Abstract
Mineral elements (copper (Cu), zinc (Zn), calcium (Ca), magnesium (Mg), iron (Fe)) play important biological roles in enzymes, hormones, vitamins, and normal metabolism. The deficiency of mineral elements can lead to abnormal physiological functions. And some elements (such as lead (Pb)) are harmful to the body. We aim to identify genetic loci which can influence the serum levels of mineral elements (Cu, Zn, Ca, Mg, Fe, and Pb). Genotyping was performed using Applied Biosystems Axiom™ PMDA in 587 individuals, and 6,423,076 single-nucleotide polymorphisms (SNPs) were available for the genome-wide association study (GWAS) analysis. The association between genotype and phenotype was analyzed using mixed linear regression (additive genetic model) adjusting by age and gender combined with identical by descent (IBD) matrix. Genetic loci in BCHE-LOC105374194, DTX2P1-UPK3BP1-PMS2P11, VAT1L, LINC00908-LINC00683, LINC01310-NONE, and rs6747410 in VWA3B were identified to be associated with serum Cu element concentration (p < 5 × 10−6). ADAMTSL1 rs17229526 (p = 4.96 × 10−6) was significantly associated with serum Zn element levels. Genetic loci in LRP1B, PIGZ-MELTF, LINC01365-LINC02502, and HAPLN3 were related to serum Ca element levels (p < 5 ×1 0−6). Three SNPs in ALPK1, ASAP1-ADCY8 and IER3IP1-SKOR2 also achieved a significant association with Mg element levels (p < 5 × 10−6). TACSTD2-MYSM1, LRP1B, and ASAP1-ADCY8 showed suggestive associations with serum Fe element levels (p < 5 × 10−6). Moreover, the two most significant SNPs associated with Pb were rs304234 in CADPS-LINC00698 (p = 2.47 × 10−6) and rs12666460 in LOC101928211-GPR37 (p = 1.81 × 10−6). In summary, we reported 19 suggestive loci associated with serum mineral elements in the Chinese Han population. These findings provided new insights into the potential mechanisms regulating serum mineral elements levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metals are essential but very limited in humans. These mineral elements play important biological roles in enzymes, hormones, vitamins, and normal metabolism [1]. The deficiency of these mineral elements can lead to abnormal physiological functions in humans, and ultimately lead to varieties of relevant diseases and disorders. Fortunately, those problems mentioned above can be prevented or reversed by appropriate supplementation [2]. Adequate intake of such elements as copper (Cu), zinc (Zn), and iron (Fe) supports Th1/Th2-mediated immune response and produces sufficient proinflammatory cytokines [3]. The concentration of calcium (Ca) is associated with maintaining normal neuromuscular activity [4]. Magnesium (Mg) is a divalent cation that is important for bone and calcium metabolism and the normal secretion of parathyroid hormone [5]. Heavy metal pollutants such as lead (Pb) may also contribute to the genesis of coronary atherosclerosis and hypertension [6]. Serum concentrations of mineral elements have been shown to have a component with heritability [7].
The identification of suggestive loci and genes associated with circulating mineral element concentrations may provide insights into physiologic regulators of mineral element homeostasis. Genome-wide association study (GWAS) has made a major contribution to our understanding of the genetics of complex disorders [8]. At present, it has been reported that some loci are closely related to serum mineral elements levels. Beben Benyamin et al. have identified eleven iron-related significant loci (HFE, TF, TFR2, and TMPRSS6) [9]. Previous population-based studies have identified two loci on chromosome 1 (rs1175550 and rs2769264) for Cu and three loci on chromosomes 8, 15, and X (rs1532423, rs2120019, and rs4826508) for Zn [10]. It was found that common loci at six genes (MUC1, TRPM6, SHROOM3, ATP2B1, DCDC5, and MDS1) were contributed to circulate Mg concentrations [7]. Esther Ng et al. reported some novel genetic variants associated with whole blood levels of toxic metals [11]. However, these loci can only be used to explain parts of the heritability of mineral elements, and many loci have not been discovered yet. In addition, previous large-scale studies are focusing on European and American populations, and the genetic contribution to circulating mineral element concentrations in the Chinese population has not been fully understood.
