Abstract
Certain amphibian species have long served as a valuable protein source for humans, in addition to being good bioindicators for environmental pollutants. Hence, to investigate the consumption outcomes leading to potential health risks, we determined the trace element (TE) levels in the hind leg and liver tissues of marsh frogs (Pelophylax ridibundus), one of the delicacies of several cuisines today. The sediment, water, and frog tissue samples were collected from 15 different locations of NE Turkey and analyzed to determine the arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), manganese (Mn), nickel (Ni), lead (Pb), vanadium (V), and zinc (Zn) concentrations. The TE concentrations in the sediment, water, and individuals were detected to show significant variations among sampling stations (p < 0.05). Yet, Cd and Pb concentrations of the hind legs cooked and enjoyed in the diets were determined below the European Commission’s permitted levels. Furthermore, based on the TEs in edible tissues, consumption of the marsh frog did not appear to pose a risk to humans in terms of provisional tolerable weekly intake (PTWI), target hazard coefficient (THQ), and hazard index (HI).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of global industrialization parallel to population growth, the ecosystem is contaminated with various anthropogenic pollutants (trace elements (TEs), polycyclic aromatic hydrocarbons, etc.). One of the most important anthropogenic pollution sources in this environment is mining activities. Mining operations may pose a pollution danger to the region in which they operate and the nearby areas, and the species that live in the surrounding ecosystem. Especially in abandoned mining areas, TE pollution can also be transported to water sources, wetlands, and agricultural areas with the help of runoff, precipitation, wind, etc. [31]. The main concern with TE pollution is the metals’ acute toxicity and their ability to accumulate in the biological systems [40, 42]. Therefore, perpetual monitoring of these pollutants with the help of sensitive keystone species (i.e., bioindicators that would help reveal pollutant presence and status) is essential for the ecosystem.
Since TEs are not biodegradable, they accumulate in the environment, which subsequently ends up in the food chain and poses severe threats to organisms [9]. In addition, revealing the current situation by examining the TE concentrations in the water and sediments where aquatic organisms live is vital for environmental quality assessment. It is also essential to investigate the TE levels in the tissues of the organisms consumed in terms of nutritional quality. Furthermore, the analysis of the natural frog populations might provide valuable information as robust bioindicators regarding the results of an ongoing TE accumulation process, thereby the pollution dynamics in the aquatic environment over a long period.
Amphibians are susceptible to environmental contamination [3]. Their all-life stages are sensitive to dermal absorption, inhalation, or ingestion of toxic substances present in the surrounding water [38]. Furthermore, they are food chain organisms with a high trophic level. Invertebrates are their primary food source, serving as an entry point into the food chain [30]. All these factors suggest that frogs are notable bioindicator candidates that could be practical models for environmental assessment and biomedical research as well as monitoring water quality and ecosystem health [15].
The literature proved that Pelophylax ridibundus and Pelophylax esculentus are great bioindicators for monitoring TEs and other pollutants [30, 37, 49]. Indeed, Vogiatzis and Loumbourdis [49] showed that these frogs meet all the criteria to be bioindicator species based on the rules listed by Lower and Kendall [24]. Furthermore, except for frog species that are potentially hazardous to humans [25], all Ranidae family species are appropriate for human consumption, such as the ones listed above. The frogs are nutritious with low-fat content and high protein and mineral values [29]. Their legs are among the most preferred recipes of traditional cuisines, especially in European countries [36]. The frog legs are served as a menu option in restaurants serving foreign tourists in several countries, including Turkey [10]. Literature highlights that the most popular culinary treats that could be cooked as a dish are the marsh frog (Pelophylax ridibundus. However, preference rates raise whether the consumption of this frog produced on farms or collected from nature poses human health risks due to the TEs that can be accumulated in its tissues. Indeed, several studies have found that frog consumption is a source of TE exposure for consumers [7, 12, 23]. However, risk assessment predictions of this frog in terms of human health and environmental contamination have not yet been investigated in the literature. Therefore, in this study, we aimed to determine the TE concentration in the tissues of P. ridibundus, a bioindicator organism, and to evaluate whether there are potential health risks that may arise from consumption.
