Abstract
Little attention has been paid to the tolerance of osteoblasts to fluoride in distinct differentiation stages, and the role of TGF-β1 in fluoride-treated osteoblast differentiation of progenitors and precursors was rarely mentioned in previous studies. The present study aimed to clarify how fluoride affected different differentiation stages of osteoblasts, and to elucidate the role of TGF-β1 in this process. We assessed cell migration, proliferation, DNA damage, and apoptosis of early-differentiated osteoblasts derived from bone marrow stem cells (BMSCs) exposed to fluoride with or without TGF-β1. Subsequently, MC3T3-E1 cells cultured with mineral induction medium were treated with fluoride to test fluoride’s effect on late-differentiated osteoblasts. The specific fluoride concentrations and treatment times were chosen to evaluate the role of TGF-β1 in fluoride-induced osteoblastic differentiation and function. Results showed early-differentiated osteoblasts treated with a low dose of fluoride grew and moved more rapidly. TGF-β1 promoted cell proliferation and inhibited cell apoptosis in early-differentiated osteoblasts exposed to a low fluoride dose, but enhanced apoptosis at higher fluoride conditions. In the late-differentiated osteoblasts, the fluorine dose range with anabolic effects was narrowed, and the fluoride range with catabolic effects was widened. Treatment with a low fluoride dose stimulated the alkaline phosphatase (ALP) expression. TGF-β1 treatment inhibited Runx2 expression but increased RANKL expression in late-differentiated osteoblasts exposed to fluoride. Meanwhile, TGF-β1 treatments activated Smad3 phosphorylation but blocked Wnt10b expression in osteoblasts. We conclude that TGF-β1 plays an essential role in fluoride-induced differentiation and osteoblast function via activation of Smad3 instead of Wnt10 signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Decades of research have shown that fluoride has both beneficial and harmful effects on bone health. Approximately 99% of fluoride in the body is found in adult calcified tissues. Fluoride concentrations in bone tend to increase with age due to continuous fluoride uptake throughout life. Consequently, excessive uptake of fluoride causes dental and skeletal fluorosis in humans and animals. Skeletal fluorosis is irreversible, and a clear understanding of the cellular mechanisms underlying disease progression can contribute to the development of interventions to prevent skeletal fluorosis. Skeletal homeostasis is sustained through the lifelong process of bone turnover. The most abundant transforming growth factor β (TGF-β) isoform, TGF-β1, is secreted by almost every cell type. Recombinant human TGF-β1 is a 25.0-kDa protein composed of two identical 112 amino acid polypeptide chains linked by a single disulfide bond. TGF-β1 signals across the plasma membrane by specific type I and type II receptors and results in phosphorylation of Smad2/3 proteins, which is known to be essential for osteoblast and osteoclast differentiation. Bone turnover is initiated through osteoclast activation and subsequent release of signaling molecules, like transforming growth factor β (TGF-β), embedded in the matrix through the proteolytic degradation of the extracellular matrix (ECM). Afterward, bone formation occurs when Wnt/β-catenin, TGF-β, and BMP signaling induce osteogenesis, through a process where bone marrow stromal cells (BMSCs) differentiate into pre-osteoblasts and mature into functional osteoblasts.
It was reported that fluoride overdose alters the balance between bone formation and resorption, leading to bone turnover disorders [1, 2]. Researchers have observed that skeletal fluorosis leads to diverse bone lesions, such as osteosclerosis, osteoporosis, and degenerative joint changes, and found bone turnover as a pathologic characteristic of skeletal fluorosis [3, 4]. Fluoride can have deleterious effects at the cellular levels, depending on concentration, duration of exposure, and cell type [5, 6]. Our previous studies indicated that fluoride regulates TGF-β1 signaling during the activation of bone turnover and BMSC differentiation via the activin receptor-like kinase 5 (ALK5)-Smad3 signaling pathway [7, 8]. The present study was designed to clarify how fluoride affected osteoblast differentiation, and to elucidate the role of TGF-β1 in this process. BMSCs were induced to early-differentiated osteoblasts by culturing in mineral induction medium. We assessed cell migration, proliferation, DNA damage, and apoptosis under fluoride combined with TGF-β1 treatment. On the other hand, MC3T3-E1 cells were differentiated into late-stage osteoblasts and exposed to fluoride with or without TGF-β1. Then, cell viability and critical proteins related to bone turnover and TGF-β1 signaling were investigated. This study demonstrated an essential role of TGF-β1 on fluoride-treated osteoblasts at different differentiation stages and preliminarily clarified the underlying signaling pathway.
