Abstract
Childhood atopic dermatitis (AD) is a chronic and recurrent health problem that involves multiple factors, particularly immunological and environmental. We evaluated the impact of docosahexaenoic acid (DHA) supplementation on prenatal arsenic exposure on the risk of atopic dermatitis in preschool children as part of the POSGRAD (Prenatal Omega-3 fatty acid Supplements, GRowth, And Development) clinical trial study in the city of Morelos, Mexico. Our study population included 300 healthy mother–child pairs. Of these, 146 were in the placebo group and 154 in the supplement group. Information on family history, health, and other variables was obtained through standardized questionnaires used during follow-up. Prenatal exposure to arsenic concentrations, which appear in maternal urine, was measured by inductively coupled plasma optical emission spectrometry. To assess the effect of prenatal arsenic exposure on AD risk, we ran a generalized estimating equation model for longitudinal data, adjusting for potential confounders, and testing for interaction by omega-3 fatty acid supplementation during pregnancy. The mean and SD (standard deviation) of arsenic concentration during pregnancy was 0.06 mg/L, SD (0.04 mg/L). We found a marginally significant association between prenatal arsenic exposure and AD (OR = 1.12, 95% CI: 0.99, 1.26); however, DHA supplementation during pregnancy modified the effect of arsenic on AD risk (p < 0.05). The results of this study strengthen the evidence that arsenic exposure during pregnancy increases the risk of atopic dermatitis early in life. However, supplementation with omega-e fatty acids during pregnancy could modify this association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis (AD) is one of the most common pediatric diseases globally and is the first manifestation of the “atopic triad” which includes AD, allergic rhinitis, and asthma. AD is a chronic, pruritic, and recurring skin disorder [1, 2]. It manifests clinically as dermatosis with erythematous and pruritic lesions and papules, which converge in large lichenified plaques [3].
AD can have a profoundly negative effect on the quality of life of children [1, 4], and imposes a significant additional burden on families, both in care and management costs. These costs are comparable to or even greater than other chronic childhood diseases such as asthma and diabetes [1, 4, 5]. In Mexico, the prevalence of eczema in children by medical diagnosis and symptoms for the year 1995 was 4.1% (95% CI 3.6–4.6) and 10.3% (confidence interval to 95% (95% CI): 9.6–11.1), respectively, and 6.5% (95% CI 5.8–7.3) and 5.6% (95% CI 4.9–6.3) for 2002 [6]. AD is evaluated mainly by its clinical symptoms; the Scoring Atopic Dermatitis (SCORAD) index is one of the most used methods for diagnosis and to assess its severity. The score is based on the evaluation of the symptoms of the skin, such as the extent and intensity of lesions, and subjective signs like pruritus and/or sleep disturbances [7, 8].
AD is a multifactorial disease, in particular genetic, immunological, and environmental factors [9] and is characterized by an increase in IgE production and/or nonspecific reactivity alterations (immunopathogenesis). These explain the cellular and biochemical changes observed in atopic skin [3]. Likewise, reports are indicating that exposure to arsenic during pregnancy may be associated with an increase in the prevalence of the disease. This is probably because arsenic can alter fetal immune regulation, causing a predominance of a Th2-type immunity response, which in turn favors an increase in the probability of allergic and/or atopic reactions [2, 10]; however, results have been contradictory and inconclusive [11,12,13].
On the other hand, experimental and epidemiological studies have shown that supplementation with polyunsaturated fatty acids (PUFAs), including docosahexaenoic acid (DHA), during pregnancy (ideally between 18 and 20 weeks of gestation) and lactation, protects against the risk of atopy. PUFAs can influence the newborn’s immune system in the prenatal and postnatal stages and protect against possible epigenetic modifications. This reduces the risk of developing allergic diseases and asthma after birth or in an early life stage [14,15,16,17,18,19,20,21,22].
For this reason, we conducted the present study to assess if exposure to arsenic during pregnancy increases the risk of developing AD during the early stages of life and evaluate whether supplementation with DHA during pregnancy could modify this effect.
