Abstract
Histopathologies are widely recognized as biomarkers of environmental pollution. In this sense, we evaluated the putative relationship of gill histopathologies and distinct ecological impacts in two regions of Todos os Santos Bay (BTS), Brazil, the largest bay in Northeastern Brazil, South Atlantic. We compared the presence and concentration of metals (Al, As, Ba, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, V, and Zn) in water, sediments, and gills and gill histopathologies of a demersal fish (Diapterus rhombeus) and a benthic fish (Ogcocephalus vespertilio). As expected, fish and sediment samples from historically contaminated areas (Aratu) showed more remarkable traces of metals than apparently low-impact areas (Jaguaripe). Likewise, the DTC (degree of tissue change) index and the volume densities were higher in fish caught in Aratu. In addition, the Diapterus rhombeus species showed more potential than Ogcocephalus vespertilio for risk assessment as it showed more responses to the environment reflected on more histopathologies. These data support the effectiveness of incorporating functional gill morphology to monitor impacts on estuarine biota that can be used as a reference to improve the management of ecosystems and prevent harm to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution in coastal areas is an actual global issue, especially in estuaries, recognized as biodiversity hotspots essential for spawning, growth, and feeding of many aquatic species [1,2,3]. Organic and metal pollutants reach the estuaries from distinct sources such as industry, mining, municipal wastewaters, port activities, and agriculture, making these areas more susceptible to effects of environmental contamination [4, 5]. Consequently, these contaminants can accumulate in sediments and marine biota, potentially impacting estuarine environments [6, 7]. Increasing concentrations of metals have caused the degradation of estuaries worldwide, causing massive loss of habitat and mortality, affecting economic activities such as fishing and other ecological services and functions, and putting human health at risk [8,9,10]. Besides metal accumulation in marine organisms, they can also reach humans through biomagnification [11]. Although the analysis of metals in fishing resources is essential for risk assessment, evaluating their accumulation effects on biological systems is still necessary to infer the health of the local biota [5, 12]. Fish have been considered one of the most critical bioindicators for assessing trace metal pollution [13, 14]. Their gills have continuous contact with the environment exposing a large surface area and are sensitive to physical and chemical alterations of the aquatic medium, allowing many contaminants to accumulate in the tissue and cause histopathologies [5, 11, 15].
Our study evaluated the impact of metals on fish gills in the Todos os Santos Bay (BTS), one of the largest estuaries in northeastern Brazil, highly affected by human activities [16]. The BTS plays a significant role in South Atlantic wildlife. Their aquatic resources are the primary source of protein and income to the region, inhabited by more than 3 million people [16]. The importance of BTS contrasts with the intensive degradation related to uncontrolled human occupation, untreated domestic effluents, tourism, agriculture, petrochemical, and chemical industries that contribute to the contamination of this estuary by metals and other xenobiotics [16,17,18]. Thus, our study presents an applicable model in estuaries with similar problems. In this sense, our objective was to evaluate the contamination levels by trace metals in two fish species in two estuaries of Todos os Santos Bay through quantitative evaluation of gill histopathologies. Furthermore, we also comment on which could be considered the bioindicator species. Thus, we expect that an estuarine habitat with industrial and naval operations will have an ichthyofauna with higher densities, volume, and severity of gill histopathologies than an estuarine habitat with no history impact polluting activities. Therefore, we analyzed the gill histopathologies in Ogcocephalus vespertilio (Teleostei, Ogcocephalidae) and Diapterus rhombeus (Teleostei, Gerreidae). They are abundant in Brazilian estuaries; O. vespertilio is benthic, closely related to estuarine sediment, while D. rhombeus is demersal and feeds on invertebrates, vegetables, and to a small degree also on fish [19, 20]. Thus, these species might potentially bioaccumulate environmental contaminants, such as trace metals. Hence, we discussed the possibility of using these species for evaluation in monitoring programs due to their vast and frequent distribution in estuarine regions on the Brazilian coast.
Material and Methods
Study Area
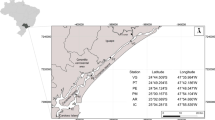
We carried out the study in two regions along Todos os Santos Bay (BTS, Brazil), Aratu and Jaguaripe. Aratu is one of the most impacted regions of BTS by intense industrial activities [6] with traffic of heavy ships to Aratu, Cotegipe, Brasquem harbors, Brazilian Navy base, and dredging processes [21, 22], besides being close to Salvador, the capital of State of Bahia, with nearly 3 million people. Jaguaripe is considered a relatively low-impacted environment, with large mangrove areas, free of nearby industrial activities, ports, and far from urban areas (Fig. 1) [16, 23].
Sampling Strategy and Studied Species
We sampled water, sediment, and fish at five sites in Jaguaripe and Aratu (Fig. 1). We collect the fish with seines (12 m × 2 m with 12-mm mesh) in shallow intertidal areas (< 2-m deep) and gill nets (100 m × 3 m with 15.30- and 60-mm mesh) in permanently flooded areas with a depth greater than 2 m. Every 2 h, we removed the trawls to prevent physical injury to the gills. After captured, we immediately fixed the second and third gill arch of the right hemibranch of the Diapterus rhombeus (total length: 10 to 13 cm; body mass: 15 to 19 g; N = 15 per estuary) and Ogcocephalus vespertilio (total length: 25 to 29 cm; body mass: 25 to 30 g; N = 15 per estuary) in 10% neutral buffered formaldehyde. After 18 h, the arches were preserved in 70% ethanol and sent to the Laboratory of Animal Physiology (LAFISA), Federal University of Bahia (UFBA), for histopathological and chemical analyses. The local ethics committee approved these procedures on animal use (CEUA, IBIO, UFBA, 23/2015).