Materials and Methods
Scratch-wound Healing Assay for BMSCs Exposed to Fluoride
Bone marrow mesenchymal stem cells (BMSCs) were isolated from the femur of young ICR mice (4 weeks old) and washed in phosphate-buffered saline (PBS, pH=7.4) at 4 °C. BMSCs were collected by centrifugation for 10 min at 1200 rpm. Cells were then cultured in DMEM/F12 medium (Hyclone Co, USA) and passaged to c third generation. Positive expression of surface antigens CD34 and CD44 (BD Biosciences, USA) was to identify and purify BMSCs [6]. After that, a wound gap was created by scratching a growing confluent culture of BMSCs induced into early-differentiated osteoblasts by culturing with mineral induction medium (0.05 g/L vitamin C, 40 ng/mL dexamethasone, and 10 mmol/L β-sodium glycerol phosphate). Cells were simultaneously treated with a medium containing fluoride ion (sodium fluoride, Sigma-Aldrich, USA) at concentrations of 0.5 and 4 mg/L. The control group was cultured in mineral induction medium. Healing of this gap by cell migration and growth was monitored and quantified at different periods between 12 h and 1~7 days.
Analysis of Cell Proliferation, Apoptosis, and DNA Damage in BMSCs Exposed to Fluoride and TGF-β1
BMSCs were seeded into a 6-well plates at a density of 5×105 cells per well and cultured in mineral induction medium for inducing early-differentiated osteoblasts. When BMSCs reached about 70% confluence, cells were treated with 0.5, 4, and 16 mg/L of fluoride with or without 10 ng/mL of TGF-β1 (PEPROTECH, Cat# 100-21c, USA) for 4 days. At the end of the period, cells were stained with fluorescent antibodies, bromodeoxyuridine (BrdU), phosphorylated H2AX, and cleaved poly [ADP-Ribose] polymerase (PARP). BrdU served as a marker of cell proliferation. On the other hand, caspase-3 cleaves PARP resulting in its inactivation and the inability of cells to repair DNA damage. Thus, cleaved PARP serves as a marker of cellular apoptosis. Phosphorylated H2AX serves as a marker of DNA damage as it promotes DNA repair maintaining genomic stability. This test is performed according to the kit protocol (BD Pharmingen, Cat #562253, USA).
Cell Viability of Osteoblasts Exposed to Fluoride
The MC3T3-E1 cell line is a model for studying in vitro osteoblast differentiation after growth in ascorbic acid and 3 to 4 mM inorganic phosphate. In this study, MC3T3-E1 cells subclone 14 were kindly provided by Stem Cell Bank, Chinese Academy of Sciences. MC3T3-E1 cells were seeded into 96-well plates at a density of 5×103 cells per well, and cultured in a medium containing mineralization induction agents for late-stage osteoblast differentiation. When cells reached about 60% confluence, they were simultaneously treated with different fluoride concentrations from 0.001 to 32 mg/L of fluorine ion for 1, 2, 4, and 7 days. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, USA) assay was performed to detect cell viability at the end of each treatment period. Cells were treated with MTT reagent (5 mg/mL), then they were incubated for 4 h at 37 °C with 5% CO2. Following incubation, dimethyl sulfoxide (DMSO, Sigma-Aldrich, USA) was added for color development. Each well’s absorbance was measured at 570 nm using the Multiskan FC spectrophotometer (Thermo Fisher, USA).
