Abstract
Apoptosis of kidney tubular epithelial cells contributes to diabetic kidney disease (DKD) pathophysiology, but the mechanisms are not fully understood. Zinc transporter protein member 8 (ZnT8, SLC30A8) is a susceptive gene in diabetes. Here, we aim to investigate whether ZnT8 has effects on pathophysiology of DKD. The animal groups include control, ZnT8KO mice, STZ-induced, and ZnT8-KO-STZ. STZ-induced DKD was performed in male C57BL/6 J mice and in ZnT8-KO mice. High glucose (HG)–induced apoptosis in a normal rat kidney tubular epithelial cell line (NRK-52E cells) was performed in vitro. Transfection of hZnT8-EGFP or TNFAIP3 siRNA was done in NRK-52E cells. Flow cytometry with Annexin V-FITC/PI double staining and TUNEL analysis was performed for the detection of apoptosis. Gene expression at mRNA and protein levels was examined with real-time RT-PCR and Western blot. Urine albumin to creatinine ratio, proinflammatory cytokines, and apoptosis were enhanced in kidneys of STZ and ZnT8-KO-STZ mice compared to control or ZnT8-KO mice. ZnT8 overexpression after hZnT8-EGFP transfection decreased HG-stimulated inflammatory activity and apoptosis in NRK-52E cells. Furthermore, treatment with ZnSO4 blunted HG-induced apoptosis and NF-κB activation. ZnSO4 increased the abundance of zinc-finger protein TNF-α-induced protein 3 (TNFAIP3). Also, ZnT8 over-expression after hZnT8-EGFP transfection significantly ameliorates HG-induced NF-κB-dependent transcriptional activity and apoptotic protein expressions in NRK-52E cells, but the inhibitory effect of ZnT8 was significantly abolished with TNFAIP3 siRNA. Our study provides evidence that ZnT8 has protective effects against apoptosis of renal tubular epithelial cells through induction of TNFAIP3 and subsequent suppression of the NF-κB pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic kidney disease [DKD; also known as diabetic nephropathy (DN)] is the primary microvascular complication of diabetes and the most common cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) [1, 2]. DKD is a multifactorial chronic disease characterized by hyperglycemia and intensive metabolic, hemodynamic, and inflammation interaction. In a clinical setting, improvements in antihyperglycemic and advances in reno-protective therapies could lead to kidney failure requiring dialysis or a kidney transplant. However, the knowledge regarding the molecular mechanisms of DKD is still limited. A crucial reason for this relentless progression is potentially associated with the incomplete understanding of the pathogenic mechanisms of DKD, which is fundamental for developing more effective preventive or therapeutic strategies.
Zinc transporter protein member 8 (ZnT8 or SLC30A8) is highly expressed in the membrane of insulin granules in pancreatic β-cells, which is responsible for the crystallization and storage of insulin by transporting Zn ions from the cytoplasm to insulin-secreting granules [3, 4]. The homology of ZnT8 between humans and mice is 82.92%, according to the GeneCards database. In 2018, our previous study revealed that ZnT8 was expressed in the mitochondria of Leydig cells and facilitated the transport of Zn ions from the cytoplasm to the mitochondria under gonadotropin stimulation [5]. Several studies have shown that ZnT8 has epigenetic, genetic, and biological effects on the pathogenesis of type 1 (T1DM) and type 2 diabetes (T2DM) [6, 7]. ZnT8 auto-antibodies have been regarded as the least-characterized islet autoantibodies to predict the risk of future T1DM [8, 9]. Genetic studies have revealed that common ZnT8 genetic polymorphisms were associated with the risk of T2DM, while the rare loss of function variants has protective effects in T2DM [6, 10, 11]. In addition, our previous epigenetic study showed that increased DNA methylation in the ZnT8 gene promoter was associated with the risk of T2DM [12]. In recent years, it has been proposed that ZnT8 could affect diabetic microvascular complications, including DKD. A previous report has also indicated that ischemic retinopathy may be mediated by abnormal Zn homeostasis caused by the down-regulation of ZnT8 [13]. Another study demonstrated that up-regulating ZnT8 expression was directly involved in the maintenance of Zn homeostasis by erythropoietin in retinas from diabetic mice via the activation of the ERK signaling pathway and the down-regulation of hypoxia-inducible factor-1α levels [14]. Our recent study demonstrated that ZnT8 protected against epithelial-to-mesenchymal transition-tubulointerstitial fibrosis in DKD by inhibiting the activation of the TGF-β1/Smad signaling pathway [15]. Thus, we hypothesized that ZnT8 might play a significant role in the pathogenesis of DKD.
The apoptosis-induced cell death pathway is an active response to altered microenvironments and features specific intracellular pathway activation [16]. Tubular apoptosis is one of the important characteristic morphological changes in human diabetic kidneys, and tubular atrophy can reflect the progress of the disease more effectively than glomerular pathology, suggesting that tubular interstitial epithelial cell apoptosis may be the initial mechanism of tubular atrophy in T2D[17]. It is well established that Zn deficiency (ZnD) is associated with multiple disorders. Particularly, ZnD often occurs in patients with diabetes or DKD. ZnD could be due to impaired intestinal absorption, hyperglycemia, or increased urinary Zn loss [18, 19]. Clinical studies have indicated that advancing DKD, with decreased glomerular filtration rates and increasing microalbuminuria, was associated with lower Zn levels in serum. Zn supplementation in patients with diabetes improved ZnD, glycemic control, and partly improved renal function [20, 21]. Changes in intracellular Zn concentration have been associated with apoptotic processes in various cell systems induced by diverse physical, chemical, and immunological stimuli. Our and other previous studies have demonstrated that Zn supplementation inhibited apoptosis in kidney cells caused by various factors and that the cells grown under ZnD conditions [22, 23]. Petersen et al. [24] demonstrated that knockdown of ZnT8 elevated insulin content and apoptosis following glucose treatment, suggesting that ZnT8 may play an essential role in β-cell survival and react to increased glucose concentrations. The Zn transporter families, Zrt- and Irt-like proteins (ZIP) and ZnT, maintain the cellular balance between cell proliferation and apoptosis by mediating Zn influx and efflux and play critical roles in cellular-physiological functions [25,26,27]. However, the cytoprotective potency of ZnT8 on renal proximal tubular epithelial cell (RPTC) apoptosis in DKD is unknown.
