Abstract
Mushrooms are a good source of protein and phenolic compounds which provides health benefits for humans. The purpose of this study was to compare the content of eight metals, protein, and total phenolics (TPC) of 5 different species (Agaricus bisporus—white and brown mushrooms, Agaricus cupreobrunneus, Auricularia cornea, Hypsizgus tesselatus, and Pleurotus eryngii species-complex) of edible mushrooms available on the Romanian market. Agaricus bisporus and Agaricus cupreobrunneus were purchased and cultivated in Romania and the other species were cultivated in other countries (Turkey and China). The metal content determined by graphite atomic absorption spectrometry (GTAAS) varied in the order Cu > Pb > Ni > Fe > Cr > Mn > Co > Cd. Almost all the samples contained a greater quantity of metals in the stalk than in the cap. In addition, the levels of toxic metals were low. The protein content of analyzed samples ranged from 0.0926 to 0.2743%, the highest value being observed in Pleurotus eryngii species-complex mushroom. TPC of extracts increased over time but there was a variability in the concentration for each mushroom species (0.25–12.25 mg GAE/g). The investigated mushroom species possess no health risk and may be potential nutritional supplements for human diets due to their phenolic compounds, protein, and mineral content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mushrooms are a valuable raw material, both for consumers and for the food industry. They are among the oldest fungi species on earth. Over the years, humans have always shown an interest in edible mushrooms. Due to the special qualities of aroma, nutritional value, and taste, mushrooms occupy an increasingly important place in the food group today [1,2,3]. Mushrooms are used in food, medicine, and industry. In medicine, they are used as a remedy for respiratory diseases, poor circulation, liver problems, heart diseases, etc. [4]. In industry, mushrooms can be used to dye wool and other natural fibers. Mushroom chromophores are organic compounds and produce strong and vivid colors. Mushrooms were the source of textile dyes until the invention of dyes.
Edible mushrooms have a very complex chemical composition and offer humans an important source of food since they can replace vegetables in periods when they are missing. Sometimes they complement meat as well, by providing a remarkable source of proteins like those in meat. Indeed, the expression “vegetable meat” is often used when talking about mushrooms. They contain glycogen, which is a polysaccharide found only in meat. They can accumulate and synthesize large amounts of B vitamins [5, 6]. Considering the mineral elements, mushrooms are very rich in phosphorus, iron, potassium, zinc, sodium, and magnesium. Since fungi are agents that help decompose organic matter and play an important role in natural changes, they have a very efficient mechanism for accumulating heavy metals from the environment [7].
Heavy metal accumulation in macrofungi has been found to be affected by environmental and fungal factors. Environmental factors such as amount of organic matter, pH, soil metal concentrations, and fungal factors such as fungal species, morphological part of the fruiting body, developmental stages, mycelial age, biochemical composition, and the interval between fruiting bodies affects the accumulation of metals in macrofungi [8, 9].
The determination of metals in mushrooms was carried out by several methods, the most common being atomic absorption spectrometry using microwave digestion for the mineralization of samples (for Cu, Cd, Cr, Ni, Mn, Fe, Zn, Pb because it is more precise and shorter than wet digestion) [10]. A problem of conventional digestion includes the formation of oxides or elements that block the silica particles in the sample, during drying-combustion digestion, the ions form oxides that are difficult to dissolve. Microwave digestion seems to be a safe and fast method for the mineralization of mushroom samples for the determination, by atomic absorption spectrometry in the flame, of the 5 elements: Fe, Mn, Zn, Co, Cd [11]. The concentrations of heavy metals in fungi vary depending on the organic matter content of the ecosystem and the soil [12].
Among recently published papers, the heavy metals concentrations (chromium, cadmium, manganese, iron, lead, zinc, copper, nickel, and mercury) have been studied and the results obtained proved that the consumption of mushrooms may result in a significant exceeding of the consumption limits of cadmium, copper, and Cs-137 [2]. In another study in which the concentrations of metals (Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, and Pb) in Nigerian mushrooms were studied using AAS (atomic absorption spectrometry) after mineralization with acid, it was observe that the mushrooms did not bioaccumulate the metals [1].
Metals including Cu, Cr, Mn, Co, Ni, Pb, Cd, and Fe have been acknowledged as one of the most dangerous types of pollutants and there are a lot of studies about the ability to accumulate metals by the fruiting bodies of mushrooms [13,14,15,16].