Environmental Analyses and Gill Treatments
The environmental parameters of the water (e.g., dissolved oxygen, temperature, salinity, and pH) were measured in situ using a Hanna® multiparameter. In each site, three water and sediment samples were placed in 500-ml plastic bottles, previously decontaminated with 10% nitric acid, and 15 gills (from 15 fish for each species) in each site to analyze trace metals. Stored samples were transported to the laboratory at 4 °C and subsequently dried at 60 °C for 48 h. The dry matter was crushed and sieved to obtain homogeneous dust. By wet decomposition in open systems, the samples were mineralized. The digested material was dissolved in HNO3 at 1.00 mol L−1 and stored at 4 °C to quantify the trace elements (Al, As, Ba, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, V, and Zn) in diluted solutions with different concentrations of metals were used to establish the analytical curves.
All elements were analyzed in triplicate using inductively coupled plasma optical emission spectrometry (Agilent Technologies 720 series ICP-OES). The values obtained for each metal in the water samples were compared to the Brazilian Environmental Council (CONAMA) [24], respectively. The standard reference materials used were Stream Sediment Reference Material (STSD1) for sediment and Oyster Tissue 1566b for gills.
Light and Electron Microscopic Analyses
Each gill arch was sectioned into ten fragments in the laboratory and kept in 70% ethanol until the histological processing. Two fragment samples per animal were randomly chosen and dehydrated in methacrylate (Historesin; Leica Biosystems, Nussloch, Germany). The gills were sagittally sectioned with a thickness of 4 μm at random positions. Three random sections were mounted and stained with toluidine blue in four histological slides, totaling 12 sections for each animal. In each section, four lamellae were photographed in at least two distinct fields of vision with a 20 × (for histopathologies severity analyses) and 40 × (for volume densities analyses) enlarged lens using a light microscope (Leica DM500) equipped with a digital camera (Leica DFC450).
For transmission electron microscopy (TEM), gill fragments were fixed in Karnovsky’s solution (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4) and post-fixed in 1% osmium tetroxide, 0.8% potassium ferrocyanide and 5 mM calcium chloride in 0.1 M sodium cacodylate buffer, pH 7.4 for 1 h. Posteriorly, the gill fragments were washed three times for 10 min in the same buffer, dehydrated in a graded series of acetone, followed by three successive baths of 100% acetone and subsequent replacement with acetone and Polybed® resin (1:1) for 6 h. Ultra-fine transverse sections of 80 nm were obtained using an automatic ultra-microtome (Leica EM UC7) and collected onto 200-mesh copper grids. The sections were then treated with uranyl acetate and lead citrate to increase contrast. Photomicrographs were obtained using a TEM (JEOL 1230).
For scanning electron microscopy, gill samples were fixed as described above, and random gill fragments were processed, washed in a 0.1 M sodium cacodylate buffer, pH 7.4 baths, and post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 h. Subsequently, the fragments were rewashed in sodium cacodylate buffer and dehydrated in an increasing ethanol series to pure ethanol, air-dried, glued to the stumps with silver glue, and coated with gold. Then, the fragments were analyzed with a JEOL JSM 639OLV SEM.
The Severity of Gill Histopathologies
The degree of tissue change (DTC) was calculated to estimate the severity of gill histopathologies into three progressive stages according to [25]. Stage I involves recoverable lesions once good environmental conditions are re-established. Otherwise, under unaltered environmental conditions, these injuries may persist. In the case of long-term exposure, the changes will likely progress to the second stage. Second-stage lesions are repairable if water quality improves, but if large areas of the gills are damaged, and the fish are exposed to chronic pollution, or increased pollution, or with deterioration in other environmental conditions (such as temperature, pH, and O2), changes can compromise gill functions and can lead to third-stage changes. When third-stage changes occur, the restoration of the gill structure is no longer possible. Even with improved water quality or cessation of toxic exposure, injuries damage vital gills and even mortality.
Gill histopathologies were identified and quantified according to Abdel-Moneim et al. [26] and Monteiro et al. [27] and considered as stage I (dilation of the marginal channel, edema, epithelial lifting, fusion between lamellae of adjacent filaments, fusion lamellar complete, hypertrophy of the pavement cells, vasodilation, proliferation of the filamentary epithelium, proliferation of the lamellar epithelium), stage II (lamellar aneurysm, rupture of epithelial cells with hemorrhage, rupture of the lamellar epithelium, rupture of pillar cells), and stage III (necrosis). A value of DTC was calculated for each specimen by the DTC index = (1.SI) + (10.SII) + (100.SIII) where I, II, and III correspond to the number of alterations of stages I, II, and III, respectively. The DTC value obtained for each fish was used to calculate the average index for each sampling site. The associated effects are as follows 0–10 (functionally normal gills), 11–20 (slightly to moderately damaged gills), 21–50 (moderately to heavily damaged gills), and > 100 (irreparably damaged gills) [25].
