Abstract
This study aimed to investigate the effect of boron on porcine mammary epithelial cells (PMECs) survival, cell cycle, and milk fat synthesis. PMECs from boron-treated groups were exposed to 0–80 mmol/L boric acid concentrations. Cell counting kit-8 and flow cytometry assays were performed to assess cell survival and the cell cycle, respectively. Triacylglycerol (TAG) levels in PMECs and culture medium were determined by a triacylglycerol kit while PMECs lipid droplet aggregation was investigated via oil red staining. Milk fat synthesis–associated mRNA levels were determined by quantitative real-time polymerase chain reaction (qPCR) while its protein expressions were determined by Western blot. Low (0.2, 0.3, 0.4 mmol/L) and high (> 10 mmol/L) boron concentrations significantly promoted and inhibited cell viabilities, respectively. Boron (0.3 mmol/L) markedly elevated the abundance of G2/M phase cells. Ten mmol/L boron significantly increased the abundances of G0/G1 and S phase cells, but markedly suppressed G2/M phase cell abundance. At 0.3 mmol/L, boron significantly enhanced ERK phosphorylation while at 0.4, 0.8, 1, and 10 mmol/L, it markedly decreased lipid droplet diameters. Boron (10 mmol/L) significantly suppressed ACACA and SREBP1 protein expressions. The FASN protein levels were markedly suppressed by 0.4, 0.8, 1, and 10 mmol/L boron. Both 1 and 10 mmol/L markedly decreased FASN and SREBP1 mRNA expressions. Ten mmol/L boron significantly decreased PPARα mRNA levels. Low concentrations of boron promoted cell viability, while high concentrations inhibited PMECS viabilities and reduced lipid droplet diameters, which shows the implications of boron in pregnancy and lactation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During lactation, the mammary glands are the most active lipid synthesis and secretion organs [1]. The mammary glands of mammals secrete milk, which triacylglycerols account for the majority of milk fat [2,3,4]. The fat in breast milk is an important source of energy and essential fatty acids for the growth and development of newborns. The late gestation period of the sow is associated with rapid mammary gland development and epithelial cell proliferation, which are important cells in milk lipid and milk protein synthesis. In the sow, the number of mammary epithelial cells may double at parturition, usually in tandem with increased milk production [5]. During lactation, milk components are majorly synthesized from small molecule substrates in mammary epithelial cells (MECs) [6]. Amino acids, fatty acids, glucose, and other hormones as well as bioactive compounds can regulate lactation by stimulating MECs through different sensors and signaling pathways [7]. Valine induces nuclear accumulation of sterol regulatory element–binding protein 1 (SREBP1), which promotes the expressions of fatty acid synthesis–associated proteins, thereby elevating intracellular triacylglycerol levels [8]. Physiologically, SREBP1 preferentially promotes the expressions of fatty acid biosynthesis–associated genes, such as acetyl coenzyme A carboxylase alpha (ACACA) and fatty acid synthase (FASN). Therefore, SREBP1 expressions in the mammary glands or MEC are correlated with milk fat levels and milk fatty acid composition [9, 10]. Acyl-CoA synthetase long-chain family member 3 (ACSL3), as a member of the long-chain acetyl coenzyme A synthase family, has the main biological function of regulating fatty acid metabolism in vivo and catalyzing the conversion of fatty acids into long-chain acetyl coenzyme A, which is important for lipid synthesis, protein modification and β-oxidation. Fatty acid–binding protein 3 (FABP3) is involved in various life activities such as lipid metabolism, energy metabolism, and immune regulation through mediating fatty acid transport. The roles of 1-acylglycerol-3-phosphate O-acyltransferase 1 (AGPAT1), glycerol-3-phosphate acyltransferase 4 (AGPAT6), and glycerol-3-phosphate acyltransferase, mitochondrial (GPAM) are TAG synthesis and lipid droplet formation. The role of peroxisome proliferator–activated receptor alpha (PPARα), insulin-induced gene 1 (INSIG1) is the transcriptional regulation of lipid. Nutritional strategies that are aimed at promoting milk fat production and mammary epithelial cell proliferation in lactating animals can ensure offspring growth and health. Boron, a mineral element that is located in the second cycle of the periodic table of elements (IIIA), has excellent physical as well as chemical properties and is widely used in agriculture, chemical industries, cosmetics, food, medical, and health fields [11]. Adequate amounts of boron in humans and animals have regulatory effects on lipid metabolism, embryonic development, and energy substrate utilization [12]. Boron can result in a significant decrease in serum total cholesterol, low-density lipoprotein (LDL), LDL cholesterol, and triacylglycerol levels [13]. Low boron concentrations have been reported to promote cell proliferation and inhibit apoptosis. Therefore, we treated PMECs with different boron concentrations to elucidate on its molecular mechanisms in milk fat synthesis and provide a reference point for dosing of boric acid during lactation.