The present study aimed to investigate the effect of ZnT8 on apoptosis in the RPTCs and to explore ZnT8 as a potential therapeutic target for treating and preventing DKD. In the present study, in vivo experiments were performed using ZnT8-knockout (KO) and ZnT8-KO-streptozotocin (STZ) mice. Then, in vitro experiments using transfection were performed to investigate high-glucose (HG)-induced apoptosis in the normal rat kidney tubular epithelial cell line (NRK-52E cells).
Material and Methods
Animals and Kidney Tissue Preparation
The animal included the control group (C57BL/6 J), the ZnT8-KO group, the STZ group (STZ-induced diabetic mice), and the ZnT8-KO-STZ group. The C57BL/6 J mice (male; 6-week-old) were purchased from the Experimental Animal Center of China Medical University (Shenyang). The mice at 8-week-old were intraperitoneally injected with STZ (150 mg/kg; Sigma-Aldrich; Merck KGaA) diluted in 0.1 M citrate buffer (pH 4.5), as previously described [28]. In the vehicle control group, the same volume of sodium citrate was intraperitoneally injected. The ZnT8-KO mice (male; 12-weeks-old) were purchased from GenOway. Genotyping was performed as previously described, using tail clips [5]. The offspring of the ZnT8-KO mice were bred by the Experimental Animal Center of China Medical University. The experiment for ZnT8-KO offspring mice started at the age of 8 weeks and was matched with the control group. All experimental animals were fed a standard diet prepared by the Laboratory Animal Center of China Medical University. Blood was obtained from the tail veins of mice, and blood glucose levels were measured weekly using an Accu-Chek glucometer (Roche Diagnostics). Blood glucose levels > 16 mmol/l were indicative of diabetes. All the mice at 24 weeks were anesthetized (1% intraperitoneal sodium pentobarbital; 50 mg/kg) and sacrificed via transcardiac perfusion with saline followed by 2.5% glutaraldehyde solution in 0.1 M phosphate buffer. Urine albumin and creatinine levels were measured by enzyme-linked immunosorbent assay (ELISA) (Albuwell and Creatinine Companion, Exocell, Inc., Philadelphia, PA). The left kidneys were processed for histology and immunostaining according to our previous study [29]. The right kidney was used for renal proximal tubules (RPTs) isolation and a Percoll gradient [30, 31]. Aliquots of freshly isolated RPTCs from the mice were used for the isolation of total protein and RNA. Histological processing of the kidneys was performed. All procedures were approved by the laboratory animal rules of China Medical University [15] (Institute Research Board nos. 201712020 and 201401112).
Cell Culture
The NRK-52E cells were purchased from the American Type Culture Collection. The cells were cultured and treated according to our previous studies [15, 29]. To examine the role of effect of transfection of ZnT8 on HG-induced apoptosis in the NRK-52E cells, the cells were divided into five groups, as follows: (1) control group, normal medium; (2) neo group, subjected to neo (empty victor) transfection; (3) hZnT8 group, subjected to hZnT8-EGFP transfection; (4) HG group, treated with 30 mM HG for 24 h; and (5) HG/ hZnT8 group, 24-h post-transfection, the cells were treated with 30 mM HG for 24 h. The control groups treated the cells with serum-free DMEM medium (normal glucose levels at 5.5 mM). In the HG group, the cells were treated with 30 mM HG for 24 h. In HG/ZnT8 siRNA or HG/TNFAIP3 siRNA group, the cells were treated with HG for 24 h following transfection with ZnT8 siRNA or TNFAIP3 siRNA.
Transfection
The NRK-52E cells were transiently transfected with hZnT-8-pcDNA3.1/myc-hisA or pcDNA3.1/myc-hisAs, according to our previous studies [5, 15]. mZnT8 (NM_172816) small interfering (si)RNA (Stealth RNAi) was prepared by Invitrogen (Thermo Fisher Scientific, Inc.). The following siRNA sequence was used: siZnT8, 5′-GAUCCAGUGUGCACAUUUATT-3′; and scrambled NC siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′. The siRNA targeting mouse tumor necrosis factor α-induced protein 3 (TNFAIP3) was purchased from Ambion, and the NRK-52E cell line was transiently transfected with TNFAIP3 siRNA, as previously described [32]. The target sequences of ZnT8 siRNA and TNFAIP3 siRNA were searched using NCBI BLAST against the GenBank database. All the transfections were performed using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) by the manufacturer’s instructions. Exogenous expression and RNAi silencing of ZnT8 or TNFAIP3 with transfection were analyzed using Western blot analysis.
Cell Viability Assay
Cell viability was analyzed using a quantitative colorimetric assay and MTT, as described in our previous study [15].
Immunofluorescence Staining
Serial paraffin Sects. (5-μm thick) were fixed with 4% paraformaldehyde overnight at 4 °C, dewaxed in xylene, and rehydrated using a gradient of alcohol solutions. Then, the cryostat sections were pre-incubated with normal donkey serum (1:20) for 1 h, then incubated overnight at room temperature with anti-interleukin 6 (IL-6) (1:200), tumor necrosis factor-α (TNF-α) (1:200; cat. no. ab252382; Abcam), and aquaporin-1 (AQP1; 1:200) Next, the sections were incubated at room temperature for 2 h with DAR-FITC (1:50) and Texas Red-DAM (1:50; Jackson Immuno Research Europe, Ltd., Newmarket, UK) after washing with PBS.
The NRK-52E cells, following various treatments, were fixed in 4% paraformaldehyde for 2 h at RT and per-mobilized in 0.1% Triton X-100 before incubation with primary mouse monoclonal anti-NF-κB p65 (1:200; cat. no. ab190589; Abcam) antibody. After three washes with PBS, the sections were incubated for 2 h with DAR-FITC (1:50) and Texas Red-DAM (1:50) at room temperature. The nuclei of the cells were labeled with DAPI for 2 h at 4 °C. Finally, the sections were mounted and examined using a confocal laser scanning microscope (SP2; Leica Microsystems, GmbH).