The aim of this study was to evaluate the content of eight metals (Cu, Cr, Mn, Co, Ni, Pb, Cd, Fe), protein and total phenolics (TPC) of 5 different species (Agaricus bisporus—white and brown mushrooms, Agaricus cupreobrunneus, Auricularia cornea, Hypsizgus tesselatus, and Pleurotus eryngii species-complex) of edible mushrooms available on the Romanian market. The metal content was determined by graphite atomic absorption spectrometry (GTAAS) after microwave digestion. TPC was determined by a modified version of the Folin–Ciocalteu method and protein content was estimated by the Kjeldahl method.
Materials and Methods
Samples
Edible mushroom corresponding to 5 different species (Agaricus bisporus—white and brown mushrooms, Agaricus cupreobrunneus, Auricularia cornea, Hypsizgus tesselatus, and Pleurotus eryngii species-complex) were purchased from the local market, Constanta, Romania, where each one of them were packaged and labeled according to their delivery site. Also, the species were authenticated using relevant literature after a short description of the studied samples. The cap of the Agaricus bisporus-white mushroom is a pale gray-brown, with broad, flat scales on a paler background and fading toward the margins. The narrow, crowded gills are free and dark brown. Agaricus bisporus-brown mushroom is like the white mushroom in shape and size but has a firmer texture. The color ranges from light tan to dark brown. Agaricus cupreobrunneus cap is 3–10 cm wide with flattened reddish-brown fibrils. The white stalk is 2–7 cm tall and 1–2 cm wide. The spores are dark brown, elliptical, and smooth. Auricularia cornea is a fungus that looks like a delicate brown ear. Hypsizgus tesselatus is a variety of mushrooms with small round caps and long, slender stems that grow from an interconnected base. Pleurotus eryngii species-complex have bright white stalks and tan or brown caps and they grow with thick, meaty stems.
The mushroom samples were divided into cap and stalk. These parts were washed with tap water and then with deionized water to remove any soil traces.
TPC Determination
Ten grams of fresh mushroom samples was macerated and kept resting after mixing with ethanol 95% (100 mL) for a week in brown recipients. According to the literature, maceration without stirring produces extracts with higher total phenols concentration [17]. The mixtures filtered through paper (Whatman No.1) were collected in glass recipients and stored at 4 °C.
The total phenolic content of mushroom samples was determined by a modified version of the Folin–Ciocalteu method [18]. In brief, mushroom extracts were mixed with 1 mL of 1:2 (v/v) Folin–Ciocalteu reagent (Merck, Darmstadt, Germany) in 50-mL calibrated flasks and 1 mL ethanol, 1 mL sodium carbonate solution 20% (Redox, Romania). The resulting mixture was maintained at room temperature for 30 min and the absorbance was measured at 675 nm against the corresponding blank, with an UV-VIS spectrophotometer (Jasco 550). The TPC concentration was calculated by the calibration curve (R2 = 0.9913) and expressed as mg gallic acid equivalents per gram of extract (mg GAE/g) witch gallic acid was purchased from Fluka (Buchs, Switzerland).
Protein Content Determination
The protein content in the mushroom samples was estimated by the Kjeldahl method, according to which the samples were digested with 20 mL of concentrated sulfuric acid (Digestion Unit, TURBO THERM, Germany). The digested material was distilled after the addition of alkali. The released ammonia was collected in 4% boric acid in the VAPODENS 20 Distilling Unit (Germany). The resultant was then titrated with 0.1 N HCl. The determined nitrogen content was multiplied by a conversion factor of 6.25 [19].
Determination of Pb, Cd, Cr, Cu, Fe, Mn, Co, and Ni by Graphite Atomic Absorption Spectrometry
The mushroom samples were digested and investigated for metal content using the system described in [20]. Briefly, mass of 0.3-g dried and powdered mushrooms sample were digested was accurately weighed into a PTFE vessel ans solution consisting of 8 ml of HNO3 65% (Merck, Darmstadt, Germany) was used in the digestion system (Berghof Speedwave®, ENTRY, Germany).
Graphite atomic absorption spectrometry (GTAAS, model: Contra 800, Analytica Jena Instruments, Germany) was used to determine the metals concentrations in the digested solution made up to 50 mL. The performance parameters of the analytical method are presented in a previous paper [21].
A multi-element standard (ICP multi-element standard solution IV, Merck, Germany) was used for the preparation of intermediate solutions to obtain calibration curves. Deionized water (Direct Q UV, Millipore, approximately 18.0 MΩ, Analytica Jena Instruments, Germany) was used in the preparation of all solutions.