Methods
Design and Study Population
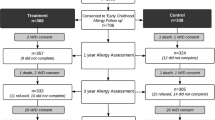
The analysis in this study is based on the POSGRAD (Prenatal Omega-3 fatty acid Supplements, GRowth, And Development) cohort study; a large double-blind, randomized controlled trial of prenatal DHA supplementation. The study design of the clinical trial has been described in detail elsewhere [23,24,25]. Briefly, women eligible for the study were 18 to 35 years of age and in their 18th to 22nd week of gestation. All participants had normal pregnancies, without any pregnancy-related diseases or complications. Of the 1,040 women who started treatment, 978 completed the study, and 973 live infants were delivered. For this report, we included a subsample of 193 mother–child pairs, of which 92 were from the placebo group and 101 were from the supplementation group, and all had provided complete information data at 5 years of age. The participant women gave informed consent for themselves and their children to participate in the study, and they were asked to sign an informed written consent letter. Their participation was completely free and voluntary. The Ethics and Investigation Committees of the Mexican National Institute of Public Health (Instituto Nacional de Salud Pública, INSP) and the Emory University Ethics Committee approved the investigation protocol.
Data Collection
Atopic Dermatitis Diagnosis
A diagnosis of AD was established by the presence of signs and symptoms on the skin, allowing for the use of the SCORAD index. This was complemented with the measurement of total and specific immunoglobulin E (IgE) levels in cord blood and again at 4 years of age (the child was considered atopic if the concentration of specific IgE levels was greater than or equal to 0.35 IU/mL for any of the allergens analyzed). Concerning the identification of signs and symptoms (presence of papules and itching, location of the lesions in folds, elbows, face, and/or behind the knees) [7, 12], we used a standardized and validated questionnaire which was applied to children at 1, 3, 6, 9, 12, 18, 24, 36, 48, and 60 months of age by a specialist medical doctor, who also clinically evaluated each child during follow-up and whose purpose was to construct the SCORAD index measurement using previously defined criteria. A child was classified as atopic when their SCORAD index was greater than 0 points [26, 27].
Assessment of Prenatal Exposure to Arsenic
First-morning urine samples were collected from the mother between 18 and 22 weeks of pregnancy; containers had been provided previously, and they had been given instructions on sample collection procedures. The urine samples were refrigerated at 4 °C and were then aliquoted and frozen at − 70 °C until the time of analysis. Arsenic concentrations were measured using inductively coupled plasma optical emission spectrometry (Thermo Scientific), after a sample digestion step in the laboratory of the Technological Institute of Sonora. In brief, 3 mL of the urine samples were placed in a linear microwave digestion vessel; 3 mL of concentrated nitric acid (HNO3) and 1.5 mL of hydrogen peroxide (H2O2) were added, and the samples were digested (CEM Corp., Matthews, NC) for 35 min at 200 °C. After microwave digestion, samples were adjusted to a final volume of 25 mL with HPLC grade water. For quality control purposes, blank and duplicate samples were analyzed during the procedure. To ensure assay quality, we used the 3669 Standard Reference Material, Arsenic Species in Frozen Human Urine (NIST, Gaithersburg, MD), and the CWW-TB Certified Wastewater Standard (High Purity, Charleston, SC). For arsenic, the recovery was between 82 and 106%. The coefficient of correlation was 0.99, and the coefficient of variation was less than 8%. The quantification limit of arsenic had an average value of 0.010 mg/L, and this limit was based on the regulation for As species in drinking water set by World Health Organization (WHO) and the US Environmental Protection Agency (USEPA). Creatinine (Cr) determination was performed using high-resolution liquid chromatography, and the Cr levels were used to correct urine dilution.
Randomization and Intervention with Omega 3 Fatty Acids
Using a computer-generated list, all eligible women were randomly placed in either the active treatment or the placebo group [24]. After signing the letter of informed consent, women were randomly assigned to receive 400 mg/d daily of algal DHA or a soy and corn oil-based placebo from between the 18th and 22nd week of pregnancy through to delivery.
Atopic Mother Status
Information on the maternal history of allergy or atopy was obtained via a questionnaire given during pregnancy. In addition, total and specific IgE levels were measured. With this information, an atopic maternal index was created using the combination of symptoms and the specific IgE levels (the mother was considered atopic if the concentration of specific IgE levels was greater than or equal to 0.35 IU/mL for any of the allergens analyzed).
