Abstract
The objective of the present study was to explore the effects of maternal iodine excess during pregnancy on infants’ neurodevelopment and physical development. A total of 143 mother–child pairs were enrolled in this cohort study. Maternal blood samples were collected during the obstetric examination. A mother–child questionnaire survey was conducted, and infants’ blood samples were collected during the newborn physical examination. Infants’ single-spot urine samples were collected, and intellectual, motor, and physical development were assessed at 2 months of age. The median (IQR) maternal serum iodine concentrations (SICs) in the first, second, and third trimesters of pregnancy were 91.2 (74.4, 102.2) μg/L, 81.2 (70.6, 94.8) μg/L, and 82.0 (68.9, 100.3) μg/L, respectively. In the first trimester of pregnancy, infants’ psychomotor developmental index (PDI), body mass index (BMI) and weight-for-length Z score (WLZ) were higher with maternal suitable SIC (40 ~ 92 μg/L) than with maternal excess SIC (P < 0.05). Infants’ PDI, BMI, weight-for-age Z score (WAZ) and WLZ were negatively correlated with maternal SIC (P < 0.05). Maternal excess SIC had a slightly negative effect on infants’ MDI (OR = 1.304, P = 0.035, 95% CI = 1.019 ~ 1.668), PDI (OR = 1.124, P = 0.001, 95% CI = 1.052 ~ 1.200) and BMI (OR = 0.790, P = 0.005, 95% CI = 0.669 ~ 0.933). In the third trimester, infants’ length-for-age Z score (LAZ) was higher with maternal high SIC (> 92 μg/L) (P = 0.015), and maternal SIC was positively correlated with infants’ urine iodine concentration (UIC) (P = 0.026). Maternal iodine excess in the first trimester had a slightly negative effect on infants’ intellectual, motor, and physical development. In the third trimester, maternal iodine excess only may have a positive impact on infants’ height. Additionally, maternal iodine status was closely related to infants’ iodine status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential nutrient for the synthesis of thyroid hormone, which supports healthy metabolism and affects normal growth and development in humans [1]. It is crucial for brain development during specific time windows, as it influences neurogenesis, neuronal and glial cell differentiation, myelination, neuronal migration, and synaptogenesis [2]. Maternal need for iodine increases substantially during pregnancy [3]. Although iodine is important, excessive iodine is harmful to health. Massive intake of iodine triggers a defense mechanism in the thyroid gland, called the Wolff‒Chaikoff effect, which inhibits the active transport mechanism of iodine and affects the synthesis of thyroid hormones [4]. Maternal iodine deficiency or excess during pregnancy has adverse effects on infants’ neurodevelopment [5].
At present, severe iodine deficiency during pregnancy has been found to cause serious body and nerve damage to the fetus, such as endemic cretinism [6, 7]. Some studies found that even mild or moderate iodine deficiency in pregnant women also increased the risk of poor offspring development with respect to verbal intelligence quotient, reading accuracy and comprehension [8–11]. However, iodine excess in pregnant women needs equal attention. In a population study, excessive maternal iodine load may cause hypothyroidism (Wolff‒Chaikoff effect) and may affect fetal thyroid development, leading to neonatal hypothyroidism [12]. A study conducted in Rio de Janeiro indicated that urinary iodine concentration (UIC) ≥ 250 μg/L was an independent risk factor for gestational diabetes and hypertension in pregnant women [13]. However, good evidence on offspring neurodevelopment and physical development is limited. A large-sample study suggested that a maternal high urinary iodine/creatinine ratio (UI/Cr) during pregnancy was associated with a smaller area of total gray matter volume among offspring and revealed potential nonlinear associations between maternal iodine excretion during pregnancy and offspring brain morphology [14].
Most studies of iodine excess have been animal experiments. When female rats are exposed to high iodine during pregnancy, their offspring show deficits in mild learning and spatial memory, which may be related to changes in the protein expression of brain-derived neurological factor (BDNF) and neuroendocrine-specific protein A (NSP-A) in the hippocampus [15]. A metabolomics study found that if mother rats had excessive iodine during pregnancy, the hippocampus of their offspring would be affected due to mild metabolic changes, which would affect the learning and memory ability of their offspring [16]. It is worth noting that after the dose conversion between humans and animals [17], it was found that the intake dose of the high iodine model constructed in animal research is far higher than the maximum dose of iodine tolerable in adults. Therefore, there are some limitations in directly extrapolating the conclusions to human research.