Health Risk Assessment
The potential health risk of consuming studied mushrooms was evaluated as target hazard quotient (THQ). This was estimated using the equation:
where EDI represents estimated daily intake; C, concentration of metal in edible mushroom (mg/kg); IR, ingestion rate (0.182 kg/day for adults and 0.118 kg/day for children); EF, exposure frequency (350 day/year); ED, exposure duration (adults = 30 years; children = 6 years); BW, body weight (adults = 70 kg; children = 15 kg); and AT, average time exposure (children = 2190 days; adults = 10,950 days). The RfD values were 4.0 × 10−2 mg kg−1 day−1 for Cu, 7.0 × 10−1 mg kg−1 day−1 for Fe, 3.5 × 10−3 mg kg−1 day−1 for Pb, 1.0 × 10−4 mg kg−1 day−1 for Cr, 1.4 × 10−2 mg kg−1 day−1 for Mn, 2.0 × 10−2 mg kg−1 day−1 for Ni and 1.0 × 10−3 mg kg−1 day−1 for Cd [16]. EPA has not established a Reference Dose (RfD) for cobalt [22].
Results
The species of mushroom and their common names are given in Table 1. The mushrooms from Agaricus bisporus were organized in two groups (white and brown varieties). These samples and Agaricus cupreobrunneus were purchased from local market in Romania and cultivated in the same country. The other samples are sold in local markets from Romania and cultivated in other countries (Turkey and China).
Total phenolic content of the mushroom extracts was determined spectrophotometrically using Folin–Ciocalteu reagent. The results for total phenolic content (expressed in mg GAE/g mushroom sample) are presented in Table 2. The highest total phenolic content was recorded in Agaricus cupreobrunneus stalk mushroom samples (12.25 mg GAE/g), while Hypsizgus tesselatus mushroom samples had relatively lower phenolic content (0.25 mg GAE/g in stalk and 2.88 mg GAE/g in cap) and in Auricularia cornea mushroom samples TPC was not detected. The other studied mushroom samples were in between 2.26 mg GAE/g and 11.24 mg GAE/g.
The data for nitrogen and protein content in the studied mushroom samples are presented in Table 3. The highest nitrogen content 0.72% was observed in Pleurotus eryngii species-complex while the lowest value of 0.11% was in the canned Agaricus bisporus - brown mushroom. For protein content, the highest value was recorded in Pleurotus eryngii species-complex (0.27 %) while the Agaricus bisporus-brown mushroom had the lowest content of protein (0.03%).
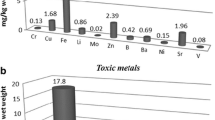
Metal concentrations obtained for tested mushroom samples were determined after microwave digestion. The Pb, Cd, Cr, Cu, Fe, Mn, Co, and Ni analyses were performed by GTAAS and their concentrations are shown in Table 4 as μg/kg dry weight. According to our data, Cu was the most abundant element in the mushroom samples, with a value of 34.46 ± 6.05 μg/kg dw, followed by Pb with a value of 18.33 ± 3.96 μg/kg dw. The Pb levels in other mushroom samples are between 2.31 ± 0.32 and 16.21 ± 2.18 μg/kg dw. Cd content in Agaricus cupreobrunneus was found to be 0.66 ± 0.03 μg /kg dw, whereas Cd content in the other studied mushroom samples ranged between 0.01 ± 0.00 and 0.47 ± 0.05 μg /kg dw. Minimum and maximum levels of Cr were measured in Agaricus bisporus as 0.35 ± 0.06 and 1.12 ± 0.26 μg /kg dw white, respectively brown mushroom samples. The highest Fe content was observed in Auricularia cornea as 4.00 ± 1.32 μg/kg dw. For the other studied mushroom, Fe ranged between 0.20 ± 0.01 and 1.89 ± 0.50 μg/kg dw. The range of Mn concentration were between 0.66 ± 0.07 and 2.45 ± 0.11 μg/kg dw. Co content was highest in Agaricus bisporus-brown mushroom samples (1.29 ± 0.56 μg/kg dw) and lowest in Agaricus cupreobrunneus (0.09 ± 0.01 μg/kg dw). Ni content was also highest in Agaricus bisporus-brown mushroom samples (7.17 ± 1.25 μg/kg dw) and lowest in Agaricus bisporus-white mushroom samples (0.03 ± 0.02 μg/kg dw).