Volume Densities of the Gill Histopathologies
Quantitatively, histopathologies were also assessed based on their volume densities in five of the 15 animal samples per species. This method is estimated by point counting the anisotropic test system in which the areas associated with each point were known. The reference volume (Vref) and the volume of each histopathology (Vl) were estimated as proposed by Cavalieri’s method [28]: V = T.a/p.ΣPi, where T is the distance between sections, a/p is the relative area of one point, and Pi is the number of points on the structure to be estimated. The equation estimated histopathological volume densities or the volume ratio of the analyzed structure to the total volume of the organ (Vv): Vv = Vl/Vref, where Vl is the estimated volume for each histopathology, Vref is the reference volume.
Statistical Analysis
The T-test was used to compare the metal levels in gills (dependent variable) with the estuarine areas (independent variable). Normality and homoscedasticity were accessed through Shapiro–Wilk’s and Levene’s tests, respectively. A two-way permutational multivariate analysis of variance (PERMANOVA) [29, 30], using the Bray–Curtis similarity index, was applied to assess the differences in the volume density of the histopathologies for both species and studied areas. To compare the volume density between the histopathologies, we use the PERMANOVA pairwise. We used PERMDISP to investigate the homogeneity of variances. T-tests were performed in the software STATISTICA® version 13.1. and PERMANOVA in PRIMER 6 version 6.1.6®. For all statistical tests, we adopted an α-value of 0.05, and the results were quantitatively described as means ± standard deviations.
Results
Water and Sediment Analysis
The water temperature and dissolved oxygen were similar for both studied areas in BTS and varied between 25.8–27.1 °C and 6.1–7.3 mg/l, respectively. The salinity varied between 26.6 and 34.3 in Jaguaripe and ranged from 26.2 to 35.1 in Aratu. The pH ranged from 7.8 to 8.2 in both areas. In both studied areas, most metals presented concentration values below or close to the quantification limit. The only exceptions were Fe: 0.20 ± 0.04 mg/l and Mo: 0.11 ± 0.01 mg/l for Aratu and Al: 0.18 ± 0.08 mg/l; Cu: 0.01 ± 0.03 mg/l; Fe: 0.36 ± 0.15 mg/l; Mn: 0.01 ± 0.02 mg/l; Mo: 0.03 ± 0.01 mg/l and Zn: 0.02 ± 0.04 mg/l for Jaguaripe. The number of trace metals recorded in water in both areas was low compared to the standards by [24].
In sediment samples, the mean concentrations of trace metals (mg/kg) in Aratu and Jaguaripe were low compared to TEL (concentration below which the probability of biological effect is low) and PEL (probable effect level: concentration above which the probability of biological effect is high values). However, the concentration of Ba in Aratu was higher than the determined limit by Chapman and Wang [31]. Al and Fe had the highest metal values in the two areas studied. In Aratu, Cu, Mn, and Zn were also increased (Table 1).
Gill Analyses
The Concentration of Metals in Gills
As for the mean concentration of trace metals in fish gills, the values were higher in Aratu. In both estuaries and both species, the highest concentrations (mg/kg) in fish gills were related to Al, Fe, and Zn. Significant differences in the levels of Al, Ba, Cr, Cu, Fe, Mn, Pb, and Zn (p < 0.05) in Diapterus rhombeus were observed between Aratu and Jaguaripe, with the highest values assigned to Aratu samples. Regarding Ogcocephalus vespertilio, significant differences between both studied areas to Al, Pb, and Zn (p < 0.05) were found (Fig. 2).
Histopathological Changes and Severity of Gill Histopathologies
The three types of microscopes were used to characterize histopathologies. We observed an intact gill structure (Fig. 3a) and gill with hypertrophy and hyperplasia of epithelial cells in the secondary lamellae and gill filament, which shows epithelial desquamation (Fig. 3b). We also identified fusions between lamellae of adjacent gill arches (Fig. 4a) and lamellae located distally from the apex of the same gill arch (Fig. 4b).
Scanning electron micrograph of Ogcocechalus vespertilio giils. a Gill arch showing filament (F) and lamellae (L) without histopathologies (bar = 100 μm). b Gill arch showing the filament with epithelial desquamation (asterisk) and secondary lamellae with hypertrophy and hyperplasia of epithelial cells (arrows); bar = 50 μm
Ogcocechalus vespertilio gills. a Light photomicrograph of gill stained with toluidine blue showing fusions between lamellae of adjacent filaments (arrowheads); bar = 100 μm. b Scanning electron micrograph showing proliferation of lamellar epithelial (arrowheads) and fusions of lamellae located distally from the apex of the same gill arch (arrow); bar = 50 μm
In addition, we made an image composition to indicate the state of functional impairment of the gills, such as the rupture of the pillar system (Fig. 5). Externally, in the extension of the secondary lamellae, we observed points of swelling (Fig. 5a), which can commonly form a dilation of the marginal canal (Fig. 5b). With the loss of the structural characteristics of the pillar cells, the blood spaces are no longer involved and even disappear. As a result, edema with blood cells and plasma disperses into the marginal canal (Fig. 5c), causing further dilation and leading to an aneurysm.