Materials and Methods
Cell Cultures
The porcine mammary epithelial cells line was a gift from Guan Wutai’s group at South China Agricultural University. The porcine mammary epithelial cells line was established as described previously [14]. Cells were cultured in modified DMEM/F12 medium containing 10% fetal bovine serum (FBS) (Gibco, USA), 5 μg/mL insulinotransferrin selenium (ITS) (ScienCell, USA), 10 ng/mL epidermal growth factor (EGF) (MCE, USA), 10 ng/mL IGF-1 (MCE, USA), 5 μg/mL hydrocortisone (MCE, USA), and 1% PSN double antibodies (Gibco, USA). The cells were cultured in an incubator at 37 °C with 5% CO2. The culture medium was changed after every 1 day. At a cell fusion rate of 90%, cells were passaged with 0.25% trypsin–EDTA.
Cell Viability Assay
The effects of boric acid on porcine mammary epithelial cells were evaluated using the CCK-8 kit (MCE, USA), as instructed by the manufacturer. Briefly, cell suspensions were inoculated in 96-well cell culture plates (100 μL/well) at a density of 5 × 103 cells/well and incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. Then, cells were exposed to different boric acid concentrations (0, 0.1, 0.2, 0.3, 0.4, 0.8, 1, 10, 20, 40, 80 mM) for 24 h. Subsequently, they were supplemented with 10 μL of CCK-8 solution and incubated at 37 °C for 2 h. Finally, absorbance was measured at 450 nm using a Full Wavelength Enzyme Labeler (Thermo Fisher Scientific, USA).
Cell Cycle Analysis
The PMECs were inoculated in 6-well cell culture plates at density of 5 × 105 cells per well and grown for 24 h. After incubation for 24 h, according to the results of CCK-8 kit, the medium was changed to a medium containing boric acid (0, 0.3, and 10 mM). Then, cells were collected by trypsin digestion and transferred into 70% ethanol for 2 h. Cell cycle analysis was performed using a MoFlo XDP flow cytometer (Beckman-Coulter, USA) and a commercial EZCell™ Cell Cycle Analysis Kit (Biovision, USA), as instructed by the manufacturers.
Intracellular Lipid Droplet Staining Assay
Intracellular lipid accumulation was assessed by Oil Red O (BBI, Canada) staining. Suspensions of PMECs were inoculated in 24-well cell culture plates at a density of 5 × 104 cells per well and incubated at 37 °C in a humidified 5% CO2 environment for 24 h. Then, according to the results of CCK-8 kit, they were exposed to media containing different concentrations of boric acid (0, 0.2, 0.3, 0.4, 0.8, 1, and 10 mM) for 24 h, rinsed twice using PBS, fixed in 4% paraformaldehyde for 30 min at room temperature, and rinsed thrice (10 min each) using PBS. Cell culture plates were supplemented with 200 μL of oil red preparation solution for 3 h, followed by several washes using PBS. Observation and imaging of cells were performed using an inverted microscope. Lipid droplet diameters were measured using the Image-Pro Plus 6.0 software (media, cybernetics, USA).