The highest metal concentration (34.46 ± 6.05 μg/kg dw) was recorded for Cu in the caps of Agaricus bisporus-brown mushrooms, while the lowest concentration (0.01 ± 0.00 μg/kg dw) was recorded for Cd in the caps of Agaricus bisporus-white mushrooms. The highest metal concentration in the stalk was recorded for Pb in Agaricus bisporus-white mushrooms (18.33 ± 3.96 μg/kg dw) while the lowest concentration was recorded for Cd in the stalks of Agaricus bisporus-white mushrooms (0.12 ± 0.01 μg/kg dw).
The results obtained for Agaricus bisporus samples cultivated and available in the same country were compared with those of other scientists. The data summarized in Table 5 indicate that the concentrations of metals found in Agaricus bisporus samples from selected countries varied considerably.
The health risk correlated with the consumption of studied metals through tested mushrooms samples was evaluated by estimating the target hazard quotient. In Table 6 are presented the results for target hazard quotient estimated for adults. And in Table 7 are presented the results for target hazard quotient estimated for children.
Discussion
Mushrooms are an important source of phenolic compounds. During the study, the evolution over time (48 h and 7 days) of the total phenolic content in the mushroom extracts was observed. It was found that the phenolic content of extracts increased over time but, what varied was the concentration for each mushroom species. In the mushroom stalks TPC was detected after 7 days of maceration unlike the mushroom caps where TPC was detected after 48 h (Table 2).
The TPC values obtained in this study ranged between 0.25 to 12.25 mg GAE/g sample. These values are smaller than those obtained by other researchers in ethanolic extracts 4.27–51.27 mg GAE/g (fresh weight) [26]. The observed variations may be due to the differences in harvesting location and climate for investigated mushroom species. According to Alispahić, TPC in mushroom ethanolic extracts was between 4.94 and 7.66 mg GAE/g (fresh weight), comparable with our results [27].
Agaricus bisporus is one of the most cultivated edible mushrooms worldwide. For this reason, we also analyzed canned Agaricus bisporus-brown mushroom samples, in which TPC content is almost the same as in raw Agaricus bisporus-brown mushroom samples. Abacan showed in his research that cooking time and salt concentration did not affect total phenolic content significantly [28].
Many mushrooms produce a large number of proteins with interesting biological activities [29]. The protein content of analyzed samples ranged from 0.03 to 0.27%, the highest value being observed in Pleurotus eryngii species-complex. The differences can be ascribed to the large genetic variation [30]. The protein contents of the analyzed mushrooms in this study were lower than those reported in the literature (1.06–3.43%) [31].
Cultivated edible mushrooms contain trace elements essential to humans and, toxic elements. Metals levels depend on mushroom species and are numerous published data but not for all species [32].
The concentration of metals in investigated fruiting bodies mushroom samples varied in order of Cu > Pb > Ni > Fe > Cr > Mn > Co > Cd (Table 4). The content of metals in the caps of studied mushroom samples varied in order of Cu > Pb > Fe > Mn > Cr > Ni > Cd > Co (Table 4), while in stalks varied in order of Pb > Cu > Ni > Cr > Mn > Co > Fe > Cd (Table 4).
The concentrations of Pb (18.33 ± 3.96), Cd (0.66 ± 0.03), Cr (1.81 ± 0.12), Co (1.29 ± 0.56), and Ni (7.17 ± 1.25) in the stalks were higher than those measured in caps. On the contrary, the concentrations of Cu (34.46 ± 6.05), Fe (4.00 ± 1.32), and Mn (2.15 ± 0.18) in caps were higher than those from the stalks. It can be observed that almost all the samples contain the greatest quantity of metals in stalk comparative with cap. Sithole et al. reported also that stalks accumulate metals more than caps [33]. Furthermore, many researchers reported differences of the heavy metal’s accumulation between the caps and stalks [16, 34,35,36]. The data shown above reveal that is possible for the investigated mushroom species in this study to accumulate metals either in their stalks or caps.
Among the eight studied metals cadmium, chromium and lead are potentially hazardous. These metals can enter the food chain and potentially harm human health.