Materials and Methods
Study Area and Sampling
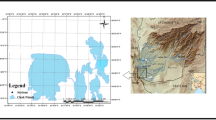
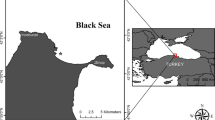
The Eastern Black Sea region (Turkey) has rich copper (Cu), lead (Pb), and zinc (Zn) reserves. However, the mining activities in the long run cause TE contamination in the region’s soil and aquatic ecosystem [14, 32, 51]. Marsh frogs were collected from 15 stations belonging to 5 different provinces and 3 different locations from each province (Fig. 1; Table S1). A total of 75 individuals belonging to 15 populations were sampled from 5 different regions within the distribution area of P. ridibundus in Turkey between June and September 2020. Marsh frogs that live in small streams or ponds were caught by hand or using a dip net and then placed in Ziploc bags. Respective water and sediments were also sampled from the same stations. Water samples were collected by hand using a polyethylene sampling bottle (500 mL). Sediments (around 1 kg) were taken using a shovel from the sediment surface (0–3 cm depths) and then transferred to Ziploc bags. All collected samples (frogs, water, and sediment) were stored in the iceboxes and transported to the laboratory. The frogs and the sediment samples were stored at − 20 °C for further experiments. Water samples (around 50 mL) were filtered through polytetrafluoroethylene (PTFE) filters (0.45 m particle size), and 5 drops of HNO3 (Suprapur, Merck) were added for decreasing pH < 2 before being stored in the refrigerator until the TE analyses.
Sampling area. Marsh frog (Pelophylax ridibundus), sediment, and water samples were collected from 15 different stations along with the NE Turkey. Letters O, G, T, R, and A indicate provinces where the frogs were collected. O: Ordu, G: Giresun, T: Trabzon, R: Rize, A: Artvin. Numbers 1, 2, and 3 indicate stations in the provinces
Sample Digestion
Sediment samples were kept at room temperature (RT) before being dried in the oven. P. ridibundus samples were defrosted and rinsed through ultrapure water at RT. The individuals’ snout-vent lengths and weights were measured (Table S1). Then, the liver and the hind leg muscles of frogs were removed using a stainless-steel dissection set. Each individual was dissected separately. The 2 g fresh weight of liver and leg tissues were placed in different digesting tubes. For sediment, digestion was performed using 0.5 g (dry weight) of the samples. Five milliliters of HNO3 (Suprapur, Merck) was added to the tubes including the tissues and sediment samples, sealed with polypropylene covers, and left overnight. The tubes were incubated for 2.5 h at 95 °C in a block heater. After cooling down to RT, the tubes were kept at 95 °C for another 2 h with 2.5 mL of H2O2 (Suprapur, Merck). The tubes were in the block heater with the caps removed until the solution contents were lowered to around 2 mL. The solutions were diluted with ultrapure H2O, filtered through PTFE syringe filters (0.45 mm pore size) (US [44], and stored at + 4 °C until measurements.
Quality Control and TE Analysis
The identical procedure used on the samples was also applied to the reference material (ERM-CE278k Mussel Tissue) to check and verify the methods’ digestion efficiency (Table 1). TE concentration was measured using an ICP-MS (Agilent, 7800). Furthermore, blank samples and the internal standards were also evaluated to check possible interference during either the sample preparation process or ICP-MS measurements.
Health Risk Evaluation
Estimated weekly intake (EWI mg/kg/week) is calculated by multiplying the weekly TE intake in the digestive tract of an adult person [13]. We calculated the EWI using the average portion size via determined TE concentrations in the leg tissues. The data were then compared to the provisional tolerable weekly intake (PTWI) values declared by the FAO/WHO Joint Expert Committee on Food Additives [18,19,20]. The following formula was used to calculate the EWI [46]:
The target hazard quotient (THQ) is used to explain long-term non-carcinogenic exposure hazards as a ratio of exposure to reference dose concentrations (RfD, mg kg−1 day−1) (US [47]. People are frequently exposed to numerous contaminants in the food they consume. Thus, the hazard index (HI) was utilized to predict the possible cumulative influence of the TEs [26] in the current study. The following formulas were used to determine THQ and HI values:
where EDI: estimated daily intake, RfD: reference dose (arsenic (As) = 3 × 10−4, cobalt (Co) = 20 × 10−3, cadmium (Cd) = 1 × 10−3, Pb = 3.5 × 10−3, Zn = 3 × 10−1, Cu = 4 × 10−2, nickel (Ni) = 2 × 10−2, chromium (Cr) = 1.5, vanadium (V) = 5 × 10−3: US [47]. The THQ and HI values > 1 mean that the TEs in the frogs can exert a potential non-carcinogenic health risk.