Enzyme-Linked Immunosorbent Assay (ELISA)
The frozen kidney tissue was homogenized on ice in 500 µl PBS, containing a 1% protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). A total of 125 µl 5% Triton X was added to the samples and then centrifuged at 14,000 × g for 15 min at 4 °C. The supernatants were measured for protein content using a Bio-Rad DC protein assay kit. The values were initially reported in picograms per milligram protein/tissue after normalization to the protein concentration for each tissue. TNF-α and IL-6 level in the tissues were analyzed using ELISA (R&D Systems Inc.). The levels of IL-6 and TNF-α in the NRK-52E cells were also analyzed using ELISA kits provided by ALPCO, according to the manufacturer’s instructions.
Western Blot Analysis
Western blot analysis was performed as previously described [5, 33, 34]. Protein was extracted from RPTCs and the NRK-52E cells, and concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc.). Total protein (20 μg) was separated using SDS-PAGE using 10% gradient Tris/glycine gels, then transferred to PVDF membranes (MilliporeSigma). Following which, the membranes were incubated with the following primary antibodies overnight at 4 °C: anti-ZnT8 (1:200; sigma, SAB2105710), anti-cleaved caspase-3 (1:400; cat. no. ab49822; Abcam), anti-cleaved caspase-9 (1:400; cat. no. ab2324; Abcam), anti-BAX (1: 400; cat. no. ab32503; Abcam), anti-AIF (1:400; cat. no. ab32516; Abcam), anti-IκBα (1:1,000; cat. no. sc-371; Santa Cruz Biotechnology, Inc.); NF-κB (1:800; cat. no. ab239882; Abcam), TNFAIP3 (1:800; cat. no. ab74037; Abcam), and mouse monoclonal anti-GAPDH (1:800; cat. no. sc-47778; Santa Cruz Biotechnology, Inc). Secondary antibodies, including rabbit anti-goat IgG (1:400, AP106, Sigma-Aldrich and rabbit anti-mouse IgG (1:400, 06–371, Sigma-Aldrich), were incubated with the membranes overnight at 4 °C. Finally, the proteins were visualized using enhanced chemiluminescence (ECL) Western blot detection system (Syngene). GAPDH served as the loading control.
Analysis of Apoptosis
The degree of NRK-52E cell apoptosis was measured using an Annexin V-FITC Apoptosis Detection kit and flow cytometry, according to the manufacturer’s instructions (Annexin V-FITC Apoptosis Detection kit I; BD Pharmingen; BD Biosciences).
TUNEL Assay
TUNEL assay using the Apoptosis Detection Kit (cat. no. ab66108; Abcam) was performed to detect cell apoptosis, according to the manufacturer’s instructions.
Intracellular Zn Measurement
As previously described, the flame mode with a Shimadzu AA-6300 atomic absorption spectrophotometer was used to evaluate the Zn levels [15].
Statistical Analysis
SPSS (version 18; SPSS, Inc.) was used for statistical analysis. Data were tested for normality using Shapiro–Wilk test, and variance homogeneity using Levene’s test. ANOVA was used when the variance was homogenous. Statistical significance was determined with one-way ANOVA followed by Tukey’s multiple comparison tests for comparisons between two or multiple groups. The data are expressed as the mean ± SD or SEM, and three independent experiments with six or eight replicates were analyzed. P < 0.05 was considered statistically significant.
Results
Metabolic and Biochemical Parameters in the Mice
First, STZ-induced diabetes and ZnT8-KO-STZ mice exhibited diabetic symptoms, including an increase in food and water intake and urine production (Supplemental Fig. 1). In addition, there was a reduction in body weight in the ZnT8-KO mice compared with that in the control group. Second, compared with that in the control group, fasting blood glucose (FBG) levels were significantly elevated in the STZ-induced and ZnT8-KO-STZ diabetic mice. Third, 16 weeks after STZ injection, the STZ-induced and ZnT8-KO-STZ diabetic mice showed increased blood serum creatinine (sCr) levels, urea nitrogen (BUN) levels, and an increased urine albumin-to-creatinine ratio (UACR), which indicated that the mice developed DKD. Notably, the ZnT8-KO-STZ mice showed the highest sCr, BUN, and UACR levels among all the groups (Table 1).
Effects of ZnT8 on Apoptosis in the RPTCs in Diabetic Mice
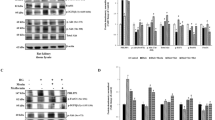
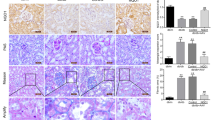
To investigate the effect of ZnT8 on apoptosis in the RPTCs in diabetic mice, apoptosis was measured using a TUNEL assay. Co-immunostaining studies showed AQP1 (a proximal tubule marker) localization in TUNEL-positive RPTCs in diabetic mice. The percentage of TUNEL-positive RPTCs was significantly higher in the STZ-induced and ZnT8-KO-STZ mice than in the wild-type (WT) mice and was highest in the ZnT8-KO-STZ mice. Notably, compared to ZnT8-KO group, ZnT8-KO-STZ mice exhibit a higher percentage of TUNEL-positive RPTCs (Fig. 1A1-D2). To further confirm the effect of ZnT8 on apoptosis in the cells in DKD, the expression levels of apoptotic-related proteins (AIF, BAX cleaved caspase-3, and cleaved caspase-9) in RPTCs were analyzed using Western blot analysis. As expected, compared with that in the control or ZnT8-KO mice, the protein expression level of AIF, BAX, cleaved caspase-3, and cleaved caspase-9 was significantly increased in the kidneys of the ZnT8-KO-STZ mice, which confirmed the findings from the TUNEL assay (Fig. 2A–E).