Information on Other Variables
At baseline, the participating women filled out a general health questionnaire that included information on weight and height before delivery, sociodemographic characteristics, health, and gynecological antecedents. In addition, an environmental questionnaire (water consumption, source of food and water, tobacco exposure, humidity, mildew, mold or saline formation on walls or floor, pet presence at home, presence of industry near home, and pesticide use), as well as a dietary questionnaire was administered during pregnancy. The child’s growth (height and weight) was measured during each follow-up visit.
Statistical Analysis
A descriptive analysis was performed to characterize the total study population by treatment group, using statistical mean differences tests (t student) and independence tests (Chi-square), depending on the variable type. For the hypotheses proposed, a probability of p < 0.05 was considered for statistical significance. To evaluate the effect of prenatal exposure to arsenic and the risk of AD, we used a generalized estimating equation model for longitudinal data with a binomial distribution and logit (p) function. The model was adjusted by maternal atopy, breastfeeding, sex of the child, and follow-up time. In addition, we built a model for all participants and each treatment group considering the arsenic level as continuous (arsenic µg per g of Cr) and divided it into terciles (arsenic, µg/L). Other variables, such as pets or animals at home; the presence of humidity, mildew, mold, or saline formation on walls or floor; use of pesticides; tobacco smoke exposure at home; source of drinking water; and industry near home, were tested as confounders; however, they did not modify the association and were not included in the final models. Statistical analyses were realized using STATA version 14.
Results
The study population involved 193 mother–child pairs, stratified by treatment group; 47.0% came from the placebo group and the rest from the supplement group (DHA). The average age of the women was 26.2 with a standard deviation of 4.8 years and a body mass index of 22.2 kg/m2 (SD 4.3) registered before pregnancy. Two-thirds of the women (72.5%) were classified as having a medium or low socioeconomic level. As to prenatal characteristics, average gestational age of 39.2 weeks (SD 2.1) was observed, 28.5% of the women reported being in their first pregnancy, and 52.3% of births were by cesarean. Regarding the history of maternal allergy, 29.5% of the mothers was classified as allergic or atopic based on the index created, and only 9.9% manifested characteristic symptoms of AD (Table 1).
Of all of the children in the study population, 54.4% were male, and they had an average weight and size of 3,195.1 g (SD 406.4) and 50.5 cm (SD 2.3), respectively. Only 1.0% of infants weighed less than 2,500 g at birth, and 6.2% showed signs of fetal distress during labor (Table 1).
No statistically significant differences were observed between the baseline characteristics of the study population by supplementation group (Table 1).
Figure 1 shows the percentage of incident cases of AD in children by supplementation group during the study follow-up. We observed that the number of cases in the first month of birth was 8.7% and 11.9% for the DHA and placebo groups, respectively. After the third month, the amount of cases drops sharply; subsequently, there is a non-constant increase in cases of this childhood condition during follow-up visits. Another point to note is the cyclically observed difference in the prevalence over time between the treatment groups, with higher values in the placebo group compared with those of the DHA supplementation group at 3, 6, 12, 24, and 36 months of age.
The geometric mean arsenic levels during pregnancy were 28.9 µg/L (confidence Interval to 95% (95% CI): 24.7, 33.7). By supplementation group, they were 29.4 µg/L (95% CI: 23.1, 37.3) for the placebo group and 28.4 µg/L (95% CI: 23.1, 34.9) for the DHA group. According to the median value, 50% or fewer mothers during pregnancy registered arsenic levels equal to or lower than 42.0 µg/L (Table 2).
Regarding other risk factors considered in the study (Table 3), no statistically significant differences were observed in AD prevalence by the supplementation group. 14.9% of mothers stated that their drinking water comes from their municipality or a private well, and in 37.0% of children’s homes, there was at least one person who smokes indoors. As for the children’s housing environment, in 34.6% of households, humidity, mildew, mold, or saline formation on walls or floor were present; 71.3% of households had a pet or animal, 36.6% of the women reported having an industry near the house where they live, and 81.8% of participants reported the presence of pests at home (spiders, cockroaches or ants).