Data Analysis
To determine normality and equal variance, the Shapiro–Wilk and Levene tests were performed. Data groups that were not normally distributed or homogeneous were transformed. The differences between the stations regarding the TE concentrations in the liver and soft tissues were determined using a one-way analysis of variance test (ANOVA, post hoc Tukey). Linear regression analysis was used to investigate the link between the TE concentrations in the liver and soft tissues of P. ridibundus along with the body lengths. The TE data in the sediments from various locations were allocated to a concentration range of high, medium, and low to establish the contamination status in the stations. Cluster (statistical) classification analysis was utilized to separate the other groups based on similarities in the TE concentrations detected in the sediments at the various stations. The relative disparities between the sampling locations were shown in each classification (low, medium, high) defined by the data collected in this study. All statistical evaluations were performed in the SigmaPlot 13 software with a 95% confidence interval.
Results and Discussion
Figure 2 shows the TE concentrations in the sediment and water collected from 15 stations across five provinces in Turkey’s Northeast Black Sea region. TE concentrations in sediments ranged (min–max) as follows (mg kg−1): As, 1.09–14.72; Cd, 4.61–13.07; Co, 8.47–51.74; Cr, 11.52–110.54; Cu, 22.50–94.25; Mn, 407.13–1876.23; Ni, 9.62–118.36; Pb, 4.75–82.45; V, 53.61–223.16; Zn, 41.03–211.07. TE concentrations in water samples are listed as follows (µg L−1): As, under detection limits (UDL)–11.141; Cd, 0.02–8.91; Co, 0.01–20.06; Cr, 0.25–3.32; Cu, 4.37–177.40; Mn, 0.94–3402.33; Ni, 0.06–14.54; Pb, UDL–50.59; V, 0.14–34.36; Zn, 0.50–366.93. According to the mean concentration, the TEs in the sediment samples and water samples were listed as follows: Mn > V > Zn > Cu > Cr > Ni > Co > Pb > Cd > As and Mn > Zn > Cu > Pb > V > Ni > As > Co > Cd > Cr respectively.
Trace element concentrations (TE average ± standard error) in sediment and water samples were collected from 15 different stations along with the NE Turkey (N = 3). X-axis shows sampling locations; y-axis shows concentration (µg kg−1 for sediment and µg L−1 for water samples). Colored circles at the stations show the cluster analysis results according to the TE concentrations in the sediment sampled from the relevant station. Each classification identified (low, medium, high) indicates relative differences between the locations
Figure 2 shows the cluster analysis results based on TE level patterns in sediment and water samples from various stations. Three different concentration ranges for TE were determined: low, medium, and high. Cluster values do not show a universal classification or categorization for human health (low, medium, high). It solely shows the status of the stations based on TE concentrations in sediments at different sites. According to Fig. 2, 47% of the stations with the greatest As concentrations were classified as high, 47% were classified as low, and the remaining stations were classified as medium. Cu concentrations were found at low and high levels in 40% and around 33% of the stations, respectively, but medium amounts were found at only four stations (Fig. 2). Seven sites showed low Cd concentrations, four exhibited medium Cd concentrations, and the remainder showed high Cd concentrations. According to the Cr concentration data, roughly 60% of the stations were classified as medium, 27% as high, and 13% as low in terms of Cr pollution. Ni levels in the stations were categorized as medium (roughly 67%), low (approximately 20%), and high (about 13%) concentrations, according to the cluster analysis results. Based on the Pb distributions, the stations were categorized as 80% high, 13% medium, and 7% low. For V, around 33% of the locations showed low amounts, 40% medium concentrations, and 27% high values. Regarding Mn concentrations in sediment samples taken from various sites, 53% of stations had medium concentrations, 27% had low concentrations, and 20% had high concentrations. Zn’s low and high values were identified in 27% and 40% of the sites, respectively, while medium levels were observed in only one station. In terms of Co content, eight sites were classified as low, six sites were classified as medium, and one site was classified as high (Fig. 2).