ZnT8 effect on renal proximal tubular cell apoptosis in diabetic mice. Co-localization of AQP1 and TUNEL-positive cells in kidneys from the (A1–3) control, (B1–3) ZnT8-KO mice, (C1–3) STZ, and (D1–3) ZnT8-KO-STZ mice. The kidney sections were analyzed using a TUNEL assay to visualize apoptotic cells (red), then incubated with anti-AQP1 antibody followed by DAR-FITC to identify AQP1 expression (green). Magnification, × 400. Scale bar, 30 μm
Apoptotic-related protein expression level in RPTCs from kidneys in animal models. Western blot analysis of apoptotic-related protein expression levels of AIF, BAX, cleaved caspase-9, and cleaved caspase-3 in RPTCs. n = 8. All experiments were performed three times. *P < 0.05 vs. control group. **P < 0.001 vs. control group. #P < 0.05 vs. STZ group. ##P < 0.001 vs. STZ group.△△P < 0.01 vs. ZnT8-KO group
IL-6 and TNF-α Level in Mice Kidneys
To evaluate the anti-inflammatory effect of ZnT8 in the kidney of diabetic mice, the levels and concentrations of the proinflammatory cytokines TNF-α and IL-6 were analyzed using immunofluorescence staining and ELISA, respectively. Immunofluorescence staining showed proteins of TNF-α and IL-6 were located in the RPTCs in STZ-induced diabetic and ZnT8-KO-STZ mice. In addition, the expression level of TNF-α and IL-6 was also higher in STZ-induced diabetic and ZnT8-KO-STZ mice compared with that in the WT mice (Fig. 3A1–B4). The results from ELISA showed a significant increase in TNF-α and IL-6 concentrations in ZnT8-KO-STZ mice compared with that in the control, diabetic or ZnT8-KO group, respectively, which is consistent with the immunofluorescence staining results (Fig. 4A and B).
Effect of ZnT8 in Intracellular Zn Concentrations in the NRK-52E Cells
It is well-known that changes in intracellular free Zn levels stimulate ZnT8 expression. To evaluate the underlying mechanism of ZnT8 in HG-induced apoptosis in RPTCs in DKD, the experiments for HG-induced apoptosis by modulating ZnT8 expression were further performed using transfection with RNAi in the NRK-52E cells. The highest expression levels of ZnT8 following transfection with hZnT8-EGFP were detected at 72 h. ZnT8 protein expression level was reduced to ~ 19.3% of the normal level using Western blot analysis following ZnT8siRNA transfection (Supplemental Fig. 3). An atomic absorption spectrophotometer was used to investigate the movement of free Zn ions in the cells following ZnT8 overexpression or hZnT8-EGFP transfection, with or without HG treatment to evaluate Zn levels. The total intracellular Zn levels were evaluated in the NRK-52E cells. The Zn level in the neo group had no significant change, while in the HG group, the level significantly decreased compared to the control group, and the highest Zn level was detected in the hZnT8-transfected cells (Fig. 5).
Intracellular Zn concentration in the NRK-52E cells. The cells were treated with neo (empty victor), HG, hZnT8, HG + hZnT8, RNAi ZnT8 and HG + RNAi ZnT8. Zn level in the cells was measured using an atomic absorption spectrophotometry assay. *P < 0.05 and **P < 0.001 vs. control group. #P < 0.05 and ##P < 0.001 vs. HG
Effect of Transfection of ZnT8 on HG-Induced Apoptosis in the NRK-52E Cells
The cells were treated as described in “Cell culture,” and cell viability was detected using MTT analysis after exposure to HG; and it was found that the cell viability of the HG/hZnT8 group was higher than that of the HG group, but lower than control group. We initially evaluated the cell apoptosis using flow cytometry with Annexin V-FITC/PI double staining and TUNEL analysis, respectively. We observed an increase in the apoptotic and dead cells treated with HG at 24 h. At the same time, hZnT-8-pcDNA3.1/myc-HisA transfection following HG treatment resulted in a substantial decrease in the number of apoptotic cells compared with the HG group (Fig. 6A and B). To further confirm the effect of ZnT8 on HG-induced apoptosis, caspase-3 and caspase-9 activity and the expression protein levels of BAX and AIF were detected by Western blot. As expected, co-treatment with HG and hZnT-8-pcDNA3.1/myc-HisA significantly reduced the level of apoptotic protein expression (AIF, BAX, cleaved caspase-3, and cleaved caspase-9) compared with the HG group (Fig. 7A–E). The results suggested that ZnT8 expression is associated with HG-induced apoptosis in NRK-52E cells, as evidenced by the reduced up-regulation of above-mentioned apoptotic protein.
Effects of ZnT8 on apoptosis in the NRK-52E cells. A Apoptosis was analyzed using a TUNEL assay in control, neo (empty victor), hZnT8, HG, HG/hZnT8 group. (A-a) DAPI, (A-b) TUNEL, and (A-c) merge. B Flow cytometry using Annexin V/PI double staining was used to analyze apoptosis. **P < 0.001 vs. Control. ##P < 0.001 vs. HG
Effect of transfection of ZnT8 in HG-induced apoptosis in the NRK-52E cells. A Western blot analysis was used to determine apoptotic-related protein expression levels of AIF, BAX, cleaved caspase-9(C-caspase-9), cleaved caspase-3(C-caspase-3), and Bcl-2 in the cells, and the results were (B–F) quantified using densitometry and statistically analyzed. The data are presented as the mean ± SEM, and all experiments were performed three times. **P < 0.001 vs. control. ##P < 0.001 vs. HG
Effects of ZnT8 on HG-Induced Inflammatory Factors in the NRK-52E Cells
Chronic inflammation is implicated in the pathogenesis of DKD and other diabetic complications, resulting in cell apoptosis. Thus, we measured the HG-induced inflammation factors (TNF-α and IL-6) in NRK-52E cells. The results indicated that TNF-α and IL-6 levels were increased after exposure to HG for 24 h, while ZnT8 overexpression after hZnT8-EGFP transfection decreased both TNF-α and IL-6 levels compared to the HG group (Fig. 8A and B).