To identify genetic loci which have an influence on the serum levels of mineral elements (Cu, Zn, Ca, Mg, Fe, and Pb), we performed a GWAS using 6,423,076 imputed single-nucleotide polymorphisms (SNPs) in 587 healthy Chinese Han population.
Material and Method
Study Cohorts
A total of 587 individuals (285 females and 302 males) were recruited from the annual checkup of Hainan Affiliated Hospital of Hainan Medical University for GWAS analyses. All participants were healthy Chinese Han population. Subjects with infections and immunological diseases, tumors, or known disease were excluded in this study. Demographic and clinical information was collected from questionnaires and/or medical records. All participating cohorts provided written informed consents. The protocols were approved by the institutional review boards of Hainan Affiliated Hospital of Hainan Medical University, and were in the Declaration of Helsinki.
GWAS Genotyping
Approximately 5 mL peripheral blood samples were collected from each participant in EDTA-coated tubes. Genomic DNA was extracted from the blood samples using a GoldMag DNA isolation Kit (GoldMag Co., Ltd., Xi’an, China). All participants were genotyped using Applied Biosystems Axiom™ Precision Medicine Diversity Array (PMDA) on GeneTitan™ Multi-Channel Instrument (Thermo Fisher, CA, USA). Genotype clustering was analyzed using the Axiom Analysis Suite 6.0 software. Quality control (QC) was performed based on sample calling rate > 0.95, maker calling rate > 0.90, and Hardy-Weinberg equilibrium (HWE) > 5 × 10−06. After removing Indel, copy number variation (CNV), duplication and loci from sex chromosome, and QC, 796,288 SNPs were available for subsequent analyses.
Genotype Imputation
Genome-wide data were imputed to 9336,679 markers from phase 3 of 1000 genome haplotype reference panel using IMPUTE2 software. Taking into account the uncertainty of imputation, Gold Helix SNP & Variation Suite 8.7 software was used for the association analysis. Loci with a minor allele frequency (MAF) < 1% and non bi-allelic were removed. After QC and imputation, 6,423,076 SNPs were available for the final analyses.
Data Analysis
The association between genotype and phenotype (mineral elements) was analyzed using mixed linear regression (additive genetic model) adjusting by age and gender combined with identical by descent (IBD) matrix using the PLINK software. The concentrations of mineral elements were normalized using rank-based inverse normal transformations. Locus regional plots were constructed by the LocusZoom 1.1 software (https://statgen.github.io/localzoom/). Manhattan plots and quantile-quantile (QQ) plots were generated by − log10 (p value) using the R-package. p < 5 × 10−6 means a statistical significance for the GWAS analysis.
Results
Overall, 587 individuals (44.39 ± 9.40 years) were recruited in the study. The characteristics of subjects including mean ages, gender distributions, body mass index (BMI), and serum levels of mineral elements (Cu, Zn, Ca, Mg, Fe, and Pb) are presented in Table 1. After having stringent QC filters, 6,423,076 imputed SNPs were analyzed to the discovery analyses of loci associated with serum concentrations of mineral elements.
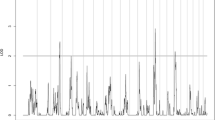
In GWAS, 105 loci in different chromosomal regions with suggestive significance of p values < 5 × 10−6 are shown in Suppl_ Table 1. Manhattan plot (Figure 1) exhibited the chromosome location of suggestive loci for serum levels of Cu (39 loci), Zn (1 locus), Ca (5 loci), Mg (21 loci), Fe (33 loci), and Pb (6 loci). QQ plots for serum levels of mineral elements are displayed in Figure 2. The p value at the point of deviation from the band in the QQ plot was used as the threshold for suggestive associations.