In addition, previous population studies mainly used the UIC or UI/Cr to reflect the iodine status, but UIC can be affected by many factors, such as diet and fluid; furthermore, although UI/Cr reduces the variation due to hydration, creatinine excretion can also be affected by differences in age, muscle mass, ethnicity, and protein intake between individuals [18]. Currently, few studies have focused on serum iodine concentration (SIC). SIC is an important biomarker of iodine metabolism. The serum iodine level in the human body is relatively stable, and the excess iodine is excreted by the kidney, which is a relatively stable indicator reflecting the long-term iodine status [18]. Maternal SIC is basically not affected by the recent fluctuation of dietary iodine intake because of iodine consumption during pregnancy [19]. A Chinese study found that SIC is more stable than UIC and has a significantly higher correlation with thyroid-related hormone levels [20]. Hlucny et al. designed experiments to determine whether a titration diet (which used known quantities of iodized salt) was reflected in the 24 h UIC and serum iodine biomarkers of iodine status and found that SIC may be more indicative of long-term (or typical) iodine status and 24 h UIC was sensitive to changes in short-term dietary intake [21]. Jin et al. also showed that the iodine level in the external environment has a strong influence on UIC, but the SIC only changes slightly. Compared with sensitive UIC, SIC can better reflect the stable state of individual iodine status [18].
In summary, there is a lack of relevant follow-up observational studies on the effects of maternal iodine excess during pregnancy on infants in terms of iodine status and intellectual, motor and physical development. This study aimed to explore the effects and hopes to provide evidence to improve infant neurodevelopment and physical development and guide iodine intake in pregnant women.
Subjects and Methods
Study Design and Participants
This prospective cohort study was conducted in a large hospital in Harbin, Heilongjiang Province, China, starting in December 2020. The national iodine deficiency disease surveillance data show that in recent years, the coverage rate of iodized salt in this area is more than 95%, the consumption rate of qualified iodized salt is more than 90%, the median urine iodine of pregnant women is within the range of 150 ~ 249 μg/L, and the median urine iodine of children is within the range of 100 ~ 300 μg/L. Pregnant women who underwent physical examination during pregnancy (first-trimester: 0 ~ 13 weeks, second-trimester: 14 ~ 27 weeks, third-trimester: 28 ~ 40 weeks) in the Department of Obstetrics were recruited as candidates. The inclusion criteria included at least 18 years old, singleton pregnancy, no communication barriers, and no intake of iodine-rich food 3 days before sampling. We excluded pregnant women with clinical/subclinical hyperthyroidism, clinical/subclinical hypothyroidism, thyroid peroxidase antibody (TPOAb), and/or thyroglobulin antibody (TGAb) positivity, treatment for thyroid diseases (e.g., radiotherapy), and visible or palpable goiter. The participants’ fasting blood samples were collected at physical examination.
Newborns who were born in this hospital and examined in the neonatal department were recruited as infant participants. Infants with premature birth (gestational age < 37 weeks at birth), low birth weight (< 2500 g), or major organic diseases caused by other reasons (severe visual, auditory, and central nervous system disorders) were excluded. A mother and infant questionnaire survey was conducted in the parents, and infants’ fasting blood samples were also collected on the day of newborn physical examination (on the 42nd day after delivery). Single-spot urine samples were collected (through disposable special urine collection bags for infants), and intellectual, motor, and physical development were assessed at 2 months of age.
Questionnaire Investigation
The questionnaire included seven parts: (1) the basic information of parents, including educational attainment; (2) details of maternal physique and fertility, including delivery age; (3) the infant’s birth situation, including the infant’s sex, neonatal thyroid stimulating hormone (TSH) level; (4) personal history of pregnant mothers, such as passive smoking exposure in pregnancy; (5) maternal iodine intake and prenatal education, including consumption of iodized salt and iodine-containing supplements during pregnancy; (6) maternal healthy lifestyle factors, including sleep quality and exercise during pregnancy; and (7) the physical growth and feeding of infants, including their weight, head circumference and body length, were measured at 2 months of age. The questionnaire survey was conducted face-to-face by a trained epidemiological investigator who was blinded to the SIC results of the pregnant women.
Neurodevelopment Assessment
At the age of 2 months, the intellectual and motor development of infants were assessed through the CDCC infant mental development scale [22], including mental developmental scale (121 items) and psycho-motor developmental scale (61 items). The average test age was 2.05 ± 0.10 months in our study. The mental developmental scale assesses perceptual acuity, attention discrimination ability and ability to respond to the outside world. The psychomotor developmental scale reflects an infant’s motor coordination and skill-behavior ability. The total number of items passed by infants was recorded to obtain the original score, which was then converted into the developmental index by correcting the infants’ age. The scale assesses infants’ overall intellectual and motor level through the mental developmental index (MDI) and psycho-motor developmental index (PDI), with high reliability and validity. The reliability coefficient of this scale varies from 0.82 to 0.98. The calculation formula of the two development indices is 100 + (x-mean)/SD × 16 (x is the original scale score, mean and SD are the mean and standard deviation of total scale score in the corresponding age group). The judgment criteria of the above two indices are as follows: ≥ 130 points are very excellent, 120 ~ 129 points are excellent, 110 ~ 119 points are upper middle (smart), 90 ~ 109 points are medium, 80 ~ 89 points are lower middle (dull), 70 ~ 79 points are critical state, and ≤ 69 points are intellectual defects or motor dysfunction. Cognitive function was assessed by specially trained personnel who were blinded to the participant study group.