From Table 4, lead concentration was generally low. The highest level of lead was 18.33 ± 3.96 μg/kg, lower than the reported lead concentration in the literature (0.029–10.18 mg/kg) [37, 38]. Also, lead concentration in studied samples was smaller than another mushroom species harvested in Romania (0.28–0.62 mg/kg) [39]. In the EU, the admissible limit for lead in cultivated mushrooms is 3.0 mg/kg dry matter [40]. Lead concentrations in our study were below this value. Regarding, cadmium, the highest concentration was found in Pleurotus eryngii species-complex (0.53 ± 0.08 μg/kg). In all samples, caps and stalks, cadmium concentration was below the recommended limit of 0.05 mg/kg in food as set by WHO [41]. Cadmium contents reported by other studies were in the range 0.11–5.35 mg/kg [42,43,44].
The level of chromium in the present studied samples did not exceed the limit established by WHO in food [45]. The highest value detected in canned Agaricus bisporus-brown mushroom (Table 4) may be due to the stainless-steel package. However, our results concerning chromium content were lower the those reported by Turkmen (0.36–6.26 mg/kg) [46]. Also, in canned Agaricus bisporus-brown mushroom was observed a high content of lead 16.21 ± 2.18 μg/kg, but it is below maximum admissible limit [40].
The analysis showed moderate differences in manganese and cobalt concentrations between the studied species of mushrooms. When compared to the data reported in the literature, the cobalt concentrations obtained in our study were lower than those recorded by other researchers (0.05–2.88 mg/kg) [44]. Also, it has been found that the manganese concentrations were lower when compared to values reported by other authors (6.96–101.60 mg/kg) [42, 44].
The concentration levels of nickel ranged from 0.03 ± 0.02 to 7.17 ± 1.25 μg/kg and the highest concentration was measured in the stalk of Agaricus bisporus-brown mushroom. Nickel concentration reported in the literature is also higher in the stalk of Agaricus bisporus-brown mushroom [33].
The highest concentration of copper was determined in Agaricus bisporus-brown mushroom caps (34.46 ± 6.05 μg/kg). Other Romanian researchers reported that copper is the most abundant element in mushrooms growing in Romania (15.8–64.1 mg/kg) [39]. The copper level detected in this study was smaller than those detected in other mushroom species from China [47].
Iron is an essential element which is required in small amounts. The obtained values (0.20–4.00 μg/kg) were smaller than that detected in mushrooms from Greece (317 μg/g) [48] and Turkey (1716 mg/kg) [49].
Agaricus bisporus is one of the most widely consumed edible mushroom species in the world and many researchers have assessed the content of metals in this specie of mushroom. For this reason, Table 5 presents data reported on studied Agaricus bisporus mushroom cultivated and bought from the same country. Metal concentrations detected in Agaricus bisporus analyzed in this study were smaller than those reported in other countries. This can be explained by large genetic species variation and by differences in harvesting location and climate [50].
If target hazard quotient > 1, the potential risk seems to be significant while at target hazard quotient ≤ 1 hazardous level is meant to be acceptable [18]. The values of THQ for adults and children were determined to be < 1; therefore, the consumers are subject to a notable non-carcinogenic health risk (Tables 6 and 7).
The values of the hazard quotient for the studied samples varies from 6∙10−8 to 2.35∙10−1 demonstrating no risk in consumption of the studied mushrooms. Other studies have been obtained for THQ, also, values lower than 1 with no potential risk in terms of heavy metals concentrations [51, 52].
Correlations between metals studied in mushroom samples purchased and cultivated in Romania (Agaricus bisporus and Agaricus cupreobrunneus) were evaluated by Pearson correlation matrix (Table 8). Positive correlations were observed for the following pairs of metals: Pb-Cr, Pb-Cu, Cd-Cr, Cd-Fe, Cd-Co, Cd-Mn, Cd-Ni, Cr-Cu, Cr-Mn, Fe–Mn, Fe-Co, Fe–Ni, Mn-Co, Mn-Ni, Co–Ni.
Statistically, between cadmium and manganese was observed the strongest positive correlation (p < 0.01). Also, high correlations were found between Pb-Cr, Cd-Fe, and Fe-Co (p < 0.05). Similarly, findings between cadmium, cobalt, and iron were reported by Sarikurkcu et al. [53]. These significant positive correlations may reflect the possibility of their common origin and could indicate for these metals an identical behavior. The moderate relationships between metal pairs could suggest similar characteristics among the Agaricus bisporus and Agaricus cupreobrunneus samples.