Table 2 presents 19 selected top loci (p-values) of each chromosome for serum mineral elements levels. Regional plots of significant loci associated with serum concentrations of mineral elements are displayed in Suppl_ Figure 1 (Cu), Suppl _Figure 2 (Zn), Suppl_ Figure 3 (Ca), Suppl_ Figure 4 (Mg), Suppl_ Figure 5 (Fe), and Suppl_ Figure 6 (Pb). Six loci were associated with serum Cu element concentration, including rs68128869 in BCHE-LOC105374194 (p = 4.47 × 10−06), rs12673824 in DTX2P1-UPK3BP1-PMS2P11 (p = 3.23 × 10−06), rs116919355 in VAT1L (p = 2.02 × 10−06), rs186084489 in LINC00908-LINC00683 (p = 3.18 × 10−07), rs17000827 in LINC01310-NONE (p = 3.75 × 10−06), and rs6747410 in VWA3B (p = 4.06 × 10−06). Zn element levels were significant associated with rs17229526 on chromosome 9 (p = 4.96 × 10−06) in the intron region of ADAMTSL1 gene. rs76917806 in LRP1B (p = 5.9 × 10−07), rs111232694 in PIGZ-MELTF (p = 3.5 × 10−06), rs72684540 in LINC01365-LINC02502 (p = 3.4 × 10−06), and rs8039131 in HAPLN3 (p = 4.4 × 10−06) were related to serum Ca element levels. Three SNPs also achieved the significant association with Mg element levels with p values of 4.2 × 10−06, 1.3 × 10−06, and 3 × 10−06 for rs2074379 in ALPK1, rs72728275 in ASAP1-ADCY8, and rs117060920 in IER3IP1-SKOR2, respectively. rs232870 in TACSTD2-MYSM1 (p = 1.9 × 10−06), rs76917806 in LRP1B (p = 5 × 10−06), and rs28428945 in ASAP1-ADCY8 (p = 1.4 × 10−07) showed suggestive associations with serum Fe element levels. Moreover, the two most significant SNPs associated with Pb were rs304234 in CADPS-LINC00698 (p = 2.47 × 10−06) and rs12666460 in LOC101928211-GPR37 (p = 1.81 × 10−06).
Discussion
In this study, our study identified 19 loci with suggestive significance of p values < 5 × 10−6 related to circulating levels of Cu (6 loci), Zn (1 locus), Ca (4 loci), Mg (3 loci), Fe (3 loci), and Pb (2 loci), which explain a part of the variation in these mineral elements. This is the first GWAS investigating the contribution of genetic loci to normal physiologic variation in serum concentrations of mineral elements in the Chinese Han population.
Cu, a cofactor of many metalloenzymes, plays an important role in the human metabolism. The disturbance of Cu homeostasis cause strong pathological manifestations, such as severe chronic liver diseases including Wilson’s disease and idiopathic toxicosis [12]. In our study, six loci (rs68128869 in BCHE-LOC105374194, rs12673824 in DTX2P1-UPK3BP1-PMS2P11, rs116919355 in VAT1L, rs186084489 in LINC00908-LINC00683, rs17000827 in LINC01310-NONE, and rs6747410 in VWA3B) were associated with serum Cu element concentration. BCHE gene is located on chromosome 3q26.1. The function of LOC105374194, DTX2P1, UPK3BP1, PMS2P11, LINC00908, LINC00683, LINC01310, and NONE genes deserve further study. In recent years, some studies have shown that flavanone derivatives complexed to Cu can act as selective cholinesterase inhibitors against butyrylcholinesterase [13]. The details of possible links between VAT1L (chromosome 16q23.1) and VWA3B (chromosome 2q11.2) genes and circulating Cu concentration are still unknown.
Zinc is a component of most enzymes, and free Zn is an essential intracellular signaling molecule [14, 15]. Zn deficiency has been related to cardiovascular disease, diabetes, immune dysfunction, and infectious diseases [16,17,18]. Zn element levels were significant associated with rs17229526 on chromosome 9 in the intron region of ADAMTSL1 gene. ADAMTS1, a secreted protein, is a member of family of ADAMTS proteases, which a disintegrin and metalloprotease with thrombospondin repeats, and the zinc-binding domain may affect the catalytic activity of ADAMTS1 [19]. The contribution of ADAMTSL1 polymorphisms to Zn concentration needs to be further studied.
