathway exhibited a significant increase in the HFD group. Existing literature indicates that NLRP3 inflammasome, caspase-1 activity, and serum IL-1β were significantly elevated in animal models of NASH induced by HFD [3]. Treating the HFD-fed rats with 5 mg/kg ZnO NPs normalized NLPR3 inflammasome pathway by reducing the levels of NLRP3, caspase-1, and IL-1β. These findings suggest that ZnO NPs can prevent inflammatory cell infiltration, proinflammatory cytokine expression, and fat accumulation [69]. Finally, morphological changes in the liver provide further evidence of ant-inflammatory, antioxidant, and anti-apoptotic effects of ZnO NPs. The regeneration properties observed in the liver of HFD-fed rats treated with ZnO NPs emphasize the advantageous impacts of this compound in the context of the NAFLD model.
Conclusions
The administration of ZnO NPs (5 mg/kg) demonstrated liver protection against damage induced by a HFD through different pathways, including (1) scavenging free radicals and/or increased antioxidant enzyme activity; (2) reducing inflammation and NLRP3 inflammasome; (3) normalizing hepatocyte apoptosis; (4) reducing liver fat accumulation; and (5) normalizing biochemical factors and histopathological parameters. The findings from this investigation suggest that low doses of ZnO NPs show potential hepatoprotective effects. However, considering the limitations of this study, further experiments are necessary to reveal the hypolipidemic and hypercholesterolemic mechanisms of ZnO NPs. Another limitation of this study is the need for additional experiments to evaluate the protein levels of apoptosis pathways.
Data Availability
No datasets were generated or analysed during the current study.
References
Ong JP, Younossi ZM (2007) Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 11(1):1–16
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M et al (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15(1):11–20
Wan X, Xu C, Yu C, Li Y (2016) Role of NLRP3 inflammasome in the progression of NAFLD to NASH. Can J Gastroenterol Hepatol 2016:6489012
Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD (2008) Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr 138(10):1866–1871
Szabo G, Petrasek J (2015) Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12(7):387–400
Summersgill H, England H, Lopez-Castejon G, Lawrence CB, Luheshi NM, Pahle J et al (2014) Zinc depletion regulates the processing and secretion of IL-1beta. Cell Death Dis 5:e1040
Clegg MS, Hanna LA, Niles BJ, Momma TY, Keen CL (2005) Zinc deficiency-induced cell death. IUBMB Life 57(10):661–669
Tang HQ, Xu M, Rong Q, Jin RW, Liu QJ, Li YL (2016) The effect of ZnO nanoparticles on liver function in rats. Int J Nanomedicine 11:4275–4285
Zhao CY, Tan SX, Xiao XY, Qiu XS, Pan JQ, Tang ZX (2014) Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res 160(3):361–367
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B et al (2020) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res Int 27(16):19151–19168
Mohammed ET, Safwat GM (2020) Grape seed proanthocyanidin extract mitigates titanium dioxide nanoparticle (TiO(2)-NPs)-induced hepatotoxicity through TLR-4/NF-κB signaling pathway. Biol Trace Elem Res 196(2):579–589
Dogra S, Kar AK, Girdhar K, Daniel PV, Chatterjee S, Choubey A et al (2019) Zinc oxide nanoparticles attenuate hepatic steatosis development in high-fat-diet fed mice through activated AMPK signaling axis. Nanomedicine 17:210–222
Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW et al (2006) Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol Lett 161(2):115–123
Afifi M, Almaghrabi OA, Kadasa NM (2015) Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int 2015:153573
Syama S, Reshma S, Sreekanth P, Varma H, Mohanan P (2013) Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Environ Toxicol Chem 95(3):495–503
Rawashdeh RY, Harb AM, AlHasan AM (2020) Biological interaction levels of zinc oxide nanoparticles; lettuce seeds as case study. Heliyon 6(5):e03983
Heckman KL, DeCoteau W, Estevez A, Reed KJ, Costanzo W, Sanford D et al (2013) Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 7(12):10582–10596
Lv H, Ji G, Yang Z, Liu Y, Zhang X, Liu W et al (2015) Enhancement photocatalytic activity of the graphite-like C 3 N 4 coated hollow pencil-like ZnO. J Colloid Interface Sci 450:381–387
Mirzaei A, Chen Z, Haghighat F, Yerushalmi L (2017) Enhanced adsorption of anionic dyes by surface fluorination of zinc oxide: a straightforward method for numerical solving of the ideal adsorbed solution theory (IAST). J Chem Eng 330:407–418