Physical Development Assessment
The body mass index (BMI) of infants was categorized as follows: 13.7 ~ 17.8 kg/m2 indicated normal physical development, < 13.7 kg/m2 indicated emaciation, 17.8 ~ 19.4 kg/m2 indicated overweight tendency, and ≥ 19.4 kg/m2 indicated overweight. The length-for-age Z score (LAZ) was categorized as follows: − 2 ~ 2 indicated infants’ body length was in the normal range, − 3 ~ − 2 indicated slight growth retardation, < − 3 indicated severe growth retardation, 2 ~ 3 indicated that infants grew more rapidly, and ≥ 3 indicated tall; The weight-for-age Z score (WAZ) was categorized as follows: − 1 ~ 2 indicated infants’ body weight was in the normal range, − 2 ~ − 1 indicated slightly malnourished, − 3 ~ − 2 indicated moderately malnourished, < − 3 indicated severely malnourished, ≥ 2 indicated overweight or obesity. The head circumference-for-age Z score (HCZ) was categorized as follows: − 2 ~ 2 indicated that infants’ cranial development was normal, < − 2 indicated microcephaly, ≥ 2 indicated macrocephaly. The weight-for-length Z score (WLZ) was categorized as follows: − 1 ~ 1 indicated that infants had normal body shape, − 2 ~ − 1 indicated lean, − 3 ~ − 2 indicated emaciated, < − 3 indicated severely emaciated, 1 ~ 2 indicated overweight, 2 ~ 3 indicated obese, ≥ 3 indicated severely obese [23].
Biochemical Evaluation
The SIC was measured by arsenic and cerium catalytic spectrophotometry (WS/T 572–2017). Referring to the reference range of adult serum iodine metabolism recommended by the Mayo Clinic (42 ~ 92 μg/L) [24], pregnant women were divided into a suitable SIC group (40 ~ 992 μg/L) and a high SIC group (> 92 μg/L), which were referred to as the SI group and HI group, respectively. UIC was also measured by arsenic and cerium catalytic spectrophotometry (WS/T 107.1–2016). Creatinine in urine was measured by a spectrophotometric method (WS/T 97–1996). The urinary iodine (UI) of infants was assessed by the iodine nutrition standard recommended by WHO/UNICEF/ICCIDD for the general population (including children); the median urinary iodine concentration (MUIC) is 100 ~ 299 μg/L [25]. Our study reduced the intraindividual variation in daily urine volume by measuring urinary creatinine concentration [26]. Because there were no clear boundary values in infants’ SCI and UI/Cr, the lowest quartile was selected as the classification standard.
Statistical Analysis
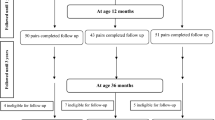
Mother–child pairs were established. We calculated that to have 80% power (1-β) to detect a 5-score difference on the infant intellectual and motor development index of the infant mental development scale from the Children’s Developmental Center of China (CDCC) between groups at 2 months of age, with a standard difference of 7 and a significance level (α) of 0.05 (two-sided). The data were divided into two groups, anticipating a loss to follow-up of no more than 10%, and at least 69 mother–child pairs should be enrolled in the study. The study initially assembled 154 pairs, but 11 infants were lost to follow-up after 2 months. Finally, 143 mother–child pairs were enrolled in this study.
Normally distributed data are described as the mean ± standard deviation, and nonnormally distributed data are described as the median (interquartile range). The categorical data were described as percentages. Normally distributed data were compared between groups by analysis of variance, and nonnormally distributed data were compared by Mann‒Whitney U test. Pearson χ2 was used to test for comparison counting data between groups. Questionnaires with missing data > 15% were deleted, and the excluded samples and lost follow-up samples were compared with the followed-up samples in population characteristics according to the same statistical method described above. Spearman rank correlation analysis was performed to test the correlation between maternal SIC and infants’ iodine status and various development indicators. The factors with statistically significant differences from any developmental index in the population characteristic information were regarded as confounding factors by single factor logistic regression. Confounders included infants’ gestational age, maternal prenatal education, average sleep time, frequency of staying up until 12 o’clock, intake of iodine-containing supplements and weekly exercise time during pregnancy, and binary logistic regression analysis was used for multivariate analysis to eliminate the influence of confounders. We used a model to adjust for potential confounders. Infants’ intellectual or motor development reached the standard when their MDI or PDI reached 90 points. Because there were only three infants with MDI below 90 points, MDI took 110 points as the dividing point. BMI was set at 17.8 kg/m2 as the dividing point to study the effect of maternal SIC on infant overweight.