Quantification of Intracellular TAG Levels
Intracellular TAG levels were determined using an enzymatic colorimetric assay kit (Solarbio, Beijing, China). Briefly, PMECs suspensions were inoculated in 6-well plates at a density of 5 × 105 cells per well and incubated for 24 h. Cells were exposed to medium containing different boric acid concentrations (0, 0.2, 0.3, 0.4, 0.8, 1, and 10 mM) for 24 h. Then, the medium was carefully collected, after which cells were rinsed thrice using PBS, collected by centrifugation in a centrifuge tube with 1 ml of n-heptane: isopropanol reagent and lysed by ultrasound for 1 min (intensity 20%, sonication for 2 s, pause for 1 s). After centrifugation at 8000 × g for 10 min at 4 °C, determination of intracellular and medium TAG levels according to kit instructions, absorbance was measured at 420 nm using a Full Wavelength Enzyme Labeler (Thermo Fisher Scientific, USA). Each experiment was performed in triplicates and repeated at least three times.
Extraction of RNA and Real-time Quantitative PCR
In this assay, PMECs were inoculated in 6-well cell culture plates at a density of 5 × 105 cells per well and incubated for 24 h. The medium was changed to a medium containing different boric acid concentrations (0, 0.2, 0.3, 0.4, 0.8, 1, and 10 mM) and incubated for 24 h. Subsequently, total RNA was extracted from cells using the commercial EZ-press RNA Purification Kit (EZBioscience, USA), as instructed by the manufacturer. Reverse transcription was performed using the commercial Color Reverse Transcription Kit (EZBioscience, USA). Expressions of adipogenic pathway–related genes and the internal reference gene (β-actin) were determined by quantitative real-time polymerase chain reaction (qPCR) and a commercial 2 × SYBR Green qPCR Master Mix kit (EZBioscience, USA), as instructed by the manufacturer. Primers (Table 1) were designed using the Primer Premier 6 software and working efficiency of each designed primer is above 92%.
Western Blot Analysis
The PMECs were inoculated in 6-well cell culture plates at a density of 5 × 105 cells per well and incubated for 24 h. Then, they were supplemented with 120 μL of RIPA lysis buffer (Beyotime, Nanjing, China) containing 1% PMSF (MCE, USA), homogenized and treated with 30 μL of 5 × SDS-PAGE protein loading buffer. Proteins were separated by 5–12% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane that was blocked with 5% BSA. The membrane was probed overnight with the following primary antibodies: anti-FASN (1:1000, ab99539), anti-ACACA (1:1000, ab72046), anti-DGAT1 (1:1000, ab118180), anti-SREBP1 (1:1000, ab3259) and anti-β-actin (1:1000, ab8226) from Abcam, UK as well as with anti-ERK (1:3000, 9102S) and anti-P-ERK (1:3000, 9101S) from Cell Signaling Technology, USA. After washing, membranes were incubated with a secondary antibody, anti-Rabbt IgG (1:5000; Abbine, USA). Chemiluminescence signals were detected using a HRP reagent (Millipore, Billerica, MA), while the fluorescent bands were quantified using the ImageJ processing software.
Data Analysis
Data are expressed as mean ± standard deviation (SD). Data analyses were performed using the SPSS 25.0 software. Comparisons of means among groups was performed using one-way analysis of variance (ANOVA), followed by the Dunnett’s post hoc test for between group comparisons. In this study, p ≤ 0.05 and p ≤ 0.01 were set as the thresholds for significant and extremely significant differences, respectively.
Results
Effects of Different Boron Concentrations on PMEC Viabilities
The effects of different boron concentrations on PMECs viabilities were evaluated by the CCK-8 assay (Fig. 1). Compared to 0 mmol/L, low boron concentrations (0.2, 0.3, and 0.4 mmol/L) significantly enhanced PMEC viabilities (p < 0.01), while high doses (10, 20, 30, 40, and 80 mmol/L) significantly inhibited PMEC viabilities (p < 0.01).