The distribution of TEs in the liver and the hind leg muscles of frogs sampled from different locations is given in Fig. 3. TE concentrations in leg tissues of frogs collected from all different stations were as follows: As, 0.24–77.82 µg kg−1, Cd, 18.76–176.70 µg kg−1, Co, 1.39–101.65 µg kg−1, Cr, 15.60–300.34 µg kg−1, Cu, 183.66–905.06 µg kg−1, Mn, 12.15–104.67 µg kg−1, Ni, 19.92–298.49 µg kg−1, Pb, 25.52–975.54 µg kg−1, V, 0.15–19.12 µg kg−1, and Zn 1889–11,450 µg kg−1 (Table 2; Fig. 3). The mean concentrations (µg kg−1) of TE were determined in the order of Zn (7392.55) > Cu (554.78) > Pb (267.97) > Mn (222.38) > Ni (109.65) > Cr (108.56) > Cd (67.92) > As (31.40) > Co (25.63) > V (8.89). The TE concentrations in liver tissues were determined as follows: As, 457–9392.65 µg kg−1; Cd, 3751.56–10,145.21 µg kg−1; Co, 148.07–1807.04 µg kg−1; Cr, 14.33–7774.24 µg kg−1; Cu, 16.52–479.79 mg kg−1; Mn, 1613.74–5401.04 µg kg−1; Ni, 31.24–442.67 µg kg−1; Pb, 5.32–1683.40 µg kg−1; V, 378.45–4779.41 µg kg−1; and Zn, 19.12–48.08 mg kg−1 (Table 2; Fig. 3). The mean concentrations of TEs in the liver were listed as follows (µg kg−1): Cu (99,652.06) > Zn (29,691.05) > Cd (6527.77) > As (3343.68) > Mn (2781.01) > V (1848.67) > Cr (1259.62) > Co (548.19) > Pb (335.72) > Ni (164.83).
Despite low TE concentration in the aquatic environment, it is not unusual to observe relatively high concentrations of certain TEs in frog tissues [7, 33, 39]. Our data corroborate similar research in the literature. The TE concentrations in the water samples were determined up to 5300-fold lower than those measured in the frog edible tissues. This difference was more dramatic in the liver tissues of frogs. Differences in the TE concentrations of the water and frog tissues might be attributed to the perception that the frogs accumulate TEs either from another anthropogenic or natural source or have been intermittently exposed to cumulative TE pollution in the past [28, 30]. Besides direct TE accumulation from water through the skin, frogs can accumulate TEs in their bodies through diet or accidental ingestion of the polluted sediment [30]. Nummelin et al. [27] stated that the frogs accumulate TEs in their tissues at much higher concentrations than in the sediments by feeding on the aquatic plants or other organisms in the food web growing in the wetlands. In a study on the same species living in another niche, Stolyar et al. [39] compared the TE levels in urban and rural individuals of P. ridibundus population in western Ukraine. Higher concentrations were detected in the frog tissues they sampled despite low TE concentrations in the water samples [39].
Since organisms are not able to always develop effective mechanisms to efflux TEs out of their bodies, e.g., resulting in accumulation in their tissues, the phenomenon of bioaccumulation occurs and continues throughout the food chain (biomagnification process) [8, 22, 39]. The Zn and Cu concentrations in particular, in the water, sediment, and the frog tissues, were found to be significantly higher than other metals, which is possibly related to the significant abundance of Zn and Cu in the earth’s crust and their widespread use in industry [17]. These two elements may also result from ongoing mining activities in the Eastern Black Sea region [1, 52] where the samples were taken [32].
Amphibians have a life cycle spending a certain part of their lives in aquatic environments and terrestrial habitats [35, 50]. They generally spend a large part of their life dependent on water [4]. In this context, they will probably be affected by TE concentrations in aquatic environments. The TE distributions in the tissues of frogs sampled from different stations are given in Fig. 4. The data showed statistically significant differences between the sampling locations in the target provinces and different sampling stations in that province (ANOVA, post hoc Tukey; Fig. 4). The variation in TE levels across sample sites might be attributed to pollution levels in the maturation habitats. The variation in TE concentrations in the tissues of frogs collected from various sites is typically proportional to pollution in the area [16, 48]. When Fig. 2 is examined with this background, the differences in TE concentrations in sediment and water samples seem critical. Considering that frogs are good bioindicators being in close contact with their environment and reflecting the environment’s conditions in terms of pollution [28, 30], TE concentration differences found in the tissues might be necessary signals showing the overall environmental quality.