Effect of Zn on HG-Induced Apoptosis and the TNFAIP3/NF-κB Signaling Pathway in the NRK-52E Cells
The nuclear factor-κB (NF-κB) pathway is an evolutionarily conserved signaling pathway with an essential role in various biological processes, including cell proliferation and apoptosis [35]. The NF-κB pathway is activated by IKK-mediated phosphorylation of the IκB subunit, which subsequently induces its polyubiquitination and proteasome degradation [35]. The p65/p50 heterodimer, NF-κB transcription factors, are then released and translocated to the nucleus, where they regulate gene transcription [36]. The Zn-finger protein, TNFAIP3 (also known as A20; encoded by the TNFAIP3 gene), is a cytoplasmic protein that plays a crucial role in the negative regulation of immunity and inflammation, which is a known endogenous inhibitor of NF-κB activation [37]. To elucidate the underlying mechanisms of the protective effects of Zn under HG, the activation of NF-κB was analyzed using immunofluorescence staining. As shown in Fig. 9A, HG treatment led to the nuclear translocation of the NF-κB p65 protein, which was significantly elevated by TPEN treatment, while the changes were significantly blunted following treatment with ZnSO4. Additional Western blot experiments were used to analyze the expression level of proteins in the TNFAIP3/NF-κB signaling pathway following ZnSO4 treatment. Western blot analysis showed HG treatment significantly increased the phosphorylation of IκBα and NF-κB p65 proteins, together with increased NF-κB-dependent transcriptional activity in the NRK-52E cells, which was further enhanced following treatment with TPEN (Fig. 9B-E). However, all the effects were significantly blunted following treatment with ZnSO4. These results, following ZnSO4 treatment, were paralleled by the increase in the expression level of the Zn-finger protein, TNFAIP3, while TPEN treatment ameliorated the protein expression level of TNFAIP3 in the NRK-52E cells (Fig. 9B-E).
Effect of Zn in the TNFAIP3/NF-κB signal pathway in the NRK-52E cells. A The cells were treated with ZnSO4 or TPEN, then NF-κB p65 expression and localization was analyzed using immunofluorescence. Representative images of three independent experiments are shown. NF-κB p65 expression is shown in green and nuclei is shown in purple. Scale bar, 30 μm. B–E Representative original Western blots of p-IκBα, IκBα, p-NF-κB, NF-κB, and TNFAIP3 protein expression in HG-induced NRK-52E cells following treatment with ZnSO4 or TPEN. **P < 0.001 vs. Control. ##P < 0.001 vs. HG
Effect of ZnT8 on TNFAIP3-Mediated Suppression of the NF-κB Signal Pathway in the NRK-52E Cells
It was subsequently investigated whether RPTC apoptosis in diabetic mice was mediated by Znt8, at least in part, via the TNFAIP3-mediated NF-κB signaling pathway. Western blot analysis showed HG treatment in the NRK-52E cells for 24 h led to an increase in the phosphorylation of NF-κB and IκBα, while the protein expression level of TNFAIP3 was decreased (Fig. 10A–D). In addition, the phosphorylation of NF-κB and IκBα was significantly increased in the HG/ZnT8 RNAi group compared with that in the control or HG group. As expected, TNFAIP3 protein expression level was significantly decreased in the HG/ZnT8 RNAi group, and these changes in the NRK-52E cells were reversed by ZnT8 overexpression after hZnT8-EGFP transfection (Fig. 10A–D).
Overexpression of ZnT8 protects the NRK-52E cell line from HG-induced apoptosis via the TNFAIP3-mediated suppression of the NF-κB signal pathway. A Representative Western blot analysis of p-IκBα, IκBα, p-NF-κB, NF-κB, and TNFAIP3 protein expression levels. B–D Relative p-IκBα, IκBα, p-NF-κB, NF-κB, and TNFAIP3 protein expression levels were calculated and normalized to the loading control. **P < 0.001 vs. control. #P < 0.05 and ##P < 0.001 vs. HG
Role of the TNFAIP3/NF-κB Signal Pathway in the Anti-apoptosis Effects of ZnT8 in the NRK-52E Cells
To further investigate the role of the TNFAIP3/NF-κB signal pathway in the anti-apoptosis effects of ZnT8, endogenous TNFAIP3 expression was knocked down in the NRK-52E cells (Supplemental Fig. 2). Knockdown of TNFAIP3 expression further increased NF-κB-dependent transcriptional activity and apoptotic-related protein expression levels in the NRK-52E cells under HG conditions compared with those only exposed to HG. Furthermore, ZnT8 overexpression after hZnT8-EGFP transfection significantly ameliorated HG-induced NF-κB-dependent transcriptional activity and expression level of apoptotic-related proteins (caspase-3, caspase-9, and Bax) and increased the anti-apoptotic protein Bcl-2 in the NRK-52E cells, but the protective effect of ZnT8 was significantly abolished in the HG-induced NRK-52E cells transfected with TNFAIP3 siRNA (Fig. 11A-G).
Role of the TNFAIP3/NF-κB signal pathway in the anti-apoptosis effects of ZnT8 in the NRK-52E cells. A Protein expression levels of p-IκBα, IκBα, p-NF-κB, NF-κB, TNFAIP3, C-caspase-3, C-caspase-9, Bax, and Bcl-2 were analyzed using Western blot analysis. B–G Relative p-IκBα, IκBα, p-NF-κB, NF-κB, TNFAIP3, C-caspase-3, C-caspase-9, Bax, and Bcl-2 protein expression levels were calculated and normalized to the loading control. The data are presented as the mean ± SEM **P < 0.01 vs. control. ##P < 0.001 and #P < 0.05 vs. HG. ▲P < 0.05 vs. HG/TNFAIP3 siRNA. ▲▲P < 0.001 vs. HG/TNFAIP3 siRNA
Discussion
DKD has been considered a major diabetic-associated microangiopathy involving both CKD and ESKD. Based upon in vivo and vitro experiments, the present study has revealed the anti-apoptosis effects of ZnT8 on DKD via TNFAIP3-mediated suppression of the NF-κB signal pathway and inflammation. First, we found that UACR in ZnT8-KO diabetic mice increased while kidney function decreased compared to non-diabetic control mice. Second, we estimated that the influence of ZnT8 in apoptotic protein expression levels was raised in the kidneys of ZnT8-KO diabetic mice compared to STZ-diabetic mice, while pro-inflammation factors levels were increased. In parallel, the protein expression of apoptosis in NRK-52E cells under HG-stimulated conditions was observed to be elevated. Overexpression of the Znt8 gene by hZnT8-EGFP reduced HG-induced apoptosis, and proinflammatory factors. Third, TNFAIP3-mediated suppression of NF-κB signal pathways was increased under HG conditions after hZnT8-EGFP transfection, but the inhibitory effect of ZnT8 was significantly abolished with TNFAIP3 siRNA on HG-induced NRK-52E cells.