Possible correlations between total phenolic content, protein content, and metals content were investigated (Table 9). TPC showed generally positive correlations with studied metals and statistically significant correlation was found between TPC with cadmium and manganese. Also, protein content was moderate positively correlated with cadmium and manganese content. Other weak positive correlations could be found in Table 9. Pearson correlation was also used to identify the relationship between TPC and protein content for studied mushrooms samples. From the calculated matrix, significant positive correlation was observed (p < 0.05; r = 0.613).
Conclusions
The mushroom samples studied showed differences in the way they accumulate metals: Agaricus bisporus (brown and white mushroom) had the highest lead, copper, cobalt, and nickel content; Auricularia cornea exhibited the highest iron content, while Pleurotus eryngii species-complex presented the highest chromium and manganese content. The mushroom species did not constitute a source of significant toxic metals levels when compared to the same and other mushrooms species collected from different countries. The investigated mushroom species possess no health risk. The positive Pearson correlations between pairs of metals may reflect the possibility of their common origin and could indicate for these metals an identical behavior.
From the obtained data, we can conclude that all mushroom studied samples may be a valuable source of phenolic compounds, protein, and mineral content. Also, these samples are characterized by low levels of toxic metals. These make mushrooms potential nutritional supplements for human diets.
References
Nnorom IC, Eze SO, Ukaogo PO (2020) Mineral contents of three wild-grown edible mushrooms collected from forests of south eastern Nigeria: an evaluation of bioaccumulation potentials and dietary intake risks. Sci Afr 8:e00163
Ronda O, Grządka E, Ostolska I, Orzeł J, Cie´slik BM (2022) Accumulation of radioisotopes and heavy metals in selected species of mushrooms. Food Chem 367:130670
Aisala H, Sola J, Hopia A, Linderborg KM, Sandell M (2019) Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem 283:566–578
Dospatliev L, Ivanova M (2017) Determination of heavy metals in mushroom samples by atomic absorption spectrometry. Bul Chem Commun 49:5–9
Chiocchetti GM, Latorre T, Clemente MJ, Jadán-Piedra C, Devesa V, Vélez D (2020) Toxic trace elements in dried mushrooms: effects of cooking and gastrointestinal digestion on food safety. Food Chem 306:125478. https://doi.org/10.1016/j.foodchem.2019.125478
Árvay J, Hauptvogl M, Demková L, Harangozo L, Šnirc M, Bobuľská L, Štefániková J, Kováčik A, Jakabová S, Jančo I, Kunca V, Relić D (2022) Mercury in scarletina bolete mushroom (Neoboletus luridiformis): intake, spatial distribution in the fruiting body, accumulation ability and health risk assessment. Ecotoxicol Environ Saf 232:113235. https://doi.org/10.1016/j.ecoenv.2022.113235
Kalacˇ P (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113:9–16
Altunay N, Tuzen M (2021) A simple and green ultrasound liquid–liquid microextraction method based on low viscous hydrophobic deep eutectic solvent for the preconcentration and separation of selenium in water and food samples prior to HG-AAS detection. Food Chem 364:130371. https://doi.org/10.1016/j.foodchem.2021.130371
Kosanic M, Rankovic B, Rancic A, Stanojkovic T (2016) Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J Food Drug Anal 24:477–484
Sesli E, Tuzen M, Soylak M (2008) Evaluation of trace metal contents of some wild edible mushrooms from Black sea region, Turkey. J Hazard Mater 160:462–467
Kučak A, Blanuša M (1998) Validation of microwave digestion method for determination of trace metals in mushrooms. Arh Hig Rada Toksikol 49(4):35–342
Mendil D, Uluőzlű ÖD, Hasdemir E, Çağlar A (2004) Determination of trace elements on some wild edible mushroom samples from Kastamonu, Turkey. Food Chem 88:281–285
Yamaç M, Yıldız D, Sarıkürkcü C, Çelikkollu M, Solak MH (2007) Heavy metals in some edible mushrooms from the Central Anatolia, Turkey. Food Chem 103(2):263–267