Trace element concentrations (TE average ± standard error) in the hind leg muscles and liver tissues of marsh frog (Pelophylax ridibundus) samples collected from 15 different stations along the NE Turkey (N = 5). X-axis shows sampling locations; y-axis shows concentration (µg kg−1). Different letters (a, b, c) show significant differences among sampling stations (ANOVA, post hoc Tukey; p < 0.05)
Another aspect that may influence TE accumulation in the tissues is that frog sizes differ at sample locations, depending on the specific variables. To that purpose, linear regression analysis was used to examine the association between frog length–weight and TE levels in the liver and flesh in the hind leg muscles (Fig. 5). Our data showed that while there was a negative correlation between the frog length and weight and As, Cd, Co, and Pb concentrations of the tissues in the hind leg muscles, a positive correlation was found between the length–weight and Cr, Cu, Mn, Ni, V, and Zn concentrations. In addition, a negative relationship was found between the length–weight and As, Cd, Pb, Cr, Ni, and Zn levels in liver tissues. In contrast, a positive relationship existed between the length–weight and Co, Cu, Mn, and V concentrations (Fig. 5). However, these relationships overall were not statistically significant (p > 0.05; r2 < 0.3).
The relationship between size and the trace element concentrations in the tissues of the marsh frog (Pelophylax ridibundus) samples collected from 15 different stations along the NE Turkey. Each dot shows the result of different samples. X-axis shows length or weight of marsh frog; y-axis shows concentration (µg kg−1)
TE concentrations of the frog’s tissues have been reported in several kinds of research given in Table 2. Indeed, in a study conducted in a river in Northern Greece [23], the levels for Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn in both liver and leg tissues were higher than those found in our study. The two frog species examined in Pakistan’s industrial areas had high amounts of Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn in their liver and leg tissue [34]. The results for P. ridibundus from Southern Bulgaria [53] matched the As, Zn, Cd, Cu, and Pb data in this study. In another research from Thailand [41], while the levels of Cd and Pb were comparable to those found in our study, the levels of As and Cr were lower. The values for X. laevis and R. esculentus from Nigeria [43] were similar in the TE data gathered throughout our research.
Turkey is an important frog supplier and trader [10]. Anatolian water frogs (Pelophylax spp.) have been collected for over 40 years, with approximately 700 tons of annual transport rate [2, 21]. Several agencies have set guidelines for maximum allowable TE levels in the food organisms to protect the consumers’ health. Among these TEs, Cu, Zn, Cr, Co, Mn, V, and Ni are involved in critical metabolic activities in the organisms. However, high doses can readily be toxic. Furthermore, As, Cd, and Pb can be toxic to humans even at very low doses [6]. Therefore, these three elements are listed in the top 10 of the “Substance Priority List” announced by ASTDR [5]. Specific international organizations have also set the maximum allowable limits for As, Cd, and Pb. For instance, the European Commission (EC) stated that the Pb and Cd values in fishery products should not exceed 1.5 and 1 mg kg−1, respectively [11]. When these limits were compared with our data, the Cd and Pb values did not exceed the specified threshold values (Table 3). For an average serving size (225 g), we computed the EWI levels using the measured value for As, Cu, Cd, Pb, and Zn. We also evaluated it according to the Joint FAO/WHO Expert Committee on Food Additives (JECFA) PTWI norms (Table 3). We also revealed that the EWI rates computed for adults were below the JECFA thresholds (Table 3).
THQ was then computed and is shown in Table 3 to assess the non-carcinogenic health risk to humans posed by each TE when hind leg muscles of frogs collected from the sample locations were consumed. The average portion size of frog consumption may not necessarily have adverse health effects on adult consumers. All THQ calculated values for TE were observed to be < 1, indicating that the average portion size of frog consumption size may not necessarily have adverse health effects on adult consumers. When we looked at the HI levels, we noticed that those lower than 1 (the limit value) ranged from 6.2 × 10−5 to 1.2 × 10−3, depending on the average portion size. Both THQ and HI levels appear to be below the limit value of 1, indicating that they are unlikely to impact human health negatively.