ZnT8 is expressed in pancreatic islets, and the study of ZnT8 in diabetes has become a hot topic [3, 38]. The ZnT8 gene is expressed in other tissues, while the kidney’s expression level is second highest. Our previous study demonstrated that ZnT8 is expressed in the Leydig cells and located in mitochondria, which transports Zn into the mitochondria in cells under gonadotropin stimulation [5]. The KO of the ZnT8 gene in mice, rather than β-cell-specific knockdown of ZnT8, has been associated with insulin resistance, glucose intolerance, and worse obesity in mice fed a high-fat diet [39]. Transgenic mice with selective Znt8 gene overexpression in β-cells had improvement in glucose tolerance compared with that in mice in the control group [40]. Currently, the role of cytosolic Zn2+ and ZnT8 in diabetic microvascular complications, particularly in DKD, is still unknown. In the present study, STZ-induced diabetic mice showed typical diabetic symptoms and kidney injury compared to the non-diabetic control mice. Notably, data from the ZnT8-KO diabetic mice showed an increase in UACR compared with that in the STZ-diabetic mice. Furthermore, Western blot results showed that the expression level of apoptosis-related proteins (AIF, BAX, cleaved caspase-9, cleaved caspase-3) was higher, while the anti-apoptosis-related protein Bcl-2 was lower in the ZnT8-KO diabetic mice than in the diabetic mice. Thus, the results from the present study represent a new investigation into the potential role of ZnT8 in the apoptosis of RPTCs in DKD. Previous studies have indicated that ZnT8 is directly involved in cytoprotection, which controls the influx and efflux of Zn within cells, and plays a vital role in maintaining the cellular balance between cell growth and apoptosis [3, 41]. Studies also provide evidence that a decrease in labile Zn is an important signaling event in proinflammatory cytokine-induced apoptosis in pulmonary endothelium [42], lung epithelial cells, and β-cells [43]. In the present study, decreased serum Zn level was found in the diabetic and ZnT8-KO diabetes mice. This is consistent with human clinical characteristics that a low Zn concentration may be critical in diabetes or patients with DKD [44].
Navarro-Gonzalez et al. [45] revealed evidence from pathological and epidemiological studies indicating that inflammation is the main pathogenic mechanism of DKD and one of the key factors for the continuous development of DKD. A previous study reported that acute cytokine exposure of pancreatic β-cells could decrease intracellular labile Zn and ZnT8 expression and subsequently impair β-cell function [39]. Other groups have demonstrated an association between Zn homeostasis and the inflammatory response in the development of diabetes and DKD [46, 47]. Furthermore, TNFAIP3 is a Zn finger protein that is crucial as a negative feedback regulator of immunopathology. Cheng et al. [40] suggested that TNFAIP3 dysfunction may be associated with the pathogenesis of diabetes via cytokine-induced down-regulation of ZnT8, while overexpression of TNFAIP3 reduced β-cell apoptosis via cytokine-induced ZnT8 down-regulation. Kahmann et al. [48] revealed that healthy elderly individuals might benefit from adjusting intracellular Zn levels by moderate supplementation, decreasing spontaneous cytokine production and defects in the termination of inflammatory activity. Wessels et al. [49] indicated that IL-1β and TNFα concentration under ZnD is regulated via epigenetic and redox-mediated mechanisms, which suggests a vital regulatory role of Zn in proinflammatory cytokines and research should be performed in the treatment of inflammatory diseases using Zn supplementation. Data from the current study revealed that HG stimulates proinflammatory protein expression, which can be decreased by overexpression of ZnT8. This may establish a novel immunological connection between HG-induced cytokine up-regulation and ZnT8 in DKD. In addition, overexpression of ZnT8 in the NRK-52E cells decreased HG-induced apoptosis, as evidenced by the improvement in apoptosis in cells and the expression level of apoptotic-related proteins (AIF, BAX, caspase-3, and caspase-9). These observations indicate that ZnT8 may inhibit, at least in part, HG-stimulated proinflammatory factor expression, which may protect RPTC apoptosis in DKD.
NF-κB is a master transcriptional factor of inflammatory responses linking hyperglycemia and inflammation in the development and progression of DKD [50]. NF-κB modulates distinct and overlapping subsets of gene expression involved in the inflammatory and immune response, cellular survival, and growth development [35]. Furthermore, NF-κB overexpression and proinflammatory-related target genes were observed in the early stages of DKD in preclinical models and kidney biopsies from patients with DKD [51]. In addition, the activated expression level of p65 was associated with the elevation of chemokines (C–C motif chemokine ligand-5 and -2) and cytokines, IL-6 and TNF-α, which regulate inflammatory cell recruitment and affect renal cells fate, including proliferation and apoptosis. The ability of Zn to adjust both positively and negatively to the TLR, IL-1R, and TNF-R signaling pathways may reflect its monitory ability on the activation of NF-κB [52]. TNFAIP3 was viewed as an important potent anti-inflammatory molecule, and mutations in the gene encoding TNFAIP3 were associated with a series of inflammatory pathologies. The binding of TNF to TNFR1 induces the NF-κB-dependent expression of TNFAIP3, as a major negative feedback mechanism preventing sustained activation of NF-κB [53]. Accordingly, elevated activation of NF-κB under calcifying conditions is paralleled by slightly increased transcription of TNFAIP3, which is strongly enhanced with Zn supplements. IκBα is a key protein in the interference of TNFAIP3 and NF-κB [54]. Zn supplementation rescued the increase in IκBα phosphorylation and decreased protein expression level under HG conditions. Very few studies investigate the transcriptional regulation of ZnT8 expression under cellular stress, particularly in the TNFAIP3/NF-κB-mediated anti-apoptosis signaling pathway. However, based on the results mentioned above and our previous study, we hypothesized that the TNFAIP3/NF-κB signaling pathway might exert a substantial effect on the anti-apoptotic role of ZnT8 and its mediated transfer of Zn into RPTC. As expected, this hypothesis was supported by the results that ZnSO4 treatment was paralleled by the increase in the protein expression level of Zn-finger protein, TNFAIP3, while TPEN treatment ameliorated the protein expression level of TNFAIP3 in the NRK-52E cells. Furthermore, ZnT8 overexpression following hZnT8-EGFP transfection significantly ameliorated HG-induced NF-κB-dependent transcriptional activity and apoptotic-related protein expression levels in the cells. Still, the inhibitory effect of ZnT8 was significantly abolished in HG-induced cells following transfection with TNFAIP3 siRNA. All these results indicated that ZnT8 exerts anti-apoptosis effects in RPTCs in DKD via TNFAIP3-mediated suppression of the NF-κB signal pathway. However, other potential mechanisms and tissues, in addition to the diabetic kidney, may contribute to the protective effects of ZnT8 in vivo and vitro. Nonetheless, the current observations show that ZnT8 may be a potential regulatory factor in anti-apoptosis.