Composite image of Ogcocephalus vespertilio gills indicating the formation of an aneurysm. a Externally, in the extension of the secondary lamellae were evidenced points of swelling (arrowheads); bar = 20 μm. b In light photomicrography, it was observed the dilation of the marginal channel (arrowheads); bar = 50 μm. c Aneurysm formation with breakdown and disappearance of the pillar cell and dispersion of blood cells (asterisks) and plasma in the marginal channel. Epithelial cell nucleus (arrow); bar = 0.5 μm
The histopathologies were higher in fish caught from Aratu than in Jaguaripe, including more severe histopathologies. In Aratu samples, the DTC was 49.19 ± 4.12 for D. rhombeus and 50.67 ± 10.95 for O. vespertilio. According to the DTC index, the values presented in D. rhombeus and O. vespertilio demonstrate moderate changes and necrosis (stage III) in gills. The histopathologies were more present in D. rhombeus than O. vespertilio showing epithelial lifting, dilation of the marginal channel, lamellar fusion (stage I), lamellar aneurysm, and rupture of pillar cells (stage II) (p < 0.01, PERMANOVA). The estimated gill DTC values presented in samples from Jaguaripe were 14.13 ± 2.04 and 17.24 ± 2.51 for D. rhombeus and O. vespertilio, respectively, indicating that the gills showed slight injuries of Stage I.
Volume Densities of Gill Histopathologies
The volume density of gills histopathologies differed significantly among collection sites and species, with the highest values found in samples from Aratu (both with p < 0.01, PERMANOVA) mainly in Diapterus rhombeus. Jaguaripe samples presented the highest volume density encompassed non-injured areas in both D. rhombeus and O. vespertilio. Analysis from both species collected in Aratu revealed a lower volume of lamella density without histopathologies than the total histopathology density (Fig. 6). Four of the six most frequent histopathologies (i.e., lamellar aneurysm, epithelial lifting of the lamellae, necrosis, and hypertrophy of the gill epithelium) found in O. vespertilio were higher in specimens from Aratu; likewise, the nine most frequent histopathologies observed in D. rhombeus were also higher in Aratu (Table 2).
Volume density of the rupture of epithelial cells (REC) with hemorrhage, lamellar aneurysm (LA), rupture of pillar cells (RPC), epithelial lifting (EL), dilation of the marginal channel (DMC), edema (ED), fusion between lamellae of adjacent filaments (FLA), lamellar fusion (LF), necrosis (NE), proliferation of the filamentary epithelium (PFE), proliferation of the lamellar epithelium (PLE), rupture of the lamellar epithelium (RLE), hypertrophy of the pavement cells (HPC), vasodilatation (VS), and no lesions (NL) regions of Diapterus rhombeus and Ogcocephalus vespertilio gills exposed to sites Aratu and Jaguaripe
Discussion
Although Aratu has a history of metal contamination, we will not establish causal relations between metal and gill histopathologies to avoid a reductionist approach because multiple sources of contamination and a mixture of xenobiotics can influence toxicity [31,32,33]. Exposure to metals, pesticides, polycyclic aromatic hydrocarbons [34, 35], or even the combination of these xenobiotics [36, 37] can cause the same types of gill lesions. However, metal-contaminated environments expose fish through direct absorption of contaminated water and food. Due to the large surface and exposure to the external environment, gills are susceptible to physical and chemical lesions by metals, being the primary target of xenobiotics and metal accumulation [38]. The first signs of gills damage induced by exposure to metal might reflect the degree of contamination by trace metals [39]. In this sense, in our study, once we determined metal concentrations in the environment and the gills, followed by quantitative morphofunctional analyses, we found possible evidence to discuss the association between environmental metal concentration and gill histopathology.
As the gills suffer injuries, their multifunctionality can be affected, resulting in impaired gas exchange and gas sensibility, osmoregulation, ion balance, pH regulation, nitrogen balance, and neural, hormonal, and paracrine control [40, 41]. In addition, the gills can accumulate metals dissolved in water through the ion-exchange process. Their surfaces function as metallic binders so that bioaccumulation can occur due to positively charged metallic species in the water with negatively charged sites in the gills. Thus, as metabolically active organs, gills have a solid tendency to store high levels of metals [42, 43].
This study revealed that fish health is negatively affected in populations from highly impacted areas with a high concentration of trace metals (Al, Ba, Cu, Fe, Mg, and Zn). The number and extension of gill histopathologies in Diapterus rhombeus and Ogcocephalus vespertilio from Aratu were higher than in Jaguaripe, a relatively well-conserved region. Indeed, previous studies revealed high levels of metallic xenobiotics in sediment, water, and biota from Aratu [6, 44, 45], putatively related to the intensified port and industrial activities [6]. Similarly, a high incidence of pathologies in fish from contaminated regions by trace metals worldwide has been reported [11, 26, 38, 46]. Moreover, even though Jaguaripe has no history of environmental contamination, as one of the less impacted areas in BTS [16], some metals were present and correlated to a certain degree of gill histopathologies in fish samples in this region.