Effects of boron on the viability of porcine mammary epithelial cells. The data are expressed as the mean ± SD (n = 6). A single asterisk (*) indicates significant difference compared to 0 mmol/L boron (p < 0.05). Double asterisks (**) indicate significant difference compared to 0 mmol/L boron (p < 0.01)
Effects of Different Boron Concentrations on PMEC Cycle
After 24 h of 10 mmol/L boron treatment, abundances of PMECs were significantly high in the G0/G1 (p < 0.01) and S (p < 0.05) phases, but were markedly low in the G2/M phase (p < 0.01). After 24 h of 0.3 mmol/L boron treatment, the abundance of PMECs in the G2/M phase was significantly high (p < 0.05) (Fig. 2).
Effect of boron on the cell cycle of porcine mammary epithelial cells assayed using flow cytometry. a Control group; b 0.3 mmol/L group; c 10 mmol/L group; d statistical graph of the effect of boron on the cell cycle of porcine mammary epithelial cells. The data are expressed as the mean ± SD (n = 4). A single asterisk (*) indicates Significant difference compared to 0 mmol/L boron (p < 0.05). Double asterisks (**) indicate significant difference compared to 0 mmol/L boron (p < 0.01)
Effects of Different Boron Concentrations on Intra- and Extracellular Triglyceride Levels
After 24 h of boron treatment, compared with 0 mM, TAG levels in the medium were significantly reduced (p < 0.01) while intracellular TAG levels did not exhibit significant differences (p > 0.05) (Fig. 3).
Effect of boron on triacylglycerol secretion by porcine mammary epithelial cells. a Intracellular; b culture fluid. The data are expressed as the mean ± SD (n = 6). A single asterisk (*) indicates significant difference compared to 0 mmol/L boron (p < 0.05). Double asterisks (**) indicate significant difference compared to 0 mmol/L boron (p < 0.01)
Effects of Different Concentrations of Boron on Maximum Lipid Droplet Diameters of PMECs
Oil red O staining confirmed that cytosolic lipid droplet formation in PMECs was suppressed by 24 h of boron treatment, and their maximum diameters decreased with increasing boron concentrations. Compared to 0 mM, the diameters were significantly reduced by 0.4, 0.8, 1, and 10 mM boron concentrations (p < 0.01) (Fig. 4).
Effects of boron on lipid droplet formation in porcine mammary epithelial cells. a 0 mmol/L boron; b 0.2 mmol/L boron; c 0.3 mmol/L boron; d 0.4 mmol/L boron; e 0.8 mmol/L boron; f 1 mmol/L boron; g 10 mmol/L boron; h statistical results of the largest lipid droplet diameter graph bar: 100 μm. The data are expressed as the mean ± SD (n = 6). A single asterisk (*) indicates significant difference compared to 0 mmol/L boron (p < 0.05). Double asterisks (**) indicate significant difference compared to 0 mmol/L boron (p < 0.01). bar: 100 μm
Expressions of Lactation-related Proteins in PMECs Treated with Different Boron Concentrations
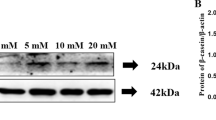
After 24 h of boron treatment, compared with the 0 mM boron treatment group, protein expressions of fatty acid ab initio synthesis of ACACA were significantly suppressed in the 10 mM boron treatment group (p < 0.01), SREBP1 protein expression was significantly inhibited in the 10 mM boron treatment group (p < 0.05), while FASN expressions decreased with increasing boron concentrations. That is, they were significantly suppressed in 0.4 and 0.8 mM boron treatment groups (p < 0.05) but were significantly suppressed in 1 and 10 mM boron treatment groups (p < 0.01). Moreover, ERK phosphorylations were enhanced in the 0.3 mM boron treatment group, compared to the 0 mM boron treatment group. All boron treatment groups of DGAT1 were not significantly different compared to the 0 mM boron treatment group (p > 0.05) (Fig. 5).