Western Blot Analysis for Bone Turnover-Related Proteins and TGF-β1 Signaling
MC3T3-E1 cells were seeded in 6-well plates at a density of 3 × 104 cells per well and cultured with mineral induction medium for late-stage osteoblast differentiation. When cells reached about 60% confluence, they were simultaneously treated with 0.5 and 4 mg/L of fluoride with or without 10 ng/mL of TGF-β1 for 7 days. At the end of the treatment period, proteins were collected with RIPA solution (Beyotime, China). Samples of total soluble protein (20 μg) were electrophoretically separated on 10% SDS-PAGE gels. Separated protein bands were transferred onto PVDF membranes (Pall, USA), which were then blocked with 5% milk-TBST (20 smmol/L Tris-HCl (pH 8.0), 8 g/L NaCl, and 0.1% Tween20) for 1 h at room temperature. After blocking, membranes were incubated with primary antibodies. Primary antibodies used in this experiment were as follows: 1:800 anti-alkaline phosphatase (ALP) (Thermo Fisher, USA); 1:100 anti-Runx2 (Santa Cruz Biotech, USA); 1:600 anti-receptor activator of nuclear factor-κB ligand (RANKL) (Abcam, USA); 1:800 anti-Wnt10b (Abcam, USA); 1:3000 anti-Smad3 (Abcam, USA); 1:2500 anti-p-Smad3 (Abcam, USA); and 1:5000 β-Actin (Proteintech, China). Secondary antibodies used were as follows: 1:5000 goat anti-rabbit IgG-HRP (Abbkine, USA); 1:3000 goat anti-mouse IgG-HRP (Abbkine, USA). Immunopositive protein bands were visualized using ECL chemiluminescence and DNR MicroChemi (DNR, Israel). Relative protein expression was calculated, using β-actin as an internal control. Integrated optical density (IOD) of protein bands was quantified by Gel Pro Analyzer software.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics version 20.0 software package; values were expressed as mean ± standard error (SEM). Differences between the two groups were analyzed using LSD and Duncan’s test; P-values < 0.05 were considered statistically significant.
Results
Effect of Fluoride on BMSC Migration
Cell migration was analyzed to observe growth trends of early-differentiated osteoblasts under different fluoride exposures. As shown in Fig. 1, the scratch was significantly narrower in cells exposed to 0.5 and 4 mg/L of fluoride compared to controls from 12 h of fluoride exposure to 7 days. The scratch became markedly thinner, along with the extension of cell growth periods. The scratch of cells exposed to 4 mg/L of fluoride group was significantly thinner than the 0.5 mg/L group from 2 to 7 days. These data suggested early-differentiated osteoblasts treated with 0.5 and 4 mg/L of fluoride grew more rapidly.
Effect of different fluoride concentrations on bone marrow mesenchymal stem cell (BMSC) migration. BMSCs were seeded in 6-well plates and cultured with mineral induction medium to differentiate them into pre-osteoblasts. A thin scratch was made in the growing confluent culture of BMSCs before they were treated with 0.5 and 4 mg/L of fluoride. The scratch width was monitored and quantified after different treatment periods. Results are expressed as mean ± SEM (n = 3). Significant changes are indicated as follows: *P < 0.05, compared to control group; **P < 0.01, compared to control group. a, P < 0.05, between two experimental groups; aa, P < 0.01, between two experimental groups
Action of TGF-β1 on Cell Proliferation, Apoptosis, and DNA Damage in BMSCs Exposed to Fluoride
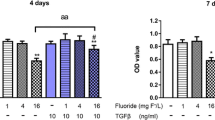
Proliferation, DNA damage, and apoptosis were analyzed via multicolor flow cytometry in individual cells. As shown in Fig. 2, cellular BrdU levels significantly increased in early-differentiated osteoblasts treated with 4 mg/L of fluoride and TGF-β1, compared to the control group. Except for the 16 mg/L of fluoride treatment groups, the other fluoride plus TGF-β1 treatments significantly increased BrdU levels compared to those of the same dose of fluoride treatment alone. Treatment of 0.5 mg/L of fluoride plus TGF-β1 significantly reduced the PARP level of early-differentiated osteoblasts compared to the same dose of fluoride treatment alone. On the contrary, 16 mg/L of fluoride combined with TGF-β1 treatment significantly enhanced PARP levels compared to the same dose of fluoride treatment alone. For DNA damage in Fig. 3, the 4 and 16 mg/L of fluoride combined with TGF-β1 treatments significantly increased H2AX levels of early-differentiated osteoblasts compared to TGF-β1 treatment alone. On the other hand, significant changes were hardly showed in every experimental group compared to the control group. These data implied that TGF-β1 prompted cell proliferation of early-differentiated osteoblasts when exposed to a low dose of fluoride. On the contrary, TGF-β1 inhibited cell apoptosis of early-differentiated osteoblasts exposed to low fluoride amounts but enhanced apoptosis under higher fluoride conditions.