All statistical analyses were performed with SPSS 24.0 software, and P < 0.05 (two-tailed) was considered statistically significant. WHO-Anthro 3.2.2 software was used to calculate the Z score of infant physical development.
Results
Characteristics of the Mother–Child Cohort
A total of 143 mother–child pairs were enrolled in this study. The characteristics of the mother–child pairs are shown in Table 1. No pregnant women smoked cigarettes or drank alcohol during pregnancy, and neonatal TSH values were all within the normal clinical range (TSH < 10 mIU/L).
As shown in Table 1, a significant difference existed in infant gender between the group with SIC in the range of 40 ~ 92 μg/L and the group with SIC > 92 μg/L in the first trimester of pregnancy (P = 0.002), and a difference existed in mother’s postpartum depression between the group with SIC in the range of 40 ~ 92 μg/L and the group with SIC > 92 μg/L in the second trimester of pregnancy (P = 0.049). The rest of the maternal and infant characteristics between these two groups were not significantly different. In addition, the survey information of infants who assessed intellectual, motor, and physical development at 2 months old was compared with that of infants who did not receive evaluation (lost follow-up samples), and there was no significant difference between the two groups (data not shown).
Biochemical Evaluation Results of Pregnant Women and Infants
The median (IQR) maternal SIC in the first, second, and third trimester of pregnancy was 91.2 (74.4, 102.2) μg/L, 81.2 (70.6, 94.8) μg/L, and 82.0 (68.9, 100.3) μg/L, respectively.
The infants’ SIC was mainly concentrated in the range of 50 ~ 150 μg/L, and the median (IQR) was 125.8 (102.4, 139.3) μg/L. The median (IQR) of infants’ UIC was 189.3 (138.5, 270.1) μg/L. A total of 13.2% (n = 12) of infants’ UIC was lower than 100 μg/L, 2.2% (n = 2) of infants’ UIC was lower than 50 μg/L, and 17.6% (n = 16) of infants’ UIC was higher than 300 μg/L. The median (IQR) of UI/Cr was 1793.6 (1186.2, 2691.6) μg/g, and 87.9% (n = 80) of infants were concentrated in the range of 0 ~ 4000 μg/g.
Neurodevelopment and Physical Assessment Results of Infants
The median (IQR) MDI in 2-month-old infants was 101 (96,106), 97.9% (n = 140) of infants reached the standard level (index ≥ 90) of intellectual development, and 2.1% (n = 3) of infants were rated as lower-middle level. The median (IQR) PDI score was 96 (89, 97). A total of 49.7% (n = 71) of infants did not reach the average motor level (medium level), and 0.7% (n = 1) of infants were in the critical state of motor development. The median (IQR) infant BMI was 16.7 (15.6, 17.4). A total of 83.9% (n = 120) of infants had normal physical development, 2.1% (n = 3) of infants were thin, 10.5% (n = 15) of infants had a tendency to be overweight, and 3.5% (n = 5) of infants were overweight. For LAZ, the median (IQR) was 0.8 (− 0.1, 1.3), 91.6% (n = 131) of infants were in the normal range, and 8.4% (n = 12) of infants grew more rapidly. For WAZ, the median (IQR) was 0.6 (− 0.1, 1.2), 93.0% (n = 133) of infants were in the normal range, 4.2% (n = 6) of infants were slightly or moderately malnourished, and 2.8% (n = 4) of infants were overweight. For HCZ, the median (IQR) was 0.1 (− 0.4, 0.7), 96.5% (n = 138) of infants had normal cranial development, and 3.5% (n = 5) of infants had microcephaly or macrocephaly. For WLZ, the median (IQR) was 0.2 (− 0.5, 0.7), 69.9% (n = 100) of infants had normal body shape, 14.0% (n = 20) of infants were lean or emaciated (recent malnutrition), 16.1% (n = 23) of infants were overweight or obese.