In conclusion, our study identified that ZnT8 has protective effects against apoptosis of renal tubular epithelial cells through induction of TNFAIP3 and subsequent suppression of the NF-κB pathway. These findings provide novel information for understanding the mechanisms underlying HG-mediated renal fibrosis. Our work hints at the possibility of a new therapeutic target for DKD, although this remains to be confirmed by future studies.
Abbreviations
- ACR:
-
Albumin-to-creatinine ratio
- AAS:
-
Atomic absorption spectrophotometry
- BUN:
-
Urea nitrogen
- CKD:
-
Chronic kidney disease
- DKD:
-
Diabetic kidney disease
- ESKD:
-
End-stage kidney disease
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBG:
-
Fasting blood glucose
- IL-6:
-
Interleukin 6
- NF-κB:
-
Nuclear factor κB
- PB:
-
Phosphate buffer
- RPTC:
-
Renal proximal tubular cells
- STZ:
-
Streptozotocin
- TGF-β1:
-
Transforming growth factor β1
- TNF-α:
-
Tumor necrosis factor-α
- TNFAIP3:
-
TNF-α-induced protein 3
- ZnT8:
-
Zinc transporter 8
- ZIP:
-
Zrt-and Irt-like proteins
- Zn:
-
Zinc
- STZ:
-
Streptozotocin
- sCr:
-
Serum creatinine
- UACR:
-
Urine albumin-to-creatinine ratio
References
Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R et al (2017) Diabetic microvascular disease: an Endocrine Society Scientific Statement. J Clin Endocrinol Metab 102:4343–4410
Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA et al (2015) Diabetic kidney disease. Nat Rev Dis Primers 1:15018
Chimienti F, Devergnas S, Favier A, Seve M (2004) Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 53:2330–2337
Flannick J, Thorleifsson G, Beer NL, Jacobs SB, Grarup N et al (2014) Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 46:57–63
Zhang X, Guan T, Yang B, Chi Z, Wang ZY, Gu HF (2018) A novel role for zinc transporter 8 in the facilitation of zinc accumulation and regulation of testosterone synthesis in Leydig cells of human and mouse testicles. Metabolism 88:40–50
Gu HF (2017) Genetic, Epigenetic and biological effects of zinc transporter (SLC30A8) in type 1 and type 2 diabetes. Curr Diabetes 13:132–140
Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM et al (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58:2070–2083
Wenzlau JM, Frisch LM, Gardner TJ, Sarkar S, Hutton JC et al (2009) Novel antigens in type 1 diabetes: the importance of ZnT8. Curr Diab Rep 9:105–112
Chimienti F, Favier A, Seve M (2005) ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals 18:313–317
Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E et al (2011) Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes 60:2624–2634
Dwivedi OP, Lehtovirta M, Hastoy B, Chandra V, Krentz NAJ, Kleiner S et al (2019) Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat Genet 51:1596–1606
Seman NA, Mohamud WN, Östenson CG, Brismar K, Gu HF (2015) Increased DNA methylation of the SLC30A8 gene promoter is associated with type 2 diabetes in a Malay population. Clin Epigenetics 7:30
Deniro M, Al-Mohanna FA (2012) Zinc transporter 8 (ZnT8) expression is reduced by ischemic insults, a potential therapeutic target to prevent ischemic retinopathy. PLoS One 7:e50360
Xu G, Kang D, Zhang C, Lou H, Sun C et al (2015) Erythropoietin protects retinal cells in diabetic rats through upregulating ZnT8 via activating ERK pathway and inhibiting HIF-1α expression. Invest Ophthalmol Vis Sci 56:8166–8178
Zhang X, Guan T, Yang B, Gu HF, Chi Z (2020) Effects of ZnT8 on epithelial-to-mesenchymal transition and tubulointerstitial fibrosis in diabetic kidney disease. Cell Death Dis 11:544
Sanchez-Niño MD, Sanz AB, Lorz C, Gnirke A, Rastaldi MP et al (2010) BASP1 promotes apoptosis in diabetic nephropathy. J Am Soc Nephrol 21:610–621
Lau GJ, Godin N, Maachi H, Lo CS, Wu SJ et al (2012) Bcl-2-modifying factor induces renal proximal tubular cell apoptosis in diabetic mice. Diabetes 61:474–484
Salgueiro MJ, Krebs N, Zubillaga MB, Weill R, Postaire E et al (2001) Zinc and diabetes mellitus: is there a need of zinc supplementation in diabetes mellitus patients? Biol Trace Elem Res 81:215–228
Batista MN, Cuppari L, de Fátima Campos Pedrosa L, Almeida Md, de Almeida JB et al (2006) Effect of end-stage renal disease and diabetes on zinc and copper status. Biol Trace Elem Res 112:1–12
Parham M, Amini M, Aminorroaya A, Heidarian E (2018) Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud 5:102–109
Al-Timimi DJ, Sulieman DM, Hussen KR (2014) Zinc status in type 2 diabetic patients: relation to the progression of diabetic nephropathy. J Clin Diagn Res 8:CC04-8
Li B, Tan Y, Sun W, Fu Y, Miao L et al (2013) The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods 23:27–33
Zhang X, Guan T, Yang B, Chi Z, Wan Q et al (2021) SLC30A7 has anti-oxidant stress effects in high glucose-induced apoptosis via the NFE2L2/HMOX1 signal transduction pathway. Diabetes Res Clin Pract 172:108445
Petersen AB, Smidt K, Magnusson NE, Moore F, Egefjord L et al (2011) siRNA-mediated knock-down of ZnT3 and ZnT8 affects production and secretion of insulin and apoptosis in INS-1E cells. APMIS 119:93–102
Chapman EM, Lant B, Ohashi Y, Yu B, Schertzberg M et al (2019) A conserved CCM complex promotes apoptosis non-autonomously by regulating zinc homeostasis. Nat Commun 10:1791
Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P et al (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem 272:18530–18533
Nolin E, Gans S, Llamas L, Bandyopadhyay S, Brittain SM, Bernasconi-Elias P et al (2019) Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat Chem Biol 15:179–188
Tesch GH, Allen TJ (2007) Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 12:261–266
Zhang X, Liang D, Fan J et al (2016) Zinc Attenuates tubulointerstitial fibrosis in diabetic nephropathy via inhibition of HIF through PI-3K signaling. Biol Trace Elem Res 173(2):372–383
Lo CS, Chang SY, Chenier I, Filep JG, Ingelfinger JR et al (2012) Heterogeneous nuclear ribonucleoprotein F suppresses angiotensinogen gene expression and attenuates hypertension and kidney injury in diabetic mice. Diabetes 61:2597–2608