The models considering the association between prenatal exposure to arsenic (continuous variable) and the risk of AD for the total population and stratified by treatment group were not significant. These results show a non-significant risk of developing AD in the children of mothers who did not receive DHA (the placebo group), contrary to results observed in those children whose mothers did receive DHA. An interquartile range increase (or a 10.6-fold increase) in arsenic µg per g of Cr level is associated with an odds ratio of AD equal to 1.05 (95% CI: 0.75, 1.48) (i.e., a 5% increase in the odds of having AD). Divided into supplementation groups, in the placebo group, we observed that the risk of developing AD increases by 20% through a rise of an interquartile range of arsenic in µg per g of Cr, whereas the risk of developing AD decreases by 9% in children whose mothers were supplemented with DHA (Table 4).
In addition, when we stratified arsenic levels in terciles, in the placebo group, we observed a significant effect on AD when maternal middle exposure (tercile 2) to arsenic was compared with low exposure (tercile 1); there was a 96% higher possibility of developing AD when the children’s mothers were exposed to middle levels of arsenic rather than low (Table 4).
Discussion
This study’s results suggest that prenatal exposure to arsenic increases the risk of AD in the early years of life: results that are consistent with those reported in other studies worldwide [28,29,30,31]. To our knowledge, few studies have been conducted to assess the effects of prenatal arsenic exposure on atopic diseases in children and the impact of DHA supplementation in a birth cohort study: a design that strengthens the results.
Although our results show a risk of developing AD at an early age when the mother has been exposed to arsenic during pregnancy, this was not statistically significant. This may be because the sample size was limited and because our study only followed the child up to 5 years of age; according to Liu et al., it is between 8 and 11 years when the highest peak of CB-tIgE (cord blood total IgE) is identified, and these levels are associated with atopic diseases including AD [30]. However, it is important to mention that concentrations of total urinary arsenic obtained from the mothers showed levels higher than those reported by the US Environmental Protection Agency during the period from 2003 to 2016, in its National Report on Human Exposure to Environmental Chemicals [32]. Geometric mean arsenic levels for our study was 28.9 µg/L; this value is relevant because the total As concentration in the mother’s urine is an exposure marker for the minimum amount of As that fetus is exposed; if a greater amount of total As than 0.010 mg/L is detected in the mother’s urine, it means that the fetus has been exposed to at least that concentration of arsenic as well. For the USEPA and the WHO, this value is the limit from which a human being could have effects of long-term, chronic exposure to arsenic [33, 34].
Exposure to arsenic and the damage it causes to health in humans has been studied for several decades; however, more research is needed to clarify these effects when they occur at an early age [11, 13].
Likewise, some studies have reported significant associations between the concentration of arsenic and alterations in the immune system, such as high concentrations of IgE in the umbilical cord, changes in isotype of immunoglobulin (Change to IgE or IgG), altered levels of some cytokines such as IL-2, and the modification of the expression of genes related to the immune response, including those that are involved in the signaling of receptors in T cells and which modify the relationships between them. Another study conducted in the USA in 2014 by Nadeau et al. found alterations in the immune systems of pregnant women exposed to high levels of arsenic (even with a relatively low intrauterine exposure) and consequently an immune fetal dysregulation. As a result, children experienced a predominance of Th2 immunity and increased activation of the immune response, increasing the risk of exacerbated allergic reactions [32].
On the other hand, Ashley-Martin and collaborators carried out a maternal-infant cohort study in ten Canadian cities and found a non-significant association between arsenic concentration and high IgE concentrations in the umbilical cord blood (OR = 1.20) [35].
The biological mechanism of how exposure to arsenic during pregnancy can affect children and lead to AD and other allergic conditions during their lives has not been clearly identified. However, studies report that arsenic could cause genetic changes through oxidative damage, modifying DNA methylation patterns, and altering genetic material packing in cell lines and animal models. This could lead to changes in gene expression and increase the risk of induced latent disease and carcinogenesis at the dermatological level [35, 36]. Likewise, there is experimental evidence of epigenetic damage in human populations, since exposure to arsenic has been associated with the activation of pathways involving the NF-κB gene, which regulates critical genes related to cellular response (inflammation, cell proliferation, stress, and apoptosis). A study conducted in Argentina found that, with high exposure to arsenic, several key genes in the immune system were hyper-methylated and showed an increase in immune system processes related to the basic functions of T CD4 + cells [37, 38]. Also, a pilot study conducted in 2015 in Bangladesh found that there is evidence that in utero exposure to arsenic may alter DNA methylation patterns of the umbilical artery tissue and placenta, but not in the endothelial cells of the umbilical vein. This study also showed that the hyper-methylation of the epidermal filaggrin gene was associated with arsenic concentrations in drinking water [35, 39].