Comparison of Infants’ Iodine Status and Development Between Different Maternal SIC Groups
As shown in Table 2, in the first trimester of pregnancy, the infants’ PDI, BMI, and WLZ of the SI group were higher than those of the HI group, and the differences were statistically significant (PPDI = 0.001, PBMI = 0.003, PWLZ = 0.021). In the third trimester of pregnancy, infants’ LAZ in the HI group was higher than that in the SI group, and the difference was significant (P = 0.015).
Relationship Between Maternal SIC and Infants’ Iodine Status and Development
As shown in Table 3, in the first trimester of pregnancy, infants’ PDI (r = − 0.634, P < 0.001), BMI (r = − 0.537, P < 0.001), WAZ (r = − 0.411, P = 0.002) and WLZ (r = − 0.472, P < 0.001) were negatively correlated with maternal SIC. The SIC of pregnant women in the third trimester of pregnancy was positively correlated with infants’ UIC (r = 0.271, P = 0.026).
Effects of Maternal SIC on Infants’ Iodine Status and Development
Binary logistic regression was first used to test the influence of infants’ UIC, SIC, and UI/Cr on their own intellectual, motor, and physical development. No significant difference was found in the results (data not shown). The single-factor logistic regression results are shown in Table 4. Table 5 shows the effects of maternal SIC during pregnancy on infants’ iodine status and development before and after adjustment for potential confounders. Confounders included infants’ gestational age, maternal prenatal education, average sleep time, frequency of staying up until 12 o’clock, intake of iodine-containing supplements and weekly exercise time during pregnancy. The results showed that the maternal SIC was high in the first trimester of pregnancy, the risk of infants’ PDI below the medium level slightly increased (OR = 1.122, P < 0.001, 95% CI = 1.055 ~ 1.193), and the risk of infants’ BMI reaching overweight tendency slightly decreased (OR = 0.847, P = 0.001, 95% CI = 0.768 ~ 0.934). After controlling for confounders, these differences in results were still significant (ORPDI = 1.124, PPDI = 0.001, 95% CIPDI = 1.052 ~ 1.200; ORBMI = 0.790, PBMI = 0.005, 95% CIBMI = 0.669 ~ 0.933), and the risk of infants’ MDI below the upper middle level slightly increased (OR = 1.304, P = 0.035, 95% CI = 1.019 ~ 1.668). In addition, in the third trimester of pregnancy, after controlling for confounders, the effect of maternal SIC on infants’ LAZ was significantly different (OR = 1.019, P = 0.049, 95% CI = 1.000 ~ 1.039), but the OR was between 1.0 and 1.1, so it was considered that there was no association in essence.
Discussion
In this study, we explored the effects of maternal high SIC on infants’ iodine status and development when maternal thyroid function was normal. This is the first study to explore the effects of maternal SIC excess during the three periods of pregnancy on infants in terms of iodine status, neurodevelopment, and physical development. The results mainly suggested that maternal excessive SIC in the first trimester of pregnancy slightly increased the risk that infants’ MDI was lower than the upper middle level, increased the risk that infants’ PDI was lower than the medium level, and slightly reduced the risk that infants’ BMI had a tendency to be overweight. In addition, in the third trimester of pregnancy, maternal SIC was positively related to infants’ UIC at 2 months of age.
The median (IQR) infant SIC on the 42nd day after delivery was 125.8 (102.4, 139.3) μg/L, mainly concentrated in the range of 50 ~ 150 μg/L. Kazi et al. [27] collected blood samples of newborns from the umbilical artery and umbilical vein after delivery, and the SIC of newborns born to mothers with normal thyroid function was 76.5 ± 9.7 μg/L. A Japanese study also determined the SIC of newborns (the first 4–7 postnatal), and the iodine level in the serum of newborns born to mothers with normal thyroid function was 10.8 ± 2.8 μg/dL [28]. Our result was slightly higher than the findings of these previous studies. This difference may be due to the proper implementation of the iodized salt popularization policy. Although these pregnant women consumed more iodine than women in the nonpregnant period, their reserve of iodine was relatively rich, and iodine was transported from the mother’s blood into the fetus through placental passage [29], so infants’ SIC was higher. The total median UIC of 2-month-old infants was 189.3 μg/L, higher than 100 μg/L. The iodine intake of the infant population was sufficient according to the WHO standard [25]. The median (IQR) of 2-month-old infants’ UI/Cr was 1793.6 (1186.2, 2691.6) μg/g. At present, there is no recognized reference range standard for UI/Cr in infants. In the study of Tachibana et al., 48 infants with an average age of 3.1 ± 2.2 years had a total UI/Cr of 3930 (182 ~ 50,000) μg/g [30], which was higher than the result of our study. The reasons may be the differences in sample size, age span, and dietary structure.