Conclusion
This study aimed to quantify the TE bioaccumulation in frog tissues obtained from NE Turkey and determine the hazards of marsh frog (Pelophylax ridibundus) consumption on human health. TE concentrations in frog tissues showed significant variation according to the sampling locations. However, Cd and Pb concentrations in edible tissues were below the allowable limits specified by the EC. Furthermore, the EWI values were lower than the PTWI levels derived for adult consumers based on the average portion size. In addition, the THQ (non-carcinogenic risk) values calculated for TEs less than one confirmed that the frog intake of the collection area would not cause possible adverse health effects in humans.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdioglu E, Arslan M (2009) Alteration mineralogy and geochemistry of the hydrothermally altered rocks of the Kutlular (Sürmene) massive sulfide deposit, NE Turkey. Turk J Earth Sci 18:139–162. https://doi.org/10.3906/yer-0806-7
Akin Ç, Bilgin CC (2010) Preliminary report on the collection, processing and export of water frogs in Turkey. (Presented to KKGM). ODTÜ, Ankara, Turkey. [in Turkish]
Altunişik A, Gül S, Özdemir N (2021) Impact of various ecological parameters on the life-history characteristics of Bufotes viridis sitibundus from Turkey. Anat Rec 304:1745–1758
Altunişik A, Kara Y (2021) Unusual winter activity of Bufo bufo (Anura: Bufonidae). Turk J Biodivers 4(2):105–108
ATSDR (2019) The ATSDR 2019 Substance Priority List. Atlanta, Georgia, USA. https://www.atsdr.cdc.gov/spl/index.html. Accessed 25 September 2021
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12(12):643972. https://doi.org/10.3389/fphar.2021.643972
Borković-Mitić SS, Prokić MD, Krizmanić II, Mutić J, Trifković J, Gavrić J, Despotović SG, Gavrilović BR, Radovanović TB, Pavlović SZ, Saičić ZS (2016) Biomarkers of oxidative stress and metal accumulation in marsh frog (Pelophylax ridibundus). Environ Sci Pollut Res 23:9649–9659. https://doi.org/10.1007/s11356-016-6194-3
Burger J, Snodgrass J (2001) Metal levels in southern leopard frogs from the Savannah River site: location and body compartment effects. Environ Res 86:157–166. https://doi.org/10.1006/enrs.2001.4245
Castiglione S, Todeschini V, Franchin C, Torrigiani P, Gastaldi D, Cicatelli A, Rinaudo C, Berta G, Biondi S, Lingua G (2009) Clonal differences in survival capacity, copper and zinc accumulation, and correlation with leaf polyamine levels in poplar: a large-scale field trial on heavily polluted soil. Environ Pollut 157:2108–2117. https://doi.org/10.1016/j.envpol.2009.02.011
Çiçek K, Ayaz D, Afsar M, Bayrakci Y, Pekşen Ç, Cumhuriyet O, İlhan Bİ, Yenmiş M, Üstündağ E, Tok CV, Bilgin CC, Akçakaya H (2021) Unsustainable harvest of water frogs in southern Turkey for the European market. Oryx 55:364–372. https://doi.org/10.1017/S0030605319000176
EC (2006) European Commission regulation no. 1881/2006 of 19 December 2006 setting 450 maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364–5-L364–24. Available at https://eur-lex.europa.eu/legal-content/EN/ALL/?uriDcelex%3A32006R1881. Accessed 30 September 2021
EFSA (2012) Cadmium dietary exposure in the European population. EFSA J 10(1):2551–2537. https://doi.org/10.2903/j.efsa.2012.2551
Gedik K (2018) Bioaccessibility of Cd, Cr, Cu, Mn, Ni, Pb, and Zn in Mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) along the southeastern Black Sea coast. Hum Ecol Risk Assess 24:754–766. https://doi.org/10.1080/10807039.2017.1398632
Gedik K, Terzi E, Yesilcicek T (2018) Biomonitoring of metal(oid) s in mining-affected Borcka Dam Lake coupled with public health outcomes. Hum Ecol Risk Assess 24(8):2247–2264. https://doi.org/10.1080/10807039.2018.1443390
Hermes-Lima M, Storey KB (1998) Role of antioxidant defences in the tolerance of severe dehydration by anurans. The case of the leopard frog Rana pipiens. Mol Cell Biochem 189:79–89. https://doi.org/10.1023/A:1006868208476
Hodkinson ID, Jackson JK (2005) Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ Manage 35(5):649–666
Hoffman DJ, Rattner BA, Burton GA, Cairns J (2003) Handbook of Ecotoxicology. Lewis Publishers, Boca Raton