Effects of boron on the expression of proteins involved in lipid synthesis in porcine mammary epithelial cells. a Representative protein bands of ACACA, FASN, DGAT1, SREBP1, ERK, and P-ERK. Bar graph of protein expression level relative to β-actin, including b ACACA; c FASN; d DGAT1; e SREBP1; f ERK/P-ERK. The data are expressed as the mean ± SD (n = 3). A single asterisk (*) indicates significant difference compared to 0 mmol/L boron (p < 0.05). Double asterisks (**) indicate significant difference compared to 0 mmol/L boron (p < 0.01)
Expressions of Lactation-associated Genes in PMECs Treated with Different Boron Concentrations
Treatment with boron for 24 h suppressed the expressions of genes involved in de novo synthesis and transcriptional regulation of fatty acids in PMECs. At 0.2–0.8 mM, boron treatment did not affect the mRNA expressions of FASN (p > 0.05); however, 1 and 10 mM boron treatment significantly downregulated the mRNA expressions of FASN (p < 0.05). Compared to 0 mM boron treatment, PPARα mRNA expressions were significantly downregulated (p < 0.05) by 10 mM boron treatment while SREBP1 mRNA expressions were significantly downregulated (p < 0.05) by both 1 and 10 mM boron treatments. Compared to 0 mM boron treatment, all boron treatment groups, boron treatment did not affect the mRNA expressions of ACACA, ACSL3, AGPAT1, AGPAT6, INSIG1, FABP3, GPAM (p > 0.05) (Fig. 6).
Effects of boron on the expression of gene involved in lipid synthesis in porcine mammary epithelial cells. a ACACA; b ACSL3; c FASN; d AGPAT1; e AGPAT6; f SREBP1; g PPARα; h INSIG1; i FABP3; j GPAM; the data are expressed as the mean ± SD (n = 3). A single asterisk (*) indicates significant difference compared to 0 mmol/L boron (p < 0.05). Double asterisks (**) indicate significant difference compared to 0 mmol/L boron (p < 0.01)
Discussion
Low concentrations of boron (0.2–0.4 mmol/L) significantly enhanced the viabilities of PMECs, which were markedly suppressed by higher boron concentrations (10 ≥ mmol/L) in current study. The finding imply that the optimal concentration range of boron for PMECs survival is 0.2–0.4 mmol/L. The flow cytometry assay found that the 0.3 mmol/L boron dose significantly increased the number of PMECs in the G2/M phase; however, higher doses of boron (concentrations above 10 mmol/L) exhibited significant toxic effects, inhibiting cell viabilities and increasing the number of cells remaining in G0/G1 and S phases. Xiaoting Zhang [15] reported that low concentrations (0.01–0.1 mmol/L) of boron promoted the proliferation and inhibited the apoptosis of ostrich spleen lymphocytes by enhancing cell membrane functions as well as intracellular catalase activities, whereas high concentrations (25–100 mmol/L) of boron exerted the opposite effects on cells. Comparable with previous studies [16, 17], boron concentrations below 0.5 mmol/L significantly improved the viabilities of testicular Sertoli cells in vitro and inhibited their apoptosis. However, high doses of boron (concentrations above 5.0 mmol/L) exhibited significant toxic effects, inhibiting cell viabilities, accelerating Sertoli cell apoptosis, and arresting the cell cycle in the G0/G1 phase. Moreover, we established that low doses of boron can achieve better results in promoting immune responses in rat duodenum, and the physiological amount of boron is a regulator of energy substrate metabolism [18]. In present experiment, 0.3 mmol/L boron significantly promoted ERK phosphorylations. The ERK signaling pathway is majorly involved in cell differentiation as well as proliferation and is highly associated with pro-proliferative and anti-apoptotic roles [19]. Therefore, promotion of cell viabilities by low boron concentrations may be related to activation of the ERK signaling pathway. High boron concentrations inhibited cell viabilities, which may be attributed to their toxic effects [20].