Abdo S, Shi Y, Otoukesh A, Ghosh A, Lo CS et al (2014) Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes 63:3483–3496
da Silva CG, Maccariello ER, Wilson SW, Putheti P, Daniel S et al (2012) Hepatocyte growth factor preferentially activates the anti-inflammatory arm of NF-κB signaling to induce A20 and protect renal proximal tubular epithelial cells from inflammation. J Cell Physiol 227:1382–1390
Zhang X, Lian X, Liang D, Zhang L, Liu S et al (2018) Protective effect of Znt7 on high glucose-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells. Kidney Blood Press Res 43:500–512
Gilda JE, Ghosh R, Cheah JX, West TM, Bodine SC, Gomes AV (2015) Western blotting inaccuracies with unverified antibodies: need for a Western Blotting Minimal Reporting Standard (WBMRS). PLoS One 19:e0135392
Martinez GP, Mijares MR, Chávez K, Suarez AI, Compagnone RS et al (2019) Caracasine acid, an ent-3,4-seco-kaurene, promotes apoptosis and cell differentiation through NFkB signal pathway inhibition in leukemia cells. Eur J Pharmacol 862:172624
Hinz M, Scheidereit C (2014) The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep 15:46–61
Verstrepen L, Verhelst K, van Loo G, Carpentier I, Ley SC et al (2010) Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem Pharmacol 80:2009–2020
Kambe T, Tsuji T, Hashimoto A, Itsumura N (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev 95:749–784
El Muayed M, Billings LK, Raja MR, Zhang X, Park PJ et al (2010) Acute cytokine-mediated downregulation of the zinc transporter ZnT8 alters pancreatic beta-cell function. J Endocrinol 2016:159–169
Cheng L, Zhang D, Chen B (2016) Tumor necrosis factor α-induced protein-3 protects zinc transporter 8 against proinflammatory cytokine-induced downregulation. Exp Ther Med 12:1509–1514
Collawn JF, Matalon S (2014) CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307:L917–L923
Thambiayya K, Wasserloos K, Kagan VE, Stoyanovsky D, Pitt BR (2012) A critical role for increased labile zinc in reducing sensitivity of cultured sheep pulmonary artery endothelial cells to LPS-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 302:L1287–L1295
Egefjord L, Jensen JL, Bang-Berthelsen CH, Petersen AB, Smidt K et al (2009) Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr Disord 9:7
Jansen J, Karges W, Rink L (2009) Zinc and diabetes–clinical links and molecular mechanisms. J Nutr Biochem 20:399–417
Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J (2011) Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7:327–340
Skupien J, Warram JH, Niewczas MA, Gohda T, Malecki M et al (2014) Synergism between circulating tumor necrosis factor receptor 2 and HbA(1c) in determining renal decline during 5–18 years of follow-up in patients with type 1 diabetes and proteinuria. Diabetes Care 37:2601–2608
Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI et al (2019) A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 25:805–813
Kahmann L, Uciechowski P, Warmuth S, Plümäkers B, Gressner AM et al (2008) Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuv Res 11:227–237
Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P (2013) Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J Nutr Biochem 24:289–297
Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B et al (2006) Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 55:2993–3003
Guijarro C, Egido J (2001) Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 59:415–424
Foster M, Samman S (2010) Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal 13:1549–1573
Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C et al (2015) Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 528:370–375
Hayashi K, Kataoka H, Minami M, Ikedo T, Miyata T et al (2020) Association of zinc administration with growth suppression of intracranial aneurysms via induction of A20. J Neurosurg 134:992–998
Acknowledgements
The authors thank Professor Dr. Harvest F. Gu, Dr. Zhaoxin Zhou and Dr. Haofei Hu for their comments and suggestions.
Funding
This study was supported by the Postdoctoral Science Foundation of China (2014MM551144), grants from the Natural Science Foundation of China (81670670), and Shenzhen Key Medical Discipline Construction Fund (SZXK009), and Key projects of the Natural Science Foundation of Liaoning Province (20180540105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary file1
Supplemental Figure 1. The food and water intake and urine production in control, ZnT8-KO, STZ and ZnT8-KO-STZ mice (PNG 251 kb)

Supplementary file2
Supplemental Figure 2. (A) Western blot analysis was performed to analyze ZnT8 protein expression levels following transfection with hZnT8 expression vector and mZnT8 RNAi. The strongest effects of transfection were observed after 72 h. (B and C). Corresponding protein expression levels were analyzed and all experiments were performed three times with virtually identical results. *P<0.05 vs. control. **P<0.001 vs. control. (PNG 144 kb)

Supplementary file3
Supplemental Figure 3. Western blot analysis was performed to analyze TNFAIP3 protein expression levels following transfection with TNFAIP3 siRNA. (B) Corresponding protein expression levels were analyzed as the relative intensities using densitometry. *P<0.05 vs. control. **P<0.001 vs. control. (PNG 144 kb)

Supplementary file4
Supplemental Figure 4. The schedule of the study design in vivo experiment (PNG 27 kb)

Supplementary file5
Supplemental Figure 5. The schedule of the study design in vitro experiment (PNG 40 kb)
Rights and permissions
About this article
Cite this article
Chi, Y., Zhang, X., Liang, D. et al. ZnT8 Exerts Anti-apoptosis of Kidney Tubular Epithelial Cell in Diabetic Kidney Disease Through TNFAIP3-NF-κB Signal Pathways. Biol Trace Elem Res 201, 2442–2457 (2023). https://doi.org/10.1007/s12011-022-03361-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03361-w