Our results showed that maternal excessive SIC in the first trimester of pregnancy slightly increased the risk of infants’ MDI less than 110 points and the risk of infants’ PDI less than 90 points. A large sample study in Spain found that maternal consumption of 150 μg/day or more of iodine from supplements during pregnancy (the highest dose group) was related to a 1.5-fold increase in the odds of a psychomotor score less than 85 and to a 1.7-fold increase in the odds of a mental score less than 85 of infants at 1 year old [31]. Mulder’s study including 990 mother–child indicated that maternal high UI/Cr during pregnancy was associated with smaller total gray matter volume of their offspring at age 10 years [14]. These results were consistent with our results. However, a study in Japan showed that there were no correlations between children’s developmental test results (at 3, 6, 9, and 12 months after birth) and UIC at a single spot during early pregnancy (7–15 weeks gestation) in their mothers [32]. This may be because they defined individual iodine status using single spot urine samples and did not take into account variations in iodine excretion.
Iodine is an important component of thyroid hormones, including thyroxine (T4) and triiodothyronine (T3) [33]. In the first 3 months of pregnancy, the fetal thyroid is not yet mature, and brain development depends on the T4 provided by the mother, which is transformed by deiodinase to provide T3 for the developing fetal brain [34]. Therefore, the iodine intake of pregnant women in the first trimester of pregnancy is quite significant for fetal brain development. When the body ingests excessive iodine that will cause a temporary reduction in thyroid hormone synthesis through a mechanism called the acute Wolff‒Chaikoff effect, and an increase in iodine load will decrease the activity of deiodinase in the thyroid gland and may also reduce the synthesis of thyroid hormone [35]. We speculated that the Wolff‒Chaikoff effect may be triggered by mothers’ excessive iodine intake in the first trimester and then affect offspring intellectual and motor development. Undoubtedly, the mechanism of the effect of maternal iodine excess on infants’ neurodevelopment is complex and multiple. The mechanism needs to be further studied.
Our results also found that maternal high SIC in the first trimester of pregnancy reduced the risk of infants’ BMI reaching an overweight tendency. The infants’ BMI of the SI group in the first trimester was higher than that of the HI group (P = 0.003), and the medians of the two groups were within the normal range. Maintaining the adequate level of maternal SIC in the first trimester of pregnancy promoted the physical growth of the fetus, the fetus was healthier than that of insufficient iodine [36], and there was no risk of other maldevelopment. This can also be proven by the results of correlation analysis: the maternal SIC in the first trimester was negatively correlated with infants’ WAZ and WLZ. In addition, there was a positive correlation between maternal SIC in the third trimester and infants’ UIC at 2 months of age, which may be because in the third trimester of pregnancy, part of the available iodine in the mother’s circulation may enter the fetal placental unit, and the placenta transports iodine to the fetal circulation through the transfer of maternal iodine and maternal thyroxine deiodination, but the degree of iodine transmission from the mother to the fetus is not clear [37]. Infants’ LAZ in the HI group was higher than that in the SI group (P = 0.015) in the third trimester of pregnancy, but logistic analysis showed that there was no correlation between them. To date, there has been no similar research, and the results and specific mechanism need to be further discussed.
Our study has some strengths. First, we enrolled pregnant women with normal thyroid function to explore whether maternal intake of excessive iodine but normal thyroid function would affect infants’ development. Second, we used SIC to define maternal individual iodine status in pregnancy. Although it was not necessarily optimal, it was more stable than single spot urine samples. We examined maternal iodine status in three periods of pregnancy. Third, we determined infants’ iodine status, including UIC, UI/Cr, and SIC, and excluded the possibility of postpartum effects of differences in the infants’ iodine status on intellectual and motor development. Fourth, we chose a follow-up time of 2 months, which can measure intellectual and motor development at the earliest, to avoid the influence of acquired education and the external environment as much as possible. After all, it was difficult to find all potential confounders in older children.
This study also has some limitations. First, it was difficult to collect blood samples of the same pregnant woman in three periods of pregnancy, as well as infants’ urine and blood samples. Second, there was no intelligence quotient (IQ) test for parents, but our study investigated the educational qualifications of parents. Third, because infants’ intellectual and motor development were assessed earlier, whether it could predict the IQ of adulthood remains to be explored.
In conclusion, maternal excess SIC in different stages of pregnancy had different effects on infant development. Our results indicated that maternal iodine excess in the first trimester of pregnancy slightly negatively affected infants’ intellectual and motor development and slightly hindered infants’ physical growth, even if maternal thyroid function was normal. In the third trimester of pregnancy, maternal iodine excess only may have a positive impact on infants’ height, and maternal iodine status was closely related to infants’ iodine status. Therefore, it is urgent to monitor maternal iodine status throughout pregnancy. In addition, there is an imperative need for recognized and clear standards for the reference range of SIC in pregnant women and infants, as well as the reference range of infants’ UI/Cr. The mechanism of the effects of maternal iodine status with normal thyroid function on infants’ development deserves further study.