Cell proliferation and apoptosis of BMSCs exposed to fluoride with or without TGF-β1. BMSCs were seeded in 6-well plates and cultured with mineral induction medium to differentiate them into pre-osteoblasts. BMSCs were treated with 0.5, 4, and 16 mg/L of fluoride with or without 10 ng/mL of TGF-β1 for 4 days. At the end of the treatment period, BMSCs were stained with BrdU and with fluorescent antibodies against cleaved PARP (Asp214). BrdU and cleaved PARP were used to measure the levels of cell proliferation and apoptosis, respectively. A Imaging flow cytometry of cells double-labelled with BrdU and cleaved PARP. B and C Quantification of data showed in A. Results are expressed as mean ± SEM (n = 3). Significant changes are indicated as follows: *P < 0.05, compared to control group; **P < 0.01, compared to control group. #P < 0.05, compared to 10 ng/mL TGF-β1 group; ##P < 0.01, compared to 10 ng/mL TGF-β1 group. a, P < 0.05, between two experimental groups; aa, P < 0.01, between two experimental groups
DNA damage of BMSCs exposed to fluoride with or without TGF-β1. BMSCs were seeded in the 6-well plates and cultured with mineral induction medium to differentiate them into pre-osteoblasts. BMSCs were treated with 0.5, 4, and 16 mg/L of fluoride with or without 10 ng/mL of TGF-β1 for 4 days. At the end of the treatment period, BMSCs were stained with fluorescent antibodies against H2AX (P139). Phosphorylated H2AX was used as a measure the level of DNA damage. A Imaging flow cytometry of phosphorylated H2AX. B Quantification of data showed in A. Results are expressed as mean ± SEM (n = 3). Significant changes are indicated as follows: #P < 0.05, compared to 10 ng/mL TGF-β1 group; ##P < 0.01, compared to 10 ng/mL TGF-β1 group
Effect of Different Conditions of Fluoride Exposure on Osteoblast Cell Viability
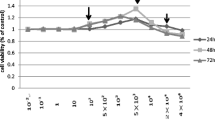
MC3T3-E1 cells, as a pre-osteoblast differentiation model, were induced into late-differentiated osteoblasts through mineral medium culture. In this study, MC3T3-E1 cells were exposed to distinct fluoride concentrations, i.e., ultra-low (0.001, 0.01, and 0.05 mg/L), low (0.1, 0.5, 1, and 2 mg/L), medium (4 and 8 mg/L), and high (16 and 32 mg/L) doses, and periods (1~7 days). As shown in Fig. 4, cell viability was mildly higher in 0.5 mg/L of fluoride treatment compared to controls after 2 days. The concentration range that slightly increased cell viability was widened from 0.5 to 8 mg/L of fluoride, and 32 mg/L significantly inhibited it compared to controls in a period of 4 days. After extending fluoride exposure to 7 days, 0.5 mg/L of fluoride still significantly enhanced cell viability. Even the concentration range of fluoride (4 to 32 mg/L) that significantly inhibited osteoblast viability was widened. These data showed that the early-differentiated osteoblasts were not as sensitive to fluoride concentration changes as late-differentiated osteoblasts. With the gradual differentiation of osteoblasts, low-dose fluoride stimulated osteoblast viability, while high-dose fluoride inhibited osteoblast viability; however, the stimulation range of fluoride gradually narrowed, while the inhibition range widened with the extension of fluoride exposure, which implied cumulative toxicity of fluoride.