Approximately 99% of Ca is usually contained in the bone; the remainder is present intracellular or circulating. Plasma Ca is mainly influenced by parathyroid hormone [20]. Ca accumulation inside mitochondria contributes to a wide range of cellular functions, including key metabolic pathways and life/death decisions [21]. Our study displayed that rs76917806 in LRP1B, rs111232694 in PIGZ-MELTF, rs72684540 in LINC01365-LINC02502, and rs8039131 in HAPLN3 were related to serum Ca element levels. LRP1B, located on chromosome 2q22.1-q22.2, may participate in extracellular signal transduction via the different phosphorylation status [22]. PIGZ and MELTF genes are located on chromosome 3q29, and the association of PIGZ and MELTF genes with serum Zn element levels is still unknown. HAPLN3 gene has been reported to be associated with calcitic biomineralization [23]. rs8039131is located on the exonic region of HAPLN3 gene. The contribution of rs8039131 to circulating Ca concentration might be related to the expression or function of HAPLN3.
Mg is the second most abundant intracellular cation and is a cofactor in nucleic acid synthesis and many enzymatic reactions [24]. It is reported that serum Mg levels are associated with several common and chronic diseases, including cardiovascular disease, neurological disorders, and hypertension [25, 26]. Here, three SNPs also achieved the significant association with Mg element levels for rs2074379 in ALPK1, rs72728275 in ASAP1-ADCY8, and rs117060920 in IER3IP1-SKOR2, respectively. The possible association of ASAP1 (chromosome 8q24.21-q24.22), ADCY8 (chromosome 8q24.22), IER3IP1 (chromosome 18q21.1), and SKOR2 (chromosome 18q21.1) genes on circulating Cu concentration are unknown. Alpha-kinase 1 (ALPK1) on chromosome 4q25, a member of the alpha-kinase family, is related to cancer and chronic diseases such as chronic kidney disease and diabetes [27, 28]. rs2074379 (Ile732Met), located on the exonic region of ALPK1 gene, has been reported to be associated with diabetes [27]. However, the functional relevance of rs2074379 variants of ALPK1 to the contribution of circulating Mg concentration has not been determined.
Fe is an essential element for numerous fundamental biologic processes, such as oxygen transport, mitochondrial respiration, and cell signaling, but the imbalances in iron homeostasis contribute to disease [29]. In the study, rs232870 in the intergenic region of TACSTD2-MYSM1, rs76917806 in the intronic region of LRP1B, and rs28428945 in the intergenic region of ASAP1-ADCY8 showed suggestive associations with serum Fe element levels. MYSM1 (chromosome 1p32.1) has emerged as an important regulator of hematopoietic stem cell function, hematogenesis, immune response, and other mammalian physiology [30]. LRP1B (chromosome 2q22.1-q22.2) mutations existed twice in patients with pure red cell aplasia [31]. The role of TACSTD2 (chromosome 1p32.1), ASAP1 (chromosome 8q24.21-q24.22) and ADCY8 (chromosome 8q24.22) on the Fe element levels need further research.
Lead chloride has a significant toxic effect on the membrane of platelets, which activates the release of platelet aggregation factors, thereby increasing its adhesion and aggregation properties [32]. Here, the two most significant SNPs associated with Pb were rs304234 in CADPS-LINC00698 and rs12666460 in LOC101928211-GPR37. LINC00698 and LOC101928211are long intergenic non-protein coding RNA whose function need further study. CADPS (chromosome 3p14.2) gene is mainly expressed in nerve and endocrine tissues and acts as a Ca sensor in regulated exocytosis [33]. GPR37 (chromosome 7q31.33) is highly expressed in the brain and has been implicated in the neurological disorders [34]. However, the contribution of CADPS and GPR37 genes to circulating Pb concentration has not been determined.