Mirzaei A, Chen Z, Haghighat F, Yerushalmi L (2018) Hierarchical magnetic petal-like Fe 3 O 4-ZnO@ gC 3 N 4 for removal of sulfamethoxazole, suppression of photocorrosion, by-products identification and toxicity assessment. Chemosphere 205:463–474
Salla S, Sunkara R, Walker LT, Verghese M (2016) Antioxidant and apoptotic activity of papaya peel extracts in HepG2 cells. Food Sci Nutr 7(06):485
Forbes-Hernandez TY, Giampieri F, Gasparrini M, Afrin S, Mazzoni L, Cordero MD et al (2017) Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients 9(6):621
Wan Y, Liu LY, Hong ZF, Peng J (2014) Ethanol extract of Cirsium japonicum attenuates hepatic lipid accumulation via AMPK activation in human HepG2 cells. Exp Ther Med 8(1):79–84
Abbasi-Oshaghi E, Mirzaei F, Mirzaei A (2018) Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine (Lond) 13(21):2791–2816
Mohammadi A, Mirzaei F, Jamshidi M, Yari R, Pak S, Sorkhani AN et al (2013) The in vivo biochemical and oxidative changes by ethanol and opium consumption in Syrian hamsters. Int J Biol 5(4):14
Lima FD, Stamm DN, Della-Pace ID, Dobrachinski F, de Carvalho NR, Royes LF et al (2013) Swimming training induces liver mitochondrial adaptations to oxidative stress in rats submitted to repeated exhaustive swimming bouts. PLoS ONE 8(2):e55668
Abbasi-Oshaghi E, Mirzaei F, Pourjafar M (2019) NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: in vivo and in vitro study. Int J Nanomedicine 14:1919–1936
Tsikas D (2007) Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci 851(1–2):51–70
Johnsen SG (1970) Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1(1):2–25
Tian J, Wang J, Dai J, Wang X, Yin Y (2009) N-doped TiO2/ZnO composite powder and its photocatalytic performance for degradation of methyl orange. Surf Coat Technol 204(5):723–730
Jiang J, Pi J, Cai J (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl 2018:1062562
Hong JS, Park MK, Kim MS, Lim JH, Park GJ, Maeng EH et al (2014) Prenatal development toxicity study of zinc oxide nanoparticles in rats. Int J Nanomedicine 9(Suppl 2):159–171
Rajput VD, Minkina TM, Behal A, Sushkova SN, Mandzhieva S, Singh R et al (2017) Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: a review. Environ Nanotechnol Monit Manag 9:76–84
Hong JS, Park MK, Kim MS, Lim JH, Park GJ, Maeng EH et al (2014) Effect of zinc oxide nanoparticles on dams and embryo-fetal development in rats. Int J Nanomedicine 9(Suppl 2):145–157
Li J, Chen H, Wang B, Cai C, Yang X, Chai Z et al (2017) ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci Rep 7:43126
Taheri S, Banaee M, Haghi BN, Mohiseni M (2017) Effects of dietary supplementation of zinc oxide nanoparticles on some biochemical biomarkers in common carp (Cyprinus carpio). Int J Aquat Biol 5(5):286–294
Hussein SA, EL-Senosi YA, El-Dawy K, Baz HA (2014) Protective effect of zinc oxide nanoparticles on oxidative stress in experimental-induced diabetes in rats. Benha med J 27(2):405–14
Bohmert L, Laux P, Luch A, Braeuning A, Lampen A (2017) Nanomaterials in foodstuffs—toxicological properties and risk assessment. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 60(7):722–727
Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H et al (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2(10):2121–2134
Kao YY, Chen YC, Cheng TJ, Chiung YM, Liu PS (2012) Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol Sci 125(2):462–472
El-Maddawy Z, El Naby WA (2019) Protective effects of zinc oxide nanoparticles against doxorubicin induced testicular toxicity and DNA damage in male rats. Toxicol Res (Camb) 8(5):654–662
Arakha M, Roy J, Nayak PS, Mallick B, Jha S (2017) Zinc oxide nanoparticle energy band gap reduction triggers the oxidative stress resulting into autophagy-mediated apoptotic cell death. Free Radic Biol Med 110:42–53
Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, Alrokayan SA (2012) Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine 7:845–857
Zhang J, Song W, Guo J, Zhang J, Sun Z, Ding F et al (2012) Toxic effect of different ZnO particles on mouse alveolar macrophages. J Hazard Mater 219–220:148–155
Reda FM, El-Saadony MT, El-Rayes TK, Attia AI, El-Sayed SAA, Ahmed SYA et al (2021) Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital J Anim Sci 20(1):324–335
John AD, Ragavee A, Selvaraj AD (2020) Protective role of biosynthesised zinc oxide nanoparticles on pancreatic beta cells: an in vitro and in vivo approach. IET Nanobiotechnol 14(9):756–760
Umrani RD, Paknikar KM (2014) Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine (Lond) 9(1):89–104