In this regard, the present study supports the evidence found in previous research on the risk associated with exposure to arsenic and the presence of AD in the early stages of life (OR = 1.05 (0.75, 1.48)). This situation has been previously reported for allergic conditions such as asthma and rhinitis, which are related to AD as part of the previously mentioned atopic triad [14, 22, 25, 40]. On the other hand, when stratifying by supplementation group, the associated risk is maintained in the placebo group (1.20 (95% CI: 0.76, 1.91)) and becomes a protector (95% CI: 0.91 (0.56, 1.48)) in the DHA group. There is evidence that suggests that DHA supplementation could mitigate the toxic effects of some metals like arsenic on allergic conditions. Romieu et al. 2007 found that there could be a protective effect of fish intake during pregnancy on the risk of AD in offspring. The protective mechanism of action of n-3 polyunsaturated fatty acids is not clearly established, although some studies suggest that supplementation with these during pregnancy and the early postnatal period protects due to their immunomodulation properties during pregnancy; they lower the levels of T-helper type 2 (Th2) cytokines, which plays an important role in the altered immune response of atopic patients [14]. Türkez et al. reported in 2012 that DHA decreases oxidative stress and that the formation of micronuclei induced by environmental contaminant 2,3,7,8-tetrachlorodibenzodioxin in rats treated with hepatocytes. Additionally, in another study in 2018, Abhilash et al. found that DHA had a protective potential to fight against the cytotoxicity induced by the arsenic cell culture of human hepatocytes [41].
Some limitations should be considered when interpreting our results. We believe that our detection power was adversely affected by several conditions that made it difficult to elucidate the negative effect on the health of individuals. These conditions include the fact that the species derived from As(III) are more toxic than those derived from As(V); free arsenic toxicity is higher than metabolized arsenic; and varying expressions of arsenic metabolizing enzymes, due to interpersonal variability. Therefore, the limited sample size and the lack of speciation made it difficult to identify the associated risk. In light of the findings mentioned above, the assessment of maternal exposure to arsenic was performed only once (single measurement) during pregnancy, which limits the study’s power to confirm that the exposure to arsenic was maintained throughout the pregnancy. Also, the arsenic in urine was measured as a total value and was not speciated. Speciation in arsenic is important since, as mentioned before, different species of arsenic have different rates of toxicity in the human body.
However, the prospective nature of this birth cohort study may support the plausibility of causal associations. In addition, it is important to highlight that—as mentioned before—high exposure to arsenic was found among participant women, which could lead to higher risks of allergic outcomes in their children.
In summary, our findings strengthen the evidence on the negative effect of arsenic exposure during pregnancy on the risk of AD in preschool age. This is a very important public health issue. There is currently a serious problem of exposure to this metal, mainly due to the natural contamination of water. Furthermore, we observed that supplementation with omega-e fatty acids during pregnancy could mitigate the damaging effects of arsenic on AD. However, it is important to continue with studies that consider larger sample sizes that permit the identification of areas of environmental exposure risk and also allow for the implementation of improved prevention strategies against this type of exposure.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available because the privacy of individual participants could be compromised but are available from the corresponding author on reasonable request.