Our results reinforce histopathological analyzes as a helpful biomarker, indicating that xenobiotics, even in trace concentrations, can affect organisms by bioaccumulation and biomagnification, leading to physiological disorders, as pointed by [1, 47]. Thus, gill histopathologies can recommend approaches to evaluate the effects of water pollution by both essential (Cu, Zn, Se, Mn, Fe) and non-essential (Al, As, Cd, Cr, Pb) trace metals [11, 48,49,50]. Although these metals may have a natural origin and the apparent lack of environmental degradation in Jaguaripe, metallic xenobiotics might have increased in this area from other sources such as the agricultural activities in the Jaguaripe river basin [23, 44].
Alterations in the external and internal gill morphology characterized the histopathologies severity stages reflecting the progressive impairment levels, changes in the organization of blood flow can lead to the formation of aneurysms, causing cell disruption and pillar system collapse, and epithelial cell rupture with hemorrhage that culminates in cell death [26, 51,52,53].
However, there is the possibility of functional recovery of the gills. Histopathologies such as epithelial lifting, hyperplasia, and hypertrophy of epithelial cells could have a defense function, as they increase the distance that water xenobiotics must diffuse to reach the bloodstream. On the other hand, lamellar fusions could reduce the vulnerable area of the gill surface [15].
Even in environments with less frequent and less severe histopathologies, the gill structure can suffer damage and compromise its organic functions. A decrease in the oxygen consumption rate may occur due to the increase in the water/blood diffusion distance [47], changes in the ammonia excretion rate, and bioaccumulation, thus affecting the energy metabolism to adapt to the metal exposure stress influencing the survival [39].
Ultrastructurally, we recorded the already ruptured pillar cell and the accumulation of blood cells that will form an aneurysm in the marginal channel of the secondary lamella. Along with epithelial changes, the disorganization of lamellar blood spaces can occur due to the degeneration of pillar cells [1, 54] that organize and separate the blood sinuses below the epithelium. The alteration of these pillar cells that direct blood flow turns gas exchange difficult and leads to the appearance of aneurysms. These changes in the gills impair gas exchange, resulting in insufficient oxygen extraction and ultimately causing fish death [54, 55]. Along with this degeneration, hypertrophy and hyperplasia of chloride cells and mucous cells can increase mucus secretion at the base of the gill filaments and secondary lamellae, interfering with ionic exchange [51].
Following the tendency observed for the concentration of trace metals, the degree of tissue change (DTC index) was higher in fish gills from samples collected in Aratu. In other studies, the DTC index has been validated in the Sincheon stream (South Korea), the effects of two effluent discharges, predominantly from sewage and wastewater treatment plants, on the gills of Carassius auratus. The DTC for upstream (reference site) of the gill samples of Carassius auratus was 16.83 ± 4.21, indicating mild damage (mainly stage I alterations). In contrast, the DTC values at the two downstream sites were 35.50 ± 3.89 and 27.33 ± 4.84, respectively, indicating moderate damage to the gills (predominantly stage II changes along with stage I lesions) [56]. Our study verified less frequent and less severe histopathologies in Jaguaripe samples, ratifying the estimated gill DTC values presented in samples from this site, which indicated slight gill injuries, probably, due to the relatively low values of metals found in the fish gills caught in this location. The volume density of gill histopathologies also differed between the collection sites. Fish samples from Aratu showed the highest percentage of lamellar lesions and the least functional tissue, reinforcing the morphofunctional approach in understanding the effects of xenobiotics, as demonstrated using the volume density in Oreochromis niloticus gills after exposure to copper [27]. Differences in the severity and density of the volume of gill lesions among the studied species also indicate that the possible effects of metal concentration on gill histopathology may be species specific. In this sense, between the two species, Diapterus rhombeus is considered an important fishing resource for local human communities and showed more sensitivity to the presence of metals. However, a state that would be a bioindicator species capable of revealing differences between low and positively impacted environments in BTS could be early.
As final considerations, we emphasize the validity of gill histopathologies as biomarkers in fish. As seen, the histopathologies indicate progressive stages, with effects considered protective in their initial stage, but which can originate degenerative processes and physiological compromises, culminating in mortality. Thus, aquatic biota health depends on actions that would promote the reduction of polluting sources and increase the recovery of aquatic environments. We further emphasize that although histopathologies are effective biomarkers, it is also advisable to use multilevel integrated biomarker responses (e.g., behavioral, biochemical, molecular) to draw more decisive conclusions. In this context, encouraging risk assessment and monitoring environmental quality are fundamental processes and must be part of public policies to conserve aquatic environments.
Data Availability
Not applicable.