Kalač P, Burda J, Stašková I (1991) Concentration of lead, cadmium, mercury and copper in mushrooms in the vicinity of a lead smelter. Sci Total Environ 105:109–119
Borovička J, Sácký J, Kaňa A, Walenta M, Ackerman L, Braeuer S, Leonhardt T, Hršelová H, Goessler W, Kotrba P (2023) Cadmium in the hyperaccumulating mushroom Thelephora penicillata: intracellular speciation and isotopic composition. Sci Total Environ 885:159002
Dowlati M, Sobhi HR, Esrafili A, FarzadKia M, Yeganeh M (2021) Heavy metals content in edible mushrooms: a systematic review, meta-analysis and health risk assessment. Trends Food Sci Technol 109:527–535
Cacique AP, Barbosa ES, de Pinh GP, Silvério FV (2020) Maceration extraction conditions for determining the phenolic compounds and the antioxidant activity of Catharanthus roseus (L.) G. Don. Sci Agrotech 44:1–12
Dobrinas S, Soceanu A (2021) Determination of total phenolic content from plant extracts used in cosmetic purpose. J Sci Arts 1(54):247–260
Association of Official Analytical Chemists (AOAC) Official Method of Analysis. 19th ed. AOAC; Washington, DC, USA: 2012
Birghila S, Matei N, Dobrinas S, Popescu V, Soceanu A, Niculescu A (2022) Assessment of heavy metal content in soil and Lycopersicon esculentum (tomato) and their health implications. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03257-9
Dobrinas S, Soceanu A, Birghila S, Birghila C, Popescu V, Matei N, Constanda LM (2022) Chemical analysis and quality assessment of honey obtained from different sources. Processes 10(12):2554. https://doi.org/10.3390/pr10122554
Environmental Protection Agency, Reference Dose (RfD): Description and Use in Health Risk Assessments, Reference Dose (RfD): Description and Use in Health Risk Assessments | US EPA
Sinha SK, Upadhyay TK, Sharma SK (2019) Heavy metals detection in white button mushroom (Agaricus Bisporus) cultivated in state of Maharashtra, India. Biochem Cell Arch 19(2):3501–3506
Bosiacki M, Siwulski M, Sobieralski K, Krzebietke S (2018) The content of selected heavy metals in fruiting bodies of Agaricus Bisporus (Lange) imbach. wild growing in Poland. J Elem 23(3):875–886
Siwulski M, Budka A, Rzymski P, Gasecka M, Kala P, Budzynska S, Magdziak Z, Niedzielski P, Mleczek P, Mleczek M (2020) Worldwide basket survey of multielemental composition of white button mushroom Agaricus bisporus. Chemosphere 239:124718
Orhan I, Ustun O (2011) Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. J Food Compos Anal 24:386–390
Alispahić A, Šapčanin A, Salihović M, Ramić E, Dedić A, Pazalja M (2015) Phenolic content and antioxidant activity of mushroom extracts from Bosnian market. Bulletin of the Chemists and Technologists of Bosnia and Herzegovina 44:5–8
Abacan SF, Hurtada WA, Devanadera MAR (2017) Effects of cooking time, temperature, and salt concentration on the phenolic content and antioxidant activity of selected edible mushrooms. Int Food Res J 24(5):2028–2032
Xu X, Yan H, Chen J, Zhang X (2011) Bioactive proteins from mushrooms. Biotechnol Adv 29:667–674
Uzun Y, Gençcelep H, Tunçtürk Y, Demirel K (2009) Determination of protein and nitrogen fractions of wild edible mushrooms. Asian J Chem 21(4):2769–2776
Al Azad S, Chong Ai Ping V (2021) Comparison of protein and amino acids in the extracts of two edible mushroom, Pleurotus sajor-caju and Schizophyllum commune. Adv Biosci Biotechnol 12(9):286–296
Falandysz J, Borovička J (2013) Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Appl Microbiol Biotechnol 97(2):477–501
Sithole SC, Mugivhisa LL, Amoo SO, Olowoyo JO (2017) Pattern and concentrations of tracemetals inmushrooms harvested from trace metal-polluted soils in Pretoria, South Africa. S Afr J Bot 108:315–320
Su J, Zhang J, Li J, Li T, Liu H, Wang Y (2018) Determination of mineral contents of wild Boletus edulis mushroom and its edible safety assessment. J Environ Sci Health B 53(7):454–463
Jarzynska G, Chojnacka A, Dryzałowska A, Nnorom IC, Falandysz J (2012) Concentrations and bioconcentration factors of minerals in yellow-cracking bolete (Xerocomus Subtomentosus) mushroom collected in Notec Forest, Poland. J Food Sci 77(9):H202–H2063
Kojta AK, Jarzynska G, Falandysz J (2012) Mineral composition and heavy metal accumulation capacity of Bay Bolete (Xerocomus badius) fruiting bodies collected near a former gold and copper mining area. J Geochem Explor 121:76–82