Data Availability
The datasets generated during and analyzed during the current study are not publicly available due to protect participant’s privacy but are available from the corresponding author on reasonable request.
References
Pearce EN, Lazarus JH, Moreno-Reyes R et al (2016) Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr 104(Suppl 3):918S-923S. https://doi.org/10.3945/ajcn.115.110429
Choudhry H, Nasrullah M (2018) Iodine consumption and cognitive performance: confirmation of adequate consumption. Food Sci Nutr 6(6):1341–1351. https://doi.org/10.1002/fsn3.694
Alexander EK, Pearce EN, Brent GA et al (2017) 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389. https://doi.org/10.1089/thy.2016.0457
Savoie JC, Leger AF (1977) Iatrogenic thyroid pathology. Sem Hop 53(24):1411–1415
Rodriguez-Diaz E, Pearce EN (2020) Iodine status and supplementation before, during, and after pregnancy. Best Pract Res Clin Endocrinol Metab 34(4):101430. https://doi.org/10.1016/j.beem.2020.101430
Pharoah P, Buttfield IH, Hetzel BS (2012) Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Int J Epidemiol 41(3):589–592. https://doi.org/10.1093/ije/dys070
Niwattisaiwong S, Burman KD, Li-Ng M (2017) Iodine deficiency: clinical implications. Cleve Clin J Med 84(3):236–244. https://doi.org/10.3949/ccjm.84a.15053
Rayman MP, Bath SC (2015) The new emergence of iodine deficiency in the UK: consequences for child neurodevelopment. Ann Clin Biochem 52(6):705–708. https://doi.org/10.1177/0004563215597249
Moleti M, Trimarchi F, Tortorella G et al (2016) Effects of maternal iodine nutrition and thyroid status on cognitive development in offspring: a pilot study. Thyroid 26(2):296–305. https://doi.org/10.1089/thy.2015.0336
Levie D, Korevaar T, Bath SC et al (2019) Association of maternal iodine status with child IQ: a meta-analysis of individual participant data. J Clin Endocrinol Metab 104(12):5957–5967. https://doi.org/10.1210/jc.2018-02559
Bath SC, Steer CD, Golding J et al (2013) Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382(9889):331–337. https://doi.org/10.1016/S0140-6736(13)60436-5
Mathews DM, Johnson NP, Sim RG et al (2021) Iodine and fertility: do we know enough? Hum Reprod 36(2):265–274. https://doi.org/10.1093/humrep
Silva DMN, Ayres SD, Corcino C et al (2020) Consequences of iodine deficiency and excess in pregnancy and neonatal outcomes: a prospective cohort study in Rio de Janeiro. Brazil Thyroid 30(12):1792–1801. https://doi.org/10.1089/thy.2019.0462
Mulder TA, Korevaar T, Peeters RP et al (2021) Urinary iodine concentrations in pregnant women and offspring brain morphology. Thyroid 31(6):964–972. https://doi.org/10.1089/thy.2020.0582
Zhang L, Teng W, Liu Y et al (2012) Effect of maternal excessive iodine intake on neurodevelopment and cognitive function in rat offspring. BMC Neurosci 13(1):121. https://doi.org/10.1186/1471-2202-13-121
Zhang L, Fan L, Li F et al (2021) Study on the effect of different iodine intake on hippocampal metabolism in offspring rats. Biol Trace Elem Res 200(10):4385–4394. https://doi.org/10.1007/s12011-021-03032-2
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Jin X, Jiang P, Liu L et al (2017) The application of serum iodine in assessing individual iodine status. Clin Endocrinol (Oxf) 87(6):807–814. https://doi.org/10.1111/cen.13421
Pan Z, Cui T, Chen W et al (2019) Serum iodine concentration in pregnant women and its association with urinary iodine concentration and thyroid function. Clin Endocrinol (Oxf) 90(5):711–718. https://doi.org/10.1111/cen.13945
Zou Y, Li H, Pang J et al (2021) An evaluation of urine and serum iodine status in the population of Tibet, China: no longer an iodine-deficient region. Nutrition 82:111033. https://doi.org/10.1016/j.nut.2020.111033
Hlucny K, Alexander BM, Gerow K et al (2021) Reflection of dietary iodine in the 24 h urinary iodine concentration, serum iodine and thyroglobulin as biomarkers of iodine status: a pilot study. Nutrients 13(8):2520. https://doi.org/10.3390/nu13082520
Fan CR (1989) The establishment of CDCC infant mental development scale. Acta Psychol Sin 02:130–140
NHFPC (2013) Assessment for growth status of children under 5 years of age. WS 423–2013. China: NHFPC. Accessed 23 Jan 2023