JECFA (1982) Evaluation of certain food additives and contaminants 26th Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series No. 683. World Health Organization, Geneva, Switzerland. Available at http://apps.who.int/iris/bitstream/10665/41546/1/WHO_TRS_683.pdf. Accessed 2 October 2021
JECFA (2011a) Evaluation of certain food additives and contaminants 72nd Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 959. World Health Organization, Rome, Italy. Available at http://apps.who.int/iris/bitstream/10665/44514/1/WHO_TRS_959_eng.pdf. Accessed 2 October 2021
JECFA 2011b. Evaluation of certain food additives and contaminants 73rd Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 960. World Health Organization, Geneva, Switzerland. Available at http://apps.who.int/iris/bitstream/10665/44515/1/WHO_TRS_960_eng.pdf. Accessed 2 October 2021
Kürüm V (2015) Statistics of frog trade in Turkey. Republic of Turkey Ministry of Agriculture and Forestry General Directorate of Fisheries and Aquaculture, Ankara, Turkey. [in Turkish]
Linder G, Grillitsch B (2000) Ecotoxicology of metals. In: Sparling DW, Linder G, Bishop CA (eds) Ecotoxicology of amphibians and reptiles. SETAC, Pensacola
Loumbourdis NS, Wray D (1998) Heavy-metal concentration in the frog Rana ridibunda from a small river of Macedonia, Northern Greece. Environ Int 24(4):427–431. https://doi.org/10.1016/S0160-4120(98)00021-X
Lower WR, Kendall RJ (1990) Sentinel species and sentinel bioassay. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. CRC Press Inc., Boca Raton, pp 309–331
Neveu A (2009) Suitability of European green frogs for intensive culture: comparison between different phenotypes of the esculenta hybridogenetic complex. Aquaculture 295:30–37. https://doi.org/10.1016/j.aquaculture.2009.06.027
Newman MC, Unger MA (2002) Fundamentals of ecotoxicology. Lewis Publishers, Boca Raton, p 480
Nummelin M, Lodenius M, Tulisalo E, Hiruonen H, Alanko T (2007) Predatory insects as bioindicators of heavy metal pollution. Environ Pollut 145:339–347. https://doi.org/10.1016/j.envpol.2006.03.002
Othman MS, Khonsue W, Kitana J, Thirakhupt K, Robson MG, Kitana N (2009) Cadmium accumulation in two populations of rice frogs (Fejervarya limnocharis) naturally exposed to different environmental cadmium levels. Bull Environ Contam Toxicol 83(5):703–707
Özogul F, Özogul Y, Olgunoglu Aİ, Boga EK (2008) Comparison of fatty acid, mineral and proximate composition of body and legs of edible frog (Rana esculenta). https://doi.org/10.1080/09637480701403277
Papadimitriou E, Loumbourdis NS (2002) Exposure of the frog Rana ridibunda to copper: the impact on two biomarkers, the lipid peroxidation and glutathione. Bull Environ Contam Toxicol 69:885–891. https://doi.org/10.1007/s00128-002-0142-2
Parraga-Aguado I, Alvarez-Rogel J, Gonzalez-Alcaraza MN, Jimenez-Carceles FJ, Conesa HM (2013) Assessment of metal(loid)s availability and their uptake by Pinus halepensis in a Mediterranean forest impacted by abandoned tailings. Ecol Eng 58:84–90. https://doi.org/10.1016/j.ecoleng.2013.06.013
Pehlivan N, Gedik K, Eltem R, Terzi E (2021) Dynamic interactions of Trichoderma harzianum TS 143 from an old mining site in Turkey for potent metal (oid) s phytoextraction and bioenergy crop farming. J Hazard Mater 403:123609. https://doi.org/10.1016/j.jhazmat.2020.123609
Prokić MD, Borković-Mitić SS, Krizmanić II, Mutić JJ, Vukojević V, Nasia M, Saičić ZS (2016) Antioxidative responses of the tissues of two wild populations of Pelophylax kl. esculentus frogs to heavy metal pollution. Ecotoxicol Environ Saf 128:21–29. https://doi.org/10.1016/j.ecoenv.2016.02.005
Qureshi IZ, Kashif Z, Hashmi MZ (2015) Assessment of heavy metals and metalloids in tissues of two frog species: Rana tigrina and Euphlyctis cyanophlyctis from industrial city Sialkot, Pakistan. Environ Sci Pollut Res 22:14157–14168. https://doi.org/10.1007/s11356-015-4454-2
Rakici E, Altunişik A, Şahin K, Özgümüş OB (2021) Determination and molecular analysis of antibiotic resistance in Gram-negative enteric bacteria isolated from Pelophylax sp. in the Eastern Black Sea Region. Acta Vet Hung 69(3):223–233