The nutrient composition and level of sow milk is regulated by nutrients. Milk fat is the main component that determines the value of sow milk [21]. We established that low boron concentrations promoted the viabilities of PMECs while TAG levels in the medium were markedly decreased after 24 h of boron treatment. Moreover, a decreasing trend of lipid droplet diameter was revealed by oil red staining. Enzymes in the biosynthesis pathway of fatty acids, triacylglycerols, and cholesterol are regulated by sterol regulatory element–binding proteins (SREBP), which are among the most important families of transcription factors involved in lipid homeostasis [22]. SREBP1 is the most physiologically relevant, and its activity indicates the lipid synthesis capacities [23]. Amino acids, fatty acids, glucose, as well as some hormones and bioactive substances, sense extracellular stimuli through different sensors and signaling pathways (mainly mTOR with SREBP1) to regulate milk synthesis [24]. Therefore, the reasons for the decrease in TAG levels and the decreasing trend of lipid droplet diameter in the present study may be attributed to various factors. First, DGAT1 protein expression and SREBP1 protein expression at low boron concentrations were unchanged. But SREBP1 protein expressions were significantly decreased at high boron concentrations (10 mmol/L). Second, expressions of ACACA and FASN, which are key proteins in fatty acid ab initio synthesis were decreased. Although the protein expression of ACACA and FASN did not change at low boron concentrations. Expressions of ACACA were significantly decreased at high boron concentration treatment, while FASN expressions gradually decreased with increasing boron concentrations. Third, no significant difference in mRNA expression of all genes in low boron treatment; meanwhile, the relative mRNA expressions of FASN and SREBP1 were significantly suppressed at high boron treatment (1 and 10 mmol/L). Moreover, mRNA expressions of PPARα, a free fatty acid receptor that plays a key role in fatty acid β-oxidation and energy metabolism, were significantly decreased in high boron concentrations. The result implies that boron inhibits TAG synthesis by suppressing the expressions of PPARα and target adipogenic genes. Fatty acids and eicosanoids are natural ligands of PPAR, and the binding of these ligands to PPARα can regulate gene expressions as well as lipogenesis rates [25].
According to our results, the reduction of triacylglycerol levels and lipid droplets in cell cultures may also be attributed to boron inhibiting fatty acid ab initio synthesis-related genes (ACACA and FASN), possibly via SREBP1 regulation. Physiologically, SREBP1 is a key regulator of genes encoding proteins involved in ab initio synthesis of fatty acids (ACACA and FASN) in mammary epithelial cells [26]. Boron inhibits adipogenesis by the Wnt/β-linked protein pathway in progenitor cells [27]. Boron was reported to significantly inhibit adipogenic differentiation of hADSCs, with progressive decreases in lipid deposition as its concentrations increased, and it suppresses the main regulators of the adipogenic transcriptional program at mRNA and protein levels [28]. These findings are consistent with the present research, whereby it is possible that boron inhibited the transcription as well as translation of ACACA and FASN by targeting SREBP1 or inhibited the activities of key enzymes, including oxidoreductase, transferase, hydrolase, and isomerase. Moreover, boron competitively inhibits pyridine as well as flavin nucleotides as coenzymes of oxidoreductase and affects energy substrate utilization by competing with these enzymes for NAD+ and flavin [29]. In current study, low boron concentrations increased the cell viabilities, and the reason of the regulation might be boron, as a mineral element, promotes cell proliferation at low concentrations. High boron concentrations are cytotoxic and inhibit the key pathway of milk lipid synthesis, SREBP1, and fatty acid synthesis from scratch (ACACA and FASN). Due to the effects of high boron concentrations, TAG levels in the cell culture medium were reduced while lipid diameters also showed gradual decreases.
Conclusions
Low boron concentrations promoted PMECs viabilities, while high concentrations inhibited PMECs viabilities and reduced lipid droplet diameters. These findings provide a basis for supplementation of boron during pregnancy and lactation as well as in feed additives.
Data Availability
The raw data supporting this study will be available by authors to any qualified researcher upon request.