Effect of different fluoride concentrations on osteoblast cell viability. MC3T3-E1 cells were cultured with mineral induction medium to differentiate them into osteoblasts. MC3T3-E1 cells were treated with different fluoride concentrations ranging from 0.001 to 32 mg/L for different periods. At the end, cell viability was measured with the MTT method. A 1-day period; B 2-day period; C 4-day period; D 7-day period. Results are expressed as mean ± SEM (n = 8). Significant changes are indicated as follows: *P < 0.05, compared to control group; **P < 0.01, compared to control group
Action of TGF-β1 on Bone Turnover-Related Protein Expression in Osteoblast Exposed to Fluoride
Proteins related to bone turnover were investigated. ALP is one of the markers of osteoblast differentiation. As shown in Fig. 5B, 0.5 and 4 mg/L of fluoride treatment markedly stimulated the expression of ALP protein compared to the control group. TGF-β1 treatment alone or combined with 4 mg/L of fluoride also significantly increased the expression of ALP protein compared to the control group. Runx2 acts as the critical regulator of bone formation by regulating early-differentiated osteoblasts. Figure 5C shows the results that TGF-β1 treatment alone or combined with fluoride significantly reduced the expression of Runx2 protein compared to the controls. Moreover, 0.5 and 4 mg/L of fluoride treatment combined with TGF-β1 markedly reduced its expression compared to the same dose of fluoride treatment alone. RANKL is generated by osteoblasts and acts as a crucial factor for osteoclast development. As shown in Fig. 5D, TGF-β1 treatment alone or combined with 4 mg/L of fluoride significantly enhanced RANKL expression compared to controls, and 4 mg/L of fluoride treatment combined with TGF-β1 markedly promoted RANKL expression compared to the same dose of fluoride treatment alone. These data suggested that a certain amount of fluoride treatment stimulated differentiation of osteoblasts, and that TGF-β1 treatment alone or a certain amount of fluoride combined with TGF-β1 treatment increased RANKL expression, but inhibited Runx2 expression.
Expression of bone turnover related-proteins in osteoblasts exposed to fluoride with or without TGF-β1. MC3T3-E1 cells were cultured with mineral induction medium to differentiate them into osteoblasts. Osteoblasts were treated with 0.5 and 4 mg/L of fluoride with or without 10 ng/mL TGF-β1 for 7 days. At the end of the treatment period, protein levels of ALP, Runx2, and RANKL were analyzed by Western blotting. Relative protein expression was calculated, with β-actin acting as an internal control. A Immunoblots for ALP, Runx2, and RANKL. B–D Quantification of protein bands showed in A. Results are expressed as mean ± SEM (n = 3). Significant changes are indicated as follows: *P < 0.05, compared to control group; P < 0.01, compared to control group. aa, P < 0.01, between two experimental groups
Action of TGF-β1 on the Expression of Proteins That Are Downstream of Its Signaling Pathway in Osteoblast Exposed to Fluoride
Smad3/p-Smad3, as a prominent TGF-β1 downstream signaling pathways, was investigated. As shown in Fig. 6, fluoride treatment alone or combined with TGF-β1 promoted phosphorylated Smad3 (p-Smad3) expression to different degrees, and 4 mg/L of fluoride combined with TGF-β1 showed a significant difference compared to the control group. Similarly, TGF-β1 combined with 4 mg/L of fluoride significantly enhanced Smad3 expression compared to controls and TGF-β1 treatment alone. Moreover, 4 mg/L of fluoride treatment combined with TGF-β1 markedly increased Smad3 expression compared to the same dose of fluoride treatment alone. Wnt10b is regulated by Runx2 in osteoblast and acts as a crucial factor for osteoblast differentiation. Results showed a significant decrease of Wnt10 expression in 0.5 mg/L of fluoride, and TGF-β1 alone or combined with fluoride also significantly reduced its protein level compared to the controls. These data showed that fluoride tended to stimulate intracellular Smad3 signaling, and that treatments with medium dose of fluoride combined with TGF-β1 significantly stimulated Smad3 signaling, and yet TGF-β1 treatment blocked Wnt10b expression in osteoblasts.