Several limitations should not be ignored. First, the major problem is that the sample size is relatively small and there are no replication study, and therefore, this study results need to be confirmed in a larger population. Second, the validity of the results was restricted to participants from Chinese Han populations, which means that our finding couldn’t be generalized to other ethnic groups. Third, we have not performed functional studies on the identified variants, so further research is required to reveal the biological mechanism behind the observed associations.
Conclusion
In summary, we reported 19 suggestive loci associated with serum mineral elements in the Chinese Han population. These findings provided new insights into the potential mechanisms regulating serum mineral element levels. Further replication studies are required to confirm these findings.
References
Fraga CG, Oteiza PI, Keen CL (2005) Trace elements and human health. Mol Asp Med 26(4-5):233–234
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Asp Med 26(4-5):235–244
Wintergerst ES, Maggini S, Hornig DH (2007) Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab 51(4):301–323
Allgrove J (2015) Physiology of calcium, phosphate, magnesium and vitamin D. Endocr Dev 28:7–32
Hoenderop JG, Bindels RJ (2005) Epithelial Ca2+ and Mg2+ channels in health and disease. J Am Soc Nephrol : JASN 16(1):15–26
Sponder M, Fritzer-Szekeres M, Marculescu R, Mittlböck M, Uhl M, Köhler-Vallant B, Strametz-Juranek J (2014) Blood and urine levels of heavy metal pollutants in female and male patients with coronary artery disease. Vasc Health Risk Manag 10:311–317
Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ et al (2010) Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet 6(8):e1001045
Dehghan A (2018) Genome-wide association studies. Methods Mol Biol (Clifton, NJ) 1793:37–49
Benyamin B, Esko T, Ried JS, Radhakrishnan A, Vermeulen SH, Traglia M et al (2014) Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun 5:4926
Evans DM, Zhu G, Dy V, Heath AC, Madden PA, Kemp JP et al (2013) Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet 22(19):3998–4006
Ng E, Lind PM, Lindgren C, Ingelsson E, Mahajan A, Morris A, Lind L (2015) Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum Mol Genet 24(16):4739–4745
Vetchý M (2018) Biological role of copper as an essential trace element in the human organism. Ceska a Slovenska farmacie : casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti 67(4):143–153
Sarria AL, Vilela AF, Frugeri BM, Fernandes JB, Carlos RM, da Silva MF et al (2016) Copper (II) and zinc (II) complexes with flavanone derivatives: Identification of potential cholinesterase inhibitors by on-flow assays. J Inorg Biochem 164:141–149
Kambe T (2011) An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem 75(6):1036–1043
Sekler I, Sensi SL, Hershfinkel M, Silverman WF (2007) Mechanism and regulation of cellular zinc transport. Mol Med (Cambridge, Mass) 13(7-8):337–343
Black RE (2003) Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 133(5 Suppl 1):1485s–1489s
Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL (2010) Zinc and cardiovascular disease. Nutrition (Burbank, Los Angeles County, Calif) 26(11-12):1050–1057
Rutter GA (2010) Think zinc: new roles for zinc in the control of insulin secretion. Islets 2(1):49–50
Rodríguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G et al (2002) ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun 293(1):501–508
Goff JP (2014) Calcium and magnesium disorders. Vet Clin North Am Food An Pract 30(2):359–381 vi
Granatiero V, De Stefani D, Rizzuto R (2017) Mitochondrial calcium handling in physiology and disease. Adv Exp Med Biol 982:25–47
Shiroshima T, Oka C, Kawaichi M (2009) Identification of LRP1B-interacting proteins and inhibition of protein kinase Calpha-phosphorylation of LRP1B by association with PICK1. FEBS Lett 583(1):43–48
Rose-Martel M, Smiley S, Hincke MT (2015) Novel identification of matrix proteins involved in calcitic biomineralization. J Proteome 116:81–96