Rahman HS, Othman HH, Abdullah R, Edin H, Al-Haj NA (2022) Beneficial and toxicological aspects of zinc oxide nanoparticles in animals. Vet Med Sci 8(4):1769–1779
Amiri A, Dehkordi RAF, Heidarnejad MS, Dehkordi MJ (2018) Effect of the zinc oxide nanoparticles and thiamine for the management of diabetes in alloxan-induced mice: a stereological and biochemical study. Biol Trace Elem Res 181(2):258–264
Bashandy SAE, El-Seidy AMA, Ibrahim FAA, Abdelrahman SS, Abdelmottaleb Moussa SA, ElBaset MA (2023) Zinc nanoparticles ameliorated obesity-induced cardiovascular disease: role of metabolic syndrome and iron overload. Sci Rep 13(1):16010
Gadoa ZA, Moustafa AH, El Rayes SM (2022) Zinc oxide nanoparticles and synthesized pyrazolopyrimidine alleviate diabetic effects in rats induced by type II diabetes. ACS Omega 7(41):36865–36872
Wei CC, Luo Z, Hogstrand C, Xu YH, Wu LX, Chen GH, et al. (2018) Zinc reduces hepatic lipid deposition and activates lipophagy via Zn(2+)/MTF-1/PPARα and Ca(2+)/CaMKKβ/AMPK pathways. FASEB J 18:fj201800463
Sassa S, Sugita O, Galbraith RA, Kappas A (1987) Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun 143(1):52–57
Guicciardi ME, Malhi H, Mott JL, Gores GJ (2013) Apoptosis and necrosis in the liver. Compr Physiol 3(2):977–1010
Liu C, Liu L, Zhu HD, Sheng JQ, Wu XL, He XX et al (2018) Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux. Sci Rep 8(1):4108
Seki S, Kitada T, Sakaguchi H (2005) Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res 33(2):132–134
Balkan J, Doğru-Abbasoğlu S, Aykaç-Toker G, Uysal M (2004) The effect of a high cholesterol diet on lipids and oxidative stress in plasma, liver and aorta of rabbits and rats. Nutr Res 24(3):229–234
Atef HA, Mansour MK, Ibrahim EM, El-Ahl RMS, Al-Kalamawey NM, El Kattan Y et al (2016) Efficacy of zinc oxide nanoparticles and curcumin in amelioration the toxic effects in aflatoxicated rabbits. Int J Curr Microbiol App Sci 5(12):795–818
Abdel-Daim MM, Eissa IAM, Abdeen A, Abdel-Latif HMR, Ismail M, Dawood MAO et al (2019) Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol 69:44–50
Burman U, Saini M, Kumar P (2013) Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem 95(4):605–612
Chimienti F, Aouffen M, Favier A, Seve M (2003) Zinc homeostasis-regulating proteins: new drug targets for triggering cell fate. Curr Drug Targets 4(4):323–338
Sun Q, Zhong W, Zhang W, Li Q, Sun X, Tan X et al (2015) Zinc deficiency mediates alcohol-induced apoptotic cell death in the liver of rats through activating ER and mitochondrial cell death pathways. Am J Physiol Gastrointest Liver Physiol 308(9):G757–G766
Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D (2006) Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol 12(38):6198–6202
Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ (2008) Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 233(5):540–548
Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A et al (2006) Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab 4(3):185–198
Iwakiri Y (2015) Nitric oxide in liver fibrosis: the role of inducible nitric oxide synthase. Clin Mol Hepatol 21(4):319–325
Mohammed ET, Safwat GM (2023) Zinc oxide nanoparticles and vitamin C ameliorate atrazine-induced hepatic apoptosis in rat via CYP450s/ROS pathway and immunomodulation. Biol Trace Elem Res 201(11):5257–5271
Szabo G, Csak T (2012) Inflammasomes in liver diseases. J Hepatol 57(3):642–654
Olechnowicz J, Tinkov A, Skalny A, Suliburska J (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. Eur J Nutr 68(1):19–31
Funding
This study was funded by the Hamadan University of Medical Sciences (No: 9805193015).
Author information
Authors and Affiliations
Contributions
F.M, E.A, A.M., N.F.H, and N.N conducted and analyzed laboratory tests. F.M. and E.A. interpreted the data. F.M wrote the manuscript. I.K., C.J. and N.M. were project supervisors. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All processes in this experiment were approved by the Institutional Animal Care and Use Committee (IACUC), Hamadan Medical University (Ethics code; IR.UMSHA.REC.1396.832). All experiments were performed in accordance with relevant guidelines and regulations.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirzaei, F., Abbasi, E., Mirzaei, A. et al. Toxicity and Hepatoprotective Effects of ZnO Nanoparticles on Normal and High-Fat Diet-Fed Rat Livers: Mechanism of Action. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04108-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04108-5