References
Odhiambo J et al (2009) Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 124:1251–8.e23. https://doi.org/10.1016/j.jaci.2009.10.009
Thomsen S (2014) Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy 2(2014):354250. https://doi.org/10.1155/2014/354250
Leung D, Soter N (2001) Cellular and immunologic mechanisms in atopic dermatitis. Acad Dermatol 44:S1–S12. https://doi.org/10.1067/mjd.2001.109815
Lewis-Jones S (2006) Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 60:984–992. https://doi.org/10.1111/j.1742-1241.2006.01047.x
Pustišek N, Vurnek Živković M, Šitum M (2016) Quality of life in families with children with atopic dermatitis. Pediatr Dermatol 33:28–32. https://doi.org/10.1111/pde.12698
Barraza-Villarreal A, Hernandez-Cadena L, Moreno-Macias H et al (2007) Trends in the prevalence of asthma and other allergic diseases in schoolchildren from Cuernavaca, Mexico. Allergy Asthma Proc 28:368–374. https://doi.org/10.2500/aap.2007.28.2998
JF Stalder A Taïeb DJ Atherton et al (1993) Severity scoring of atopic dermatitis: The SCORAD index: Consensus report of the European task force on atopic dermatitis Dermatology 186.https://doi.org/10.1159/000247298
Oranje AP (2011) Practical issues on interpretation of scoring atopic dermatitis: SCORAD Index, objective SCORAD, patient-oriented SCORAD and Three-Item Severity score. Curr Probl Dermatol 41:149–155. https://doi.org/10.1159/000323308
Kantor R, Silverberg JI (2017) Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol 13:15–26. https://doi.org/10.1080/1744666X.2016.1212660
Schäfer T, Nienhaus A, Vieluf D et al (1998) Prevalence of atopic eczema and body burden of arsenic. Epidemiology 9(S4):S151
Schäfer T, Heinrich J, Wjst M et al (1999) Indoor risk factors for atopic eczema in school children from East Germany. Environ Res 81:151–158. https://doi.org/10.1006/enrs.1999.3964
Ring J, Przybilla B, Ruzicka T (2006) Handbook of atopic eczema. Springer-Verlag, Berlin Heidelberg, New York, Second
Kathuria P, Silverberg JI (2016) Association of pollution and climate with atopic eczema in US children. Pediatr Allergy Immunol 27(5):478–485. https://doi.org/10.1111/pai.12543
Romieu I, Torrent M, Garcia-Esteban R et al (2007) Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy 37:518–525. https://doi.org/10.1111/j.1365-2222.2007.02685.x
Furuhjelm C, Warstedt K, Larsson J et al (2009) Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 98:1461–1467. https://doi.org/10.1111/j.1651-2227.2009.01355.x
Lumia M, Luukkainen P, Tapanainen H et al (2011) Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr Allergy Immunol 22:827–835. https://doi.org/10.1111/j.1399-3038.2011.01202.x
D’Vaz N, Meldrum SJ, Dunstan JA et al (2012) Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics 130:674–682. https://doi.org/10.1542/peds.2011-3104
D’Vaz N, Meldrum SJ, Dunstan JA et al (2012) Fish oil supplementation in early infancy modulates developing infant immune responses. ClinExp Allergy 42:1206–1216. https://doi.org/10.1111/j.1365-2222.2012.04031.x
Lee H-S, Barraza-Villarreal A, Hernandez-Vargas H et al (2013) Modulation of DNA methylation states and infant immune system by dietary supplementation with ω-3 PUFA during pregnancy in an intervention study. Am J Clin Nutri 98:480–487. https://doi.org/10.3945/ajcn.112.052241
Miyake Y, Tanaka K, Okubo H et al (2013) Maternal fat intake during pregnancy and wheeze and eczema in Japanese infants: the Kyushu Okinawa Maternal and Child Health Study. Ann Epidemiol 23:674–680. https://doi.org/10.1016/j.annepidem.2013.08.004
Willemsen LEM (2016) Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur J Pharmacol 785:174–186. https://doi.org/10.1016/j.ejphar.2016.03.062
Bisgaard H, Stokholm J, Chawes BL et al (2016) Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl JMed 375:2530–2539. https://doi.org/10.1056/NEJMoa1503734
Ramakrishnan U, Stein AD, Parra-Cabrera S et al (2010) Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull 31:S108–S116. https://doi.org/10.1177/15648265100312S203
Imhoff-Kunsch B, Stein AD, Villalpando S et al (2011) Docosahexaenoic acid supplementation from mid-pregnancy to parturition influenced breast milk fatty acid concentrations at 1 month postpartum in Mexican Women. J Nutr 141:321–326. https://doi.org/10.1177/15648265100312S203
Hernández E, Barraza-Villarreal A, Escamilla-Núñez MC et al (2013) Prenatal determinants of cord blood total immunoglobulin E levels in Mexican newborns. Allergy Asthma Proc 34:e27-34. https://doi.org/10.2500/aap.2013.34.3688
Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB (2007) Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol 157:645–648. https://doi.org/10.1111/j.1365-2133.2007.08112.x
Gorozave-Car K, Barraza-Villarreal A, Escamilla-Núñez MC et al (2013) Validation of the ISAAC standardized questionnaire used by schoolchildren from Mexicali, Baja California. Mexico Epidemiol Res Inter 2(6760):1–6
Yang C-Y, Chang C-C, Tsai S-S et al (2003) Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res 91:29–34. https://doi.org/10.1016/s0013-9351(02)00015-4
Abdul KSM, Jayasinghe SS, Chandana EPS et al (2015) Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40:828–846. https://doi.org/10.1016/j.etap.2015.09.016
Liu K-L, Tsai T-L, Tsai W-C et al (2021) Prenatal heavy metal exposure, total immunoglobulin E, trajectory, and atopic diseases: a 15-year follow-up study of a Taiwanese birth cohort. J Dermatol 48:1542–1549. https://doi.org/10.1111/1346-8138.16058
Center for Diseases Control and Prevention (2019) Fourth national report on human exposure to environmental chemicals updated tables, January 2019. Atlanta, GA.