Liu B, Huang Q, Cai H, Guo X, Wang T, Gui M (2015) Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem 188:294–300
Aloupi M, Koutrotsios G, Koulousaris M, Kalogeropoulos N (2012) Trace metal contents in wild edible mushrooms growing on serpentineand volcanic soils on the island of Lesvos, Greece. Ecotoxicol Environ Saf 78:184–194
Zavastin DE, Biliuta G, Dodi G, Macsim AM, Lisa G, Gherman SP, Breaban IG, Miron A, Coseri S (2018) Metal content and crude polysaccharide characterization of selected mushrooms growing in Romania. J Food Compos Anal 67:149–158
EEC Directive 2001/22/EC, European Commission (EC). European Commission, Office for Official publications of the European Communities, Luxembourg, Council Directive 66/278/EEC on the protection of environment, and in particular of soil, when sewage sludge is used in agriculture (1986)
World Health Organization (WHO) (1996) World Health Organization technical series. Heavy elements in human nutrition and health. World Health Organization, Geneva, pp 199–205
Ronda O, Grządka E, Ostolska I, Orzeł J, Cieslik BM (2022) Accumulation of radioisotopes and heavy metals in selected species of mushrooms. Food Chem 367:130670
Barea-Sepúlveda M, Espada-Bellido E, Ferreiro-Gonzalez M, Benítez-Rodríguez A, Lopez-Castillo JG, Palma M, Barbero GF (2021) Metal concentrations in Lactarius mushroom species collected from Southern Spain and Northern Morocco: evaluation of health risks and benefits. J Food Compos Anal 99:103859
Sarikurkcu C, Popović-Djordjevi J, Solak MH (2020) Wild edible mushrooms from Mediterranean region: metal concentrations and health risk assessment. Ecotoxicol Environ Saf 190:110058
World Health Organization (WHO) (2001) Toxicological evaluation of certain food additives. Joint FAO/WHO Expert Committee on Food Additives. Food Additive Series No. 683, World Health Organization, Geneva
Türkmen M, Budur D (2018) Heavy metal contaminations in edible wild mushroom species from Turkey’s Black Sea region. Food Chem 254:256–259
Wang C, Hou Y (2011) Determination of trace elements in three mushroom samples of Basidiomycetes from Shandong, China. Biol Trace Elem Res 142(3):843–847
Ouzounia PK, Veltsistasb PG, Paleologosa EK, Riganakosa KA (2007) Determination of metal content in wild edible mushroom species from regions of Greece. J Food Compos Anal 20:480–486
Sarikurkcu C, Copur M, Yildiz D, Akata I (2011) Metal concentration of wild edible mushrooms in Soguksu National Park in Turkey. Food Chem 128:731–734
Liu H, Zhang J, Li T, Shi Y, Wang Y (2012) Mineral element levels in wild edible mushrooms from Yunnan, China. Biol Trace Elem Res 147:341–345
Sithole SC, Agboola OO, Mugivhisa LL, Amoo SO, Olowoyo JO (2022) Elemental concentration of heavy metals in oyster mushrooms grown on mine polluted soils in Pretoria, South Africa. Journal of King Saud University – Science 34(2):101763. https://doi.org/10.1016/j.jksus.2021.101763
Ihugba UA, Nwoko CO, Tony-Njoku FR, Ojiaku AA, Izunobi L (2018) Heavy metal determination and health risk assessment of oyster mushroom Pleurotus tuberregium (Fr.) Singer, collected from selected markets in Imo State. NIGERIA Am J Environ Prot 6(1):22–27
Sarikurkcu C, Yildiz D, Akata I, Tepe B (2021) Evaluation of the metal concentrations of wild mushroom species with their health risk assessments. Environ Sci Pollut Res Int 28(17):21437–21454
Author information
Authors and Affiliations
Contributions
Conceptualization, S. D. and A. S.; methodology, S. D. and S. B.; software, N. M. and A. S.; validation, S. D. and A. S.; formal analysis, V. P. and S. B.; investigation, S. D. and A. S.; resources, G. C. and N. M.; data curation, G. C. and C. B.; writing—original draft preparation, S. D. and N. M.; writing—review and editing, A. S.; visualization, V. P.; supervision, S. B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soceanu, A., Matei, N., Dobrinas, S. et al. Metal Content in Caps and Stalks of Edible Mushrooms: Health Benefits and Risk Evaluation. Biol Trace Elem Res 202, 2347–2356 (2024). https://doi.org/10.1007/s12011-023-03800-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03800-2