References
Lu Y, Yuan J, Lu X, Su C et al (2018) Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environ Pollut 239:670–680. https://doi.org/10.1016/j.envpol.2018.04.016
Whitfield AK, Elliott M (2002) Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. J Fish Biol 61:229–250. https://doi.org/10.1111/j.1095-8649.2002.tb01773.x
Wild-Allen K, Skerratt J, Whitehead J, Rizwi F, Parslow J (2013) Mechanisms driving estuarine water quality: a 3-D biogeochemical model for informed management. Estuar Coast Shelf Sci 135:33–45. https://doi.org/10.1016/j.ecss.2013.04.009
Arizzi Novelli A, Losso C, Libralato G, Tagliapietra D, Pantanib C, Volpi GA (2006) Is the 1:4 elutriation ratio reliable? Ecotoxicological comparison of four different sediment: water proportions. Ecotoxicol Environ Saf 65:306–313. https://doi.org/10.1016/j.ecoenv.2005.08.010
Barišić J, Dragun Z, Ramani S, Filipović VM, Krasnići N, Čož-Rakovac R, Kostov V, Rebok K, Jordanova M (2015) Evaluation of histopathological alterations in the gills of Vardar chub (Squalius vardarensis Karaman) as an indicator of river pollution. Ecotoxicol Environ Saf 118:158–166. https://doi.org/10.1016/j.ecoenv.2015.04.027
Araújo CFS, Lopes MV, Mirian RV, Menezes-Filho JA (2016) Cadmium and lead in seafood from the Aratu Bay, Brazil and the human health risk assessment. Environ Monit Assess 188:259. https://doi.org/10.1007/s10661-016-5262-y
Vieira LR, Gravato C, Soares AMVM, Morgado F, Guilhermino F (2009) Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: Linking biomarkers to behavior. Chemosphere 76:1416–1427. https://doi.org/10.1016/j.chemosphere.2009.06.005
Guntenspergen GR, Baldwin AH, Hogan DM, Neckles HA, Nielsen MG (2009) Valuing urban wetlands: modification, preservation and restoration. In: McDonnell MJ, Hahs AK, 24. Breuste JH (ed) Ecology of cities and towns: a comparative approach. Cambridge University, pp 516–520
Hatje V, Andrade RLB, de Oliveira CC, Polejack A, Gxaba T (2021) Pollutants in the South Atlantic Ocean: sources, knowledge gaps and perspectives for the decade of Ocean Science. Front Mar Sci 8:1–17. https://doi.org/10.3389/fmars.2021.644569
Mathews T, Fisher NS (2008) Evaluating the trophic transfer of cadmium, polonium, and methylmercury in an estuarine food chain. Environ Toxicol Chem 27:1093–1101. https://doi.org/10.1897/07-318.1
Vasanthi LA, Revathi P, Mini J, Munuswamy N (2013) Integrated use of histological and ultrastructural biomarkers in Mugil cephalus for assessing heavy metal pollution in Ennore estuary, Chennai. Chemosphere 91:1156–1164. https://doi.org/10.1016/j.chemosphere.2013.01.021
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5:288–295. https://doi.org/10.1016/j.toxrep.2018.01.007
Has-Schon E, Bogut I, Strelec I (2006) Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch Environ Contam Toxicol 50:545–551. https://doi.org/10.1007/s00244-005-0047-2
Plessl C, Otachi EO, Körner W, Avenant-Oldewage A, Jirsa F (2017) Fish as bioindicators for trace element pollution from two contrasting lakes in the Eastern Rift Valley, Kenya: spatial and temporal aspects. Environ Sci Pollut Res 24:19767–19776. https://doi.org/10.1007/s11356-017-9518-z
Mallat J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648. https://doi.org/10.1139/f85-083
Hatje V, Barros F (2012) Overview of the 20th century impact of trace metal contamination in the estuaries of Todos os Santos Bay: past, present and future 418 scenarios. Mar Pollut Bull 64:2603–2614. https://doi.org/10.1016/j.marpolbul.2012.07.009
Barros F, Hatje V, Figueiredo MB, Magalhães WF, Dórea HS, Emídio ES (2008) The structure of the benthic macrofaunal assemblages and sediments characteristics of the Paraguaçu estuarine system, NE, Brazil. Estuar Coast Shelf Sci 78:753–762. https://doi.org/10.1016/j.ecss.2008.02.016
Reis-Filho JA, Giarrizzo T (2016) Microgobius meeki as a potential bio-indicator of habitat disturbance in shallow estuarine areas: a useful tool for the assessment of estuarine quality. J Fish Biol 23:126–134. https://doi.org/10.1111/jfb.13007
Chaves PTCC, Otto G (1999) Aspectos biológicos de Diapterus rhombeus (Cuvier) (Teleostei, Gerreidae) na baía de Guaratuba, Paraná, Brasil. Rev Bras Zool 15:289–295
Gibran FZ, Castro RMC (1999) Activity, feeding behaviour and diet of Ogcocephalus vespertilio in southern west Atlantic. J Fish Biol 55:588–595. https://doi.org/10.1111/j.1095-8649.1999.tb00701.x
Ferreira AN, Beretta M, Mafalda PO Jr (2012) Assessing the impact of dredging on phytoplankton association of Aratu harbor, in Todos os Santos Bay, Bahia state. Arquivos de Ciências do Mar, Fortaleza 45:30–46
Rocha TS, Sales EA, Beretta M, Oliveira IB (2016) Effects of dredging at Aratu port in All Saints Bay, Brazil: monitoring the metal content in water and sediments. Environ Monit Assess 188:394. https://doi.org/10.1007/s10661-016-5396-y