Costello R, Wallace TC, Rosanoff A (2016) Magnesium. Adv Nutr (Bethesda, Md) 7(1):199–201
Houston M (2011) The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens (Greenwich, Conn) 13(11):843–847
Kirkland AE, Sarlo GL, Holton KF (2018) The Role of Magnesium in Neurological Disorders. Nutrients 10(6):730
Yamada Y, Matsui K, Takeuchi I, Oguri M, Fujimaki T (2015) Association of genetic variants of the α-kinase 1 gene with type 2 diabetes mellitus in a longitudinal population-based genetic epidemiological study. Biomed Rep 3(3):347–354
Yamada Y, Nishida T, Ichihara S, Kato K, Fujimaki T, Oguri M, Horibe H, Yoshida T, Watanabe S, Satoh K, Aoyagi Y, Fukuda M, Sawabe M (2013) Identification of chromosome 3q28 and ALPK1 as susceptibility loci for chronic kidney disease in Japanese individuals by a genome-wide association study. J Med Genet 50(6):410–418
Dev S, Babitt JL (2017) Overview of iron metabolism in health and disease. Hemodial Int Int Symp Home Hemodial 21 Suppl 1(Suppl 1):S6–s20
Fiore A, Liang Y, Lin YH (2020) Deubiquitinase MYSM1 in the hematopoietic system and beyond: a current review. Int J Mol Sci 21(8):24
Zhang X, Shi Y, Song L, Shen C, Cai Q, Zhang Z, Wu J, Fu G, Shen W (2018) Identification of mutations in patients with acquired pure red cell aplasia. Acta Biochim Biophys Sin 50(7):685–692
Myslyts’kyĭ VF, Podolian SK (1999) Pathological changes in thrombocyte-vascular and coagulative hemostasis under the influence of lead chloride and their correction by using a synthetic analog of prostacyclin. Fiziolohichnyi zhurnal (Kiev, Ukraine : 1994) 45(4):99–104
Cisternas FA, Vincent JB, Scherer SW, Ray PN (2003) Cloning and characterization of human CADPS and CADPS2, new members of the Ca2+-dependent activator for secretion protein family. Genomics 81(3):279–291
Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR (2018) GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 128(8):3568–3582
Acknowledgements
The authors thank all participants and volunteers in this study.
Availability of Data and Materials
All the data regarding the findings are available within the manuscript. Anyone who is interested in the information should contact the corresponding author.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81660013 and No.81860015) and Key Research and Development Plan of Hainan Province (No. ZDYF2018116).
Author information
Authors and Affiliations
Contributions
Duojian Guo and Yu zhou: writing; Xingwei Wei, Shanshan Zhang, Tianbo Jin: methodology; Yutian Zhang, Mei Lin, Xiaoli Zhou: data curation; Yufei Xie, Chanyi He, Qi Lin: Sample collection; Ping He and Yipeng Ding: conceptualization.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All of the participating cohort provided written informed consents. The protocols were approved by the institutional review boards of Hainan Affiliated Hospital of Hainan Medical University, and were in the Declaration of Helsinki.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Duojian Guo and Yu Zhou are co-first authors
Supplementary Information
ESM 1
(DOCX 25 kb)
ESM 2.
Locus regional plots of six loci associated with serum Cu level. (PNG 9387 kb)
ESM 3.
Locus regional plots of one locus associated with serum Zn level. (PNG 1311 kb)
ESM 4.
Locus regional plots of four loci associated with serum Ca level. (PNG 7105 kb)
ESM 5.
Locus regional plots of three loci associated with serum Mg level. (PNG 4990 kb)
ESM 6.
Locus regional plots of three loci associated with serum Fe level. (PNG 4853 kb)
ESM 7.
Locus regional plots of two loci associated with serum Pb level. (PNG 3201 kb)
Rights and permissions
About this article
Cite this article
Guo, D., Zhou, Y., Wei, X. et al. Preliminary study of genome-wide association identifies novel susceptibility genes for serum mineral elements in the Chinese Han population. Biol Trace Elem Res 200, 2549–2555 (2022). https://doi.org/10.1007/s12011-021-02854-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02854-4