Han JH, Wu L, Yu SL et al (2015) Values of iodine metabolism biomarkers in assessing the iodine nutrition status in surgically treated patients with thyroid disease. Acta Acad Med Sinicae 37(2):221–225. https://doi.org/10.3881/j.issn.1000-503X.2015.02.014
WHO/UNICEF/ICCIDD (2007) Assessment of iodine deficiency disorders and monitoring their elimination : a guide for programme managers, 3rd edn. WHO, Geneva
Bourdoux P (1998) Evaluation of the iodine intake: problems of the iodine/creatinine ratio–comparison with iodine excretion and daily fluctuations of iodine concentration. Exp Clin Endocrinol Diabetes 106(Suppl 3):S17–S20. https://doi.org/10.1055/s-0029-1212039
Kazi TG, Kandhro GA et al (2010) Evaluation of iodine, iron, and selenium in biological samples of thyroid mother and their newly born babies. Early Hum Dev 86(10):649–655. https://doi.org/10.1016/j.earlhumdev.2010.07.010
Nishiyama S, Mikeda T, Okada T et al (2004) Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid 14(12):1077. https://doi.org/10.1089/thy.2004.14.1077
Trofimiuk-Mudlner M, Hubalewska-Dydejczyk A (2017) Iodine deficiency and iodine prophylaxis in pregnancy. Recent Pat Endocr Metab Immune Drug Discov 10(2):85–95. https://doi.org/10.2174/1872214811666170309151538
Tachibana M, Miyoshi Y, Fukui M et al (2021) Urinary iodine and thyroglobulin are useful markers in infants suspected of congenital hypothyroidism based on newborn screening. J Pediatr Endocrinol Metab 34(11):1411–1418. https://doi.org/10.1515/jpem-2021-0205
Rebagliato M, Murcia M, Alvarez-Pedrerol M et al (2013) Iodine supplementation during pregnancy and infant neuropsychological development. INMA Mother and Child Cohort Study. Am J Epidemiol 177(9):944–953. https://doi.org/10.1093/aje/kws333
Orito Y, Oku H, Kubota S et al (2009) Thyroid function in early pregnancy in Japanese healthy women: relation to urinary iodine excretion, emesis, and fetal and child development. J Clin Endocrinol Metab 94(5):1683–1688. https://doi.org/10.1210/jc.2008-2111
Santana LM, Jacome DCJ, Marcelino M et al (2012) Iodine and thyroid: what a clinic should know. Acta Med Port 25(3):174–178
Melse-Boonstra A, Jaiswal N (2010) Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 24(1):29–38. https://doi.org/10.1016/j.beem.2009.09.002
Leung AM, Braverman LE (2014) Consequences of excess iodine. Nat Rev Endocrinol 10(3):136–142. https://doi.org/10.1038/nrendo.2013.251
Charoenratana C, Leelapat P, Traisrisilp K et al (2016) Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr 12(4):680–687. https://doi.org/10.1111/mcn.12211
Glinoer D (1997) The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 18(3):404–433. https://doi.org/10.1210/edrv.18.3.0300
Acknowledgements
We are sincerely grateful to all participants and the Fourth Affiliated Hospital of Harbin Medical University for their cooperation.
Funding
This study was supported by the National Natural Science Foundation of China (Grant no.81830098) and the Natural Science Foundation of Heilongjiang Province of China (Grant no.TD2019H001).
Author information
Authors and Affiliations
Contributions
Sihan Wang and Ye Bu conceived and designed the study, conducted the participants, samples and data collection, and completed the laboratory analysis. Sihan Wang analyzed the data and wrote the paper. Qingliang Shao and Yan Cai contributed to collect participants. Dianjun Sun and Lijun Fan contributed to revise the paper. All authors read and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
The present study was approved by the Ethics Committee of the Center for Endemic Disease Control of the Harbin Medical University (HRBMUECDC20211002). All procedures performed in the study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments.
Consent to Participate
Informed consent was obtained from all individual participants included in the study. Written informed consent was signed by participants.
Consent for Publication
The authors affirm that human research participants provided informed consent for the publication of their data results.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Bu, Y., Shao, Q. et al. A Cohort Study on the Effects of Maternal High Serum Iodine Status During Pregnancy on Infants in Terms of Iodine Status and Intellectual, Motor, and Physical Development. Biol Trace Elem Res 202, 133–144 (2024). https://doi.org/10.1007/s12011-023-03677-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03677-1