Krull M, Abessa DMS, Hatje V, Barros F (2014) Integrated assessment of metal contamination in sediments from two tropical estuaries. Ecotoxicol Environ Saf 106:195–203. https://doi.org/10.1016/j.ecoenv.2014.04.038
Conama. Conselho Nacional do Meio Ambiente/Ministério do Meio Ambiente (2012) Resolução n° 454, de 1° de novembro de 2012 http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=693
Poleksić V, Mitrović-Tutundžić V (1994) Fish gills as a monitor of sublethal and chronic effects of pollution. In: Müller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Fishing News Books, Oxford, pp 339–352
Abdel-Moneim AM, Mohamed A, Al-Kahtani A, Elmenshawy OM (2012) Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 88:1028–1035. https://doi.org/10.1016/j.chemosphere.2012.04.001
Monteiro SM, Rocha E, Fontaínhas-Fernandes A, Sousa M (2008) Quantitative histopathology of Oreochromis niloticus gills after copper exposure. J Fish Biol 73:1376–1392. https://doi.org/10.1111/j.1095-8649.2008.02009.x
Howard CV, Reed MG (2005) Unbiased stereology: three-dimensional measurement in microscopy. Bios Scientific Publishers, Oxford
Anderson MJ, Gorley RN, Clarke KR (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Chapman PM, Wang F (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22. https://doi.org/10.1002/etc.5620200102
Macêdo AKS, dos Santos KPE, Brighenti LS, Windmöller CC, Barbosa FAR, Ribeiro RIMA, dos Santos HB, Thomé RG (2020) Histological and molecular changes in gill and liver of fish (Astyanax lacustris Lütken, 1875) exposed to water from the Doce basin after the rupture of a mining tailings dam in Mariana MG, Brazil. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.139505
Mebane CA, Chowdhury MJM, De Schamphelaere KAC, Lofts S, Paquin PR, Santore RC, Wood CM (2020) Metal bioavailability models: current status, lessons learned, considerations for regulatory use, and the path forward. Environ Toxicol Chem 39:60–84. https://doi.org/10.1002/etc.4560
Ardeshir RA, Zolgharnein H, Movahedinia A, Salamat N, Zabihi E (2017) Comparison of waterborne and intraperitoneal exposure to fipronil in the Caspian white fish (Rutilus frisii) on acute toxicity and histopathology. Toxicol Rep 4:348–357. https://doi.org/10.1016/j.toxrep.2017.06.010
Martins M, Santos JM, Costa MH, Costa PM (2016) Applying quantitative and semi-quantitative histopathology to address the interaction between sediment-bound polycyclic aromatic hydrocarbons in fish gills. Ecotoxicol Environ Saf 131:164–171. https://doi.org/10.1016/j.ecoenv.2016.04.019
Gauthier PT, Norwood WP, Prepas EE, Pyle GG (2014) Metal–PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat Toxicol 154:253–269. https://doi.org/10.1016/j.aquatox.2014.05.026
Oliveira Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquatic Toxicol 74:53–69. https://doi.org/10.1016/j.aquatox.2005.04.008
Cruz AL, Prado TM, Maciel LAS, Couto RD (2015) Environmental effects on the gills and blood of Oreochromis niloticus exposed to rivers of Bahia, Brazil. Ecotoxicol Environ Saf 111:23–31. https://doi.org/10.1016/j.ecoenv.2014.09.022
Bao J, Xing Y, Feng C, Kou S, Jiang H, Li X (2020) Acute and sub-chronic effects of copper on survival, respiratory metabolism, and metal accumulation in Cambaroides dauricus. Sci Rep 10:16700. https://doi.org/10.1038/s41598-020-73940-1
Araujo HRM, Fernandes MN, Cruz AL (2019) Gill morphology and Na+/K+-Atpase activity of Gobionellus oceanicus (Teleostei: Gobiidae) in an estuarine system. Biol Trace Elem Res 187:526–535. https://doi.org/10.1007/s12011-018-1393-z
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. https://doi.org/10.1152/physrev.00050.2003
Jia YJ, Wang L, Qu Z, Wang C, Yang Z (2017) Effects on heavy metal accumulation in freshwater fishes: species, tissues, and sizes. Environ Sci Pollut Res 24:9379–9386. https://doi.org/10.1007/s11356-017-8606-4
Teien H-C, Standring WJF, Salbu B (2006) Mobilization of river transported colloidal aluminium upon mixing with seawater and subsequent deposition in fish gills. Sci Total Environ 364:149–164. https://doi.org/10.1016/j.scitotenv.2006.01.005
CRA. Centro de Recursos Ambientais (2004) Diagnóstico da concentração de metais pesados e hidrocarbonetos de petróleo nos sedimentos e biota da Baía de Todos os Santos. Consórcio BTS Hydros CH2MHILL. Governo do Estado da Bahia
Hatje V, Bícego MC, Carvalho GC, Andrade JB (2009) Todos os Santos Bay: oceanographic aspects/chemical contamination. EDUFBA, Salvador
Beg MU, Al-Jandal N, Al-Subiai S, Karam Q, Husain S, Butt SA, Ali A, Al-Hasan E, Al-Dufaileej S, Al-Husaini M (2015) Metallothionein, oxidative stress and trace metals in gills and liver of demersal and pelagic fish species from Kuwaits’ marine area. Mar Pollut Bull 75:344–356. https://doi.org/10.1016/j.marpolbul.2015.07.058