References
Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM (2003) Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res 44(6):1100–12. https://doi.org/10.1194/jlr.M300045-JLR200
Russell TD, Palmer CA, Orlicky DJ, Fischer A, Rudolph MC, Neville MC, McManaman JL (2007) Cytoplasmic lipid droplet accumulation in developing mammary epithelial cells: roles of adipophilin and lipid metabolism. J Lipid Res 48(7):1463–75. https://doi.org/10.1194/jlr.M600474-JLR200
Lv Y, Guan W, Qiao H, Wang C, Chen F, Zhang Y, Liao Z (2015) Veterinary medicine and omics (veterinomics): metabolic transition of milk triacylglycerol synthesis in sows from late pregnancy to lactation. Omics : a J Integrative Biol 19(10):602–16. https://doi.org/10.1089/omi.2015.0102
Craig A, Henry W, Magowan E (2016) Effect of phase feeding and valine-to-lysine ratio during lactation on sow and piglet performance. J Anim Sci 94(9):3835–3843. https://doi.org/10.2527/jas.2016-0648
Anthony VC, Steven EE (2013) Comparative aspects of mammary gland development and homeostasis. Annu Rev Anim Biosci 1:179–202. https://doi.org/10.1146/annurev-animal-031412-103632
Macias H, Hinck L (2012) Mammary gland development. Wiley Interdiscip Rev Dev Biol 1(4):533–57. https://doi.org/10.1002/wdev.35
Anthony VC, Ratan KC (2020) Symposium review: determinants of milk production: understanding population dynamics in the bovine mammary epithelium. J Dairy Sci 103(3):2928–2940. https://doi.org/10.3168/jds.2019-17241
Che L, Xu M, Gao K, Zhu C, Wang L, Yang X, Wen X, Xiao H, Jiang Z, Wu D (2019) Valine increases milk fat synthesis in mammary gland of gilts through stimulating AKT/MTOR/SREBP1 pathwaydagger. Biol Reprod 101(1):126–137. https://doi.org/10.1093/biolre/ioz065
Bionaz M, Loor J (2008) Gene networks driving bovine milk fat synthesis during the lactation cycle. Bmc Genomics 9(1):1–21. https://doi.org/10.1186/1471-2164-9-366
Rudolph MC, Monks J, Burns V, Phistry M, Marians R, Foote MR, Bauman DE, Anderson SM, Neville MC (2010) Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol-Endocrino Metabol 299(6):E918-27. https://doi.org/10.1152/ajpendo.00376.2010
Devirian TA, Volpe SL (2003) The physiological effects of dietary boron. Crit Rev Food Sci 43(2):219–31. https://doi.org/10.1080/10408690390826491
Forrest HN (2009) Boron deprivation decreases liver S -adenosylmethionine and spermidine and increases plasma homocysteine and cysteine in rats. J Trace Elem Med Bio 23(3):204–13. https://doi.org/10.1016/j.jtemb.2009.03.001
Ruya K, Sahin Y, Gulsah B, Burcin AT, Pakize NT, Serap A, Feyza YO, Yuksel A, Aysen Y, Fikrettin S (2019) Boron-rich diet may regulate blood lipid profile and prevent obesity: a non-drug and self-controlled clinical trial. J Trace Elem Med Bio 54:191–198. https://doi.org/10.1016/j.jtemb.2019.04.021
Zheng Y, He X (2010) Characteristics and EGFP expression of porcine mammary gland epithelial cells. Res Vet Sci 89(3):383–390. https://doi.org/10.1016/j.rvsc.2010.03.023
Zhang X, Xiao K, Qiu W, Wang J, Li P, Peng K (2020) The immune regulatory rffect of boron on ostrich chick splenic lymphocytes. Biol Trace Elem Res 199(7):2695–2706. https://doi.org/10.1007/s12011-020-02392-5
Lu L, Zhang Q, JinE, Hu Q, Zhao C, Li S, Ren M (2020) Effects of boron on cytotoxicity, apoptosis, and cell cycle of cultured rat sertoli cells in Vitro. Biol Trace Elem Res 196(1):223–230. https://doi.org/10.1007/s12011-019-01911-3
Zhao C, Han Y, Wang C, Ren M, Hu Q, Gu Y, Ye P, Li S, Jin E (2021) Transcriptome profiling of duodenum reveals the importance of boron supplementation in modulating immune activities in rats. Biol Trace Elem Res 200(8):3762–3773. https://doi.org/10.1007/s12011-021-02983-w