Schlaepfer PM, Hoover C, Dodd CK (2005) Challenges in evaluating the impact of the trade in amphibians and reptiles on wild populations. Bioscience 55:256–264. https://doi.org/10.1641/0006-3568(2005)055[0256:CIETIO]2.0.CO;2
Shaapera U, Nnamonu LA, Eneji IS (2013) Assessment of heavy metals in Rana esculenta organs from River Guma, Benue State Nigeria. AIM 4:496–500. https://doi.org/10.4236/ajac.2013.49063
Sotomayor V, Lascano C, D’angelo DE, Venturino AMP (2012) Developmental and polyamine metabolism alterations in Rhinella arenarum embryos exposed to the organophosphate chlorpyrifos. Environ Toxicol Chem 31:2052–2058. https://doi.org/10.1002/etc.1921
Stolyar OB, Loumbourdis NN, Falfushinska HI, Romanchuk LD (2008) Comparison of metal bioavailability in frogs from urban and rural sites of western Ukraine. Arch Environ Contam Toxicol 54:107–113
Taiwo IE, Amaeze NH, Imbufe AP, Adetoro OO (2014) Heavy metal bioaccumulation and biomarkers of oxidative stress in the wild African tiger frog, Hoplobatrachus occipitalis. Afr J Environ Sci Technol 8:6–15. https://doi.org/10.5897/AJEST2013.603
Thanomsangad P, Tengjaroenkul B, Sriuttha M, Neeratanaphan L (2020) Heavy metal accumulation in frogs surrounding an e-waste dump site and human health risk assessment. Hum Ecol Risk Assess Int J 26(5):1313–1328. https://doi.org/10.1080/10807039.2019.1575181
Torre A, Trischitta F, Faggio C (2013) Effect of CdCl2 on regulatory volume decrease (RVD) in Mytilus galloprovincialis digestive cells. Toxicol in Vitro 27:1260–1266. https://doi.org/10.1016/j.tiv.2013.02.017
Tyokumbur ET, Okorie TG (2011) Macro and trace element accumulation in edible crabs and frogs in Alaro Stream Ecosystem, Ibadan. J Res Natl Dev 9(2):439–446
US EPA (1996) Acid digestion of sediments, sludges, and soils. US Environmental Protection Agency. EPA Method 3050B. Washington, DC, USA
US EPA (2000) Guidance for assessing chemical contaminant data for use in fish advisories volume 2 risk assessment and fish consumption limits Third Edition. US Environmental Protection Agency
US EPA (2011) Exposure factors handbook: 2011 edition. US Environmental Protection Agency. National Center for Environmental Assessment, Washington, DC; EPA/600/R-09/052F. Available from the National Technical Information Service, Springfield, VA, and online at http://www.epa.gov/ncea/efh
US EPA (2020) Regional screening level (RSLs) Generic Tables, May 2020. US Environmental Protection Agency. Available at https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables. Accessed 2 October 2021
Vershinin VL (2007) Biota of urban areas. UroRAN, Ekatirinburg, Russia, 85
Vogiatzis A, Loumbourdis NS (1998) Cadmium accumulation in liver and kidneys and hepatic metallothionein and glutathione levels in Rana ridibunda, after exposure to CdCl2. Arch Environ Contam Toxicol 34:64–68. https://doi.org/10.1007/s002449900286
Wells KD (2007) The Ecology and Behaviour of Amphibians. Chicago University Press, Chicago
Yaylali AG, Tüysüz N (2009) Heavy metal contamination of soils and tea plants in the eastern Black Sea region, NE Turkey. Environ Earth Sci 59:131–144. https://doi.org/10.1007/s12665-009-0011-y
Yumlu M (2001) Çayeli Underground Cu-Zn Mine. 17th International Mining Congress and Exhibition of Turkey- IMCET2001. ISBN 975–395–417–4
Zhelev ZM, Arnaudova DN, Popgeorgiev GS, Tsonev SV (2020) In situ assessment of health status and heavy metal bioaccumulation of adult Pelophylax ridibundus (Anura: Ranidae) individuals inhabiting polluted area in southern Bulgaria. Ecol Ind 115:106413. https://doi.org/10.1016/j.ecolind.2020.106413
Funding
This work was supported by the Recep Tayyip Erdogan University Research Funds (grant number: FYL-2019–1043).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All specimens used in this study were treated humanely and ethically following permission to perform the study by Recep Tayyip Erdogan University’s Local Ethics Committee (decision number: RTEÜ.2020/06).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mani, M., Altunışık, A. & Gedik, K. Bioaccumulation of Trace Elements and Health Risk Predictions in Edible Tissues of the Marsh Frog. Biol Trace Elem Res 200, 4493–4504 (2022). https://doi.org/10.1007/s12011-021-03017-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03017-1