Cruz AL, Prado RLP, Klein W (2019) The potential respiratory surfaces of a fish living in a historically polluted river. Anim Biol. https://doi.org/10.1163/15707563-20191109
Al-Yousuf MH, El-Shahawi MS, Al-Ghais SM (2000) Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ 256:87–94. https://doi.org/10.1016/s0048-9697(99)00363-0
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121:129–136. https://doi.org/10.1016/S0269-7491(02)00194-X
Ural M, Yildirim N, Danabas D, Kaplan O, Yildirim NC, Ozcelik M, Kurekci EF (2012) Some heavy metals accumulation in tissues in Capoeta umbla (Heckel, 1843) from Uzuncayir Dam Lake (Tunceli, Turkey). Environ Contam Tox 88:172–176. https://doi.org/10.1007/s00128-011-0474-x
Brunelli E, Mauceri A, Maisano M, Bernab I, Giannetto A, DeDomenico E, Corapi B, Tripepi S, Fasulo S (2011) Ultrastructural and immunohistochemical investigation on the gills of the teleost, Thalassoma pavo L., exposed to cadmium. Acta histochem 113:201–213. https://doi.org/10.1016/j.acthis.2009.10.002
Pane EF, Haque A, Wood CM (2004) Mechanistic analysis of acute, Ni-induced respiratory toxicity in the rainbow trout (Oncorhynchus mykiss): an exclusively branchial phenomenon. Aquat Toxicol 69:11–24. https://doi.org/10.1016/j.aquatox.2004.04.009
Thophon S, Kruatachue M, Upatham ES, Pokethityook P, Sahaphong S, Jaritkhuan S (2003) Histopathological alterations of whiteseabass, Lates calarifera, in acute and subchronic cadmium exposure. Environ Pollut 121:307–320. https://doi.org/10.1016/S0269-7491(02)00270-1
Massar B, Dey S, Dutta K (2013) Electron microscopy of fish gill ultra-structure with reference to water pollution by municipal wastes. J Adv Microsc Res 7:1–7. https://doi.org/10.1166/jamr.2012.1109
Macirella R, Brunelli R (2017) Morphofunctional alterations in zebrafish (Danio rerio) gills after exposure to mercury chloride. Int J Mol Sci 18:1–19. https://doi.org/10.3390/ijms18040824
Samanta P, Im H, Na J, Jung J (2018) Ecological risk assessment of a contaminated stream using multi-level integrated biomarker response in Carassius auratus. Environ Pollut 233:429–438. https://doi.org/10.1016/j.envpol.2017.10.061
Acknowledgements
H.H.Q.O. and L.A.S.M. thank the National Council for Scientific and Technological Development, Brazil (CNPq) and Foundation for Research Support of the state of Bahia, Brazil (FAPESB) for the master’s fellowships. R.M.S. and E.F.E.S. thank CNPq for the scientific initiation scholarships. J.A.C.C.N. and J.A.R.F. thank Coordination of Improvement of Higher-Level Personnel, Brazil (CAPES), Finance Code 001, for PhD fellowships. The authors also thank Ralph Gruppi Thomé (UFSJ, Divinópolis, MG, Brazil) and Marisa Narciso Fernandes (UFSCar, São Carlos, SP, Brazil) for their comments and suggestions, Domingos Cardoso for computer assistance, and the Laboratório de Microscopia Eletrônica, FIOCRUZ, Salvador, Bahia, for the assistance on microscopy electronic transmission.
Funding
This study was financially supported by the National Institute of Science and Technology in Comparative Physiology (INCT-Comparative Physiology, Brazil) to ALC, and Fapesb (Foundation for Research Support of the State of Bahia, Brazil) (RED0037/2014) on behalf of ALC and PRAMA.
Author information
Authors and Affiliations
Contributions
H.H.Q.O.: conceptualization, formal analysis, investigation, methodology, and writing—original draft
J.A.R.F.: conceptualization, formal analysis, investigation, methodology, resources, supervision and writing—original draft.
J.A.C.C.N.: formal analysis, investigation, resources and writing—original draft
R.M.S.: investigation, formal analysis and writing—review & editing
E.F.E.S.: investigation and formal analysis
L.A.: investigation, formal analysis and writing—review & editing
P.R.A.M.A.: conceptualization, funding acquisition, project administration and resources
A.L.C.: conceptualization, formal analysis, investigation, funding acquisition, methodology, project administration, resources, supervision and writing—review & editing
Corresponding author
Ethics declarations
Ethical Approval
The local ethics committee approved these procedures on animal use (Comitê de Ética no Uso de Animais, Instituto de Biologia, Universidade Federal da Bahia, 23/2015).
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, H.H.Q., Reis-Filho, J.A., Nunes, J.A.C.C. et al. Gill Histopathological Biomarkers in Fish Exposed to Trace Metals in the Todos os Santos Bay, Brazil. Biol Trace Elem Res 200, 3388–3399 (2022). https://doi.org/10.1007/s12011-021-02930-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02930-9