Protein expression of TGF-β1 signaling pathway downstream factors in osteoblasts exposed to fluoride with or without TGF-β1. MC3T3-E1 cells were cultured with mineral induction medium to differentiate them into osteoblasts. Osteoblasts were treated with 0.5 and 4 mg/L fluoride with or without 10 ng/mL TGF-β1 for 7 days, respectively. At the end of the treatment period, protein levels of p-Smad3, Smad3, and Wnt10b were analyzed by Western blotting. Relative protein expression was calculated, with β-actin acting as an internal control. A Immunoblots for p-Smad3, Smad3, and Wnt10b. B–D Quantification of protein bands showed in A. Results are expressed as mean ± SEM (n = 3). Significant changes are indicated as follows: *P < 0.05, compared to control group; P < 0.01, compared to control group. ##P < 0.01, compared to 10 ng/mL TGF-β1 group. a, P < 0.05, between two experimental groups; aa, P < 0.01, between two experimental groups
Discussion
This study analyzed how a specific dose of fluoride affected osteoblast progenitor and precursor differentiation and investigated the roles of TGF-β1 in the mechanism underlying fluoride-modulated osteoblast differentiation. The ingestion of excessive fluoride causes dental and skeletal fluorosis in human beings and animals. It is essential to clarify the signaling molecules in a specific osteoblast phase or cell phenotype in skeletal fluorosis. Yang et al. [9] found that a low dose of fluoride increased cell viability, but a high dose inhibited it. Co-exposure to TGF-β1 inhibitor and fluoride significantly influenced cell viability. The current study exerted 0.5 and 4 mg/L of fluoride on BMSCs and found two fluoride conditions that stimulated growth and migration of cells, which implied that the higher the fluoride concentration, the faster the migration. BMSC migration is an essential step of bone formation, and studies focused on BMSC migration are important to develop novel strategies to develop effective therapeutics against bone disease [10]. Stimulation of fluoride on BMSC migration revealed that low doses of fluoride have an anabolic effect on bone formation.
This study found cell proliferation trends, apoptosis, and DNA damage in early differentiation of BMSCs under co-exposure of fluoride and TGF-β1. Results showed that co-treatment with a low dose of fluoride and TGF-β1 significantly prompted cell proliferation, more than during early differentiation of BMSCs. TGF-β signaling stimulates proliferation, early differentiation, and commitment to the osteoblastic lineage through the selective MAPKs and Smad2/3 pathways [11]. Administration of TGF-β1 increased PARP levels of BMSCs, which suggested that TGF-β1 plays an essential role in regulating cell apoptosis induced by fluoride. Grafe et al. [12] summarized multiple roles of TGF-β in osteoblast differentiation. Linked with our results, a specific low dose of fluoride treatment slightly influenced cell proliferation and apoptosis, and the presence of TGF-β1 altered the effect of fluoride on early osteoblast differentiation.
In addition, the present study showed that the fluoride concentration range that increased cell viability of late-differentiated osteoblasts was widened with exposure to 4 days, which was similar to the viability observed for the early-differentiated osteoblasts. Conversely, the fluoride concentration range that promoted cell viability narrowed, while the fluoride concentration range that reduced cell viability widened during the 7-day period. These changes suggest that the more differentiated osteoblasts become, the lower their tolerance to fluoride. Most experiments demonstrated that fluoride exerted a dual effect on bone cells that was dependent on the exposure concentration [13, 14]. This study suggested the extent of differentiation influenced the tolerance of osteoblasts to fluoride concentrations, which was rarely mentioned in previous studies.
This study analyzed bone turnover-related protein expression in late-differentiated osteoblasts. Multiple studies have demonstrated that TGF-β1 plays a crucial role in coupling bone formation and resorption [15]. In this study, fluoride treatments significantly increased ALP expression. TGF-β1 treatment alone or combined with 4 mg/L of fluoride also significantly increased ALP expression. Conversely, TGF-β1 treatment reduced Runx2 expression, and yet fluoride treatment showed little effect on it. TGF-β can activate a negative feedback loop to inhibit its signaling via Runx2 to reduce TGF-β responsiveness [16]. Suzuki et al. [17] demonstrated that TGF-β1 could be an inducer or an inhibitor of osteoblastic differentiation of MC3T3-E1 cells depending on the phosphorylation state of Akt. Taken together, these results are consistent with the multiple roles of TGF-β1 on osteoblast differentiation.