Nadeau KC, Li Z, Farzan S et al (2014) In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin Immunol 155:188–197. https://doi.org/10.1016/j.clim.2014.09.004
US EPA, “Drinking water standard for arsenic.” p. 2, 2001, Accessed: Aug. 17, 2022. [Online]. Available: https://nepis.epa.gov/Exe/ZyPdf.cgi?Dockey=20001XXC.txt.
WHO (2011) Guidelines for Drinking Water Quality. 4th Edition World Health Organization, Geneva, Switzerland. http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf
Ashley-Martin J, Levy AR, Arbuckle TE et al (2015) Maternal exposure to metals and persistent pollutants and cord blood immune system biomarkers. Environ Health 14:52. https://doi.org/10.1186/s12940-015-0046-3
Farzan SF, Karagas MR, Chen Y (2013) In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol 272:384–390. https://doi.org/10.1016/j.taap.2013.06.030
Cardenas A, Houseman EA, Baccarelli AA et al (2015) In utero arsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics 10:1054–1063. https://doi.org/10.1080/15592294.2015.1105424
Perera F, Herbstman J (2011) Prenatal environmental exposures, epigenetics, and disease. Reproduc Toxicol 31:363–373. https://doi.org/10.1016/j.reprotox.2010.12.055
Engström K, Wojdacz TK, Marabita F et al (2017) Transcriptomics and methylomics of CD4-positive T cells in arsenic-exposed women. Arch Toxicol 91:2067–2078. https://doi.org/10.1007/s00204-016-1879-4
DaVeiga SP (2012) Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc 33:227–234. https://doi.org/10.2500/aap.2012.33.3569
Escamilla-Nuñez MC, Barraza-Villarreal A, Hernández-Cadena L et al (2014) Omega-3 fatty acid supplementation during pregnancy and respiratory symptoms in children. Chest 146:373–382
Türkez H, Geyikoglu F, Yousef MI (2012) Ameliorative effect of docosahexaenoic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress, and DNA damage in rat liver. Toxicol Ind Health 28:687–696. https://doi.org/10.1177/0748233711420475
Acknowledgements
We would like to express our sincere appreciation to the participants in this project.
Funding
This study was supported by Mexico’s National Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología, CONACYT), Grant 87121, and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Award R01HD058818.
Author information
Authors and Affiliations
Contributions
CEN was the general coordinator and participated in conceptualization; methodology; validation; formal analysis; investigation; writing, original draft; writing, review and editing; visualization; and supervision. IFG: Conceptualization; methodology; data curation; writing, original draft; visualization. ABV: Methodology; investigation; writing, original draft; resources; supervision; writing, review and editing; project administration. LHC: Writing, original draft; writing, review and editing. ENOP: Writing, original draft; writing, review and editing. IR: Writing, original draft; writing, review and editing; funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were under the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics and Investigation Committees of the Mexican National Institute of Public Health (Instituto Nacional de Salud Pública, INSP), and the Emory University Ethics Committee approved the investigation protocol (CI:418).
Consent to Participate
Informed consent was obtained from all individual participants included in the study. All children participated in the study with their parent’s written informed consent.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Figueroa-Garduño, I., Escamilla-Núñez, C., Barraza-Villarreal, A. et al. Docosahexaenoic Acid Effect on Prenatal Exposure to Arsenic and Atopic Dermatitis in Mexican Preschoolers. Biol Trace Elem Res 201, 3152–3161 (2023). https://doi.org/10.1007/s12011-022-03411-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03411-3