Hunt CD (1994) The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ Health Persp 102(suppl 7):35–43. https://doi.org/10.1289/ehp.94102s735
Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Aoyama M, Niwa A, Nakura T, Takayasu M (2012) Activation of Ras/MEK/ERK signaling in chronic subdural hematoma outer membranes. Brain Res 13(1489):98–103. https://doi.org/10.1016/j.brainres.2012.10.013
Hadrup N, Frederiksen M, Sharma AK (2021) Toxicity of boric acid, borax and other boron containing compounds: a review. Regul Toxicol Pharm 121:104873. https://doi.org/10.1016/j.yrtph.2021.104873
Harvatine KJ, Boisclair YR, Bauman DE (2009) Recent advances in the regulation of milk fat synthesis. Animal 3(1):40–54. https://doi.org/10.1017/S1751731108003133
Li J, Huang Q, Long X, Zhang J, Huang X, Aa J, Yang H, Chen Z, Xing J (2015) CD147 Reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARalpha pathways. J Hepatol 63(6):1378–89. https://doi.org/10.1016/j.jhep.2015.07.039
Kim YM, Shin HT, Seo YH, Byun HO, Yoon SH, Lee IK, Hyun DH, Chung HY, Yoon G (2010) Sterol regulatory element-binding protein (SREBP)-1-mediated lipogenesis is involved in cell senescence. J Biol Chem 285(38):29069–77. https://doi.org/10.1074/jbc.M110.120386
Han M, Zhang M (2021) The regulatory mechanism of amino acids on milk protein and fat synthesis in mammary epithelial cells: a mini review. Anim Biotechnol 1–11. https://doi.org/10.1080/10495398.2021.1950743.
Joel B, David EM (2002) The mechanisms of action of ppars. Annu Rev Med 53:409–35. https://doi.org/10.1146/annurev.med.53.082901.104018
Xu H, Luo J, Zhao W, Yang Y, Tian H, Shi H, Bionaz M (2016) Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J Dairy Sci 99(1):783–95. https://doi.org/10.3168/jds.2015-9736
Doğan A, Demirci S, Apdik H, Bayrak OF, Gulluoglu S, Tuysuz EC, Gusev O, Rizvanov AA, Nikerel E, Şahin F (2017) A new hope for obesity management: boron inhibits adipogenesis in progenitor cells through the Wnt/β-catenin pathway. Metabolism 69:130–142. https://doi.org/10.1016/j.metabol.2017.01.021
Abdik EA, Abdik H, Taşlı PN, Deniz AAH, Şahin F (2019) Suppressive role of boron on adipogenic differentiation and fat deposition in human mesenchymal stem cells. Biol Trace Elem Res 188(2):384–392. https://doi.org/10.1007/s12011-018-1428-5
Hunt CD, Stoecker BJ (1996) Deliberations and evaluations of the approaches, endpoints and paradigms for boron, chromium and fluoride dietary recommendations. J Nutri 126(9 Suppl):2441S-2451S. https://doi.org/10.1093/jn/126.suppl_9.2441S
Funding
This study was funded by the National Natural Science Foundation of China (project no.31501968), Major Special Projects of Science and Technology in Anhui Province (project no. 201903a06020002), Academic Support Project for University Top Talents in Anhui Province (gxbjZD2020080), Chuzhou Municipal Science and Technology Project (2022ZN002), and Anhui Provincial Scientific Research Planning Project (Excellent Youth Scientific Research Project) (2022AH030146).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yanan Yang and Ya Yang. The first draft of the manuscript was written by Yanan Yang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Yang, Y., Li, X. et al. Effects of Boron on Fat Synthesis in Porcine Mammary Epithelial Cells. Biol Trace Elem Res 202, 190–198 (2024). https://doi.org/10.1007/s12011-023-03663-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03663-7