In the present study, fluoride treatment also showed a slight effect on RANKL expression, but TGF-β1 treatment increased its expression. A previous study reported that TGF-β enhanced osteoblast-lineage RANKL expression, and recruited osteoclast precursor [18]. Morikawa et al. [19] proposed that TGF-β1 differentially affects certain stages of osteoblast differentiation. Here, TGF-β1 induces proliferation of osteoblasts and increased ALP and RANKL expression, but inhibited Runx2 expression in late-differentiated osteoblasts exposed to fluoride. This study precisely indicated that TGF-β acted as a critical modulator of the process in which fluoride affects osteoblast and osteoclast differentiation.
This study probed into Smad2/3 and Wnt10b signaling in osteoblasts exposed to fluoride. Results showed that fluoride mildly increased phosphorylation of Smad3, and that a combination of fluoride and TGF-β1 significantly stimulated Smad3/p-Smad3 expression. The increasing trend of p-Smad3 expression in this study implied the cumulative effect of TGF-β1 on fluoride-induced osteoblast differentiation. It has been demonstrated that TGF-β increases ALP expression and reduces Runx2 expression via the upregulation of Smad3 expression [12]. Wnt10b is regulated by Runx2 in osteoblasts and acts as a crucial factor for osteoblast differentiation. On the other hand, TGF-β stimulates sclerostin expression, which inhibits Wnt signaling in bone [20]. This study showed that fluoride treatment substantially reduced Wnt10b expression, and that TGF-β1 alone or combined with fluoride significantly inhibited it. These results implied that TGF-β probably modulates late-stage osteoblast differentiation under fluoride treatment via activation of Smad3 and inhibition of the Wnt10b pathway.
In conclusion, low fluoride doses stimulated growth and migration of early differentiated osteoblast. There was a disparity of fluoride tolerance in distinct osteoblast differentiation stages, and the early-differentiated cells were more resistant to fluoride. TGF-β1 influenced cell proliferation and apoptosis in early-differentiated osteoblasts exposed to fluoride. In late-differentiated osteoblasts, TGF-β1 played an essential role in stimulating osteoblast and osteoclast differentiation under fluoride treatment. The activation of Smad3 was involved in the mechanism underlying TGF-β1 modulation of fluoride-treated osteoblast differentiation and function.
References
Nawata S, Kaneta T, Ogawa M (2017) Differences in sodium fluoride-18 uptake in the normal skeleton depending on the location and characteristics of the bone. Nuklearmedizin. 56(3):91–96. https://doi.org/10.3413/Nukmed-0867-16-12
Wang XL, Ming J, Qiu B et al (2019) Relationship between fluoride exposure, orthopedic injuries and bone formation markers in patients with coal-burning fluorosis. Ying Yong Sheng Tai Xue Bao 30(1):43–48. https://doi.org/10.13287/j.1001-9332.201901.026
Nelson EA, Halling CL, Buikstra JE (2019) Evidence of skeletal fluorosis at the Ray Site, Illinois, USA: a pathological assessment and discussion of environmental factors. Int J Paleopathol 26:48–60. https://doi.org/10.1016/j.ijpp.2019.05.003
Tamer MN, Kale Köroğlu B, Arslan C, Akdoğan M, Köroğlu M, Çam H, Yildiz M (2007) Osteosclerosis due to endemic fluorosis. Sci Total Environ 373(1):43–48. https://doi.org/10.1016/j.scitotenv.2006.10.051
Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90(5):552–560. https://doi.org/10.1177/0022034510384626
Jiang N, Guo F, Sun B et al (2020) Different effects of fluoride exposure on the three major bone cell types. Biol Trace Elem Res 193(1):226–233. https://doi.org/10.1016/j.tox.2020.152429
Zhang J, Jiang N, Yu H, Yu X, Guo F, Zhao Z, Xu H (2019) Requirement of TGFβ signaling for effect of fluoride on osteoblastic differentiation. Biol Trace Elem Res 187(2):492–498. https://doi.org/10.1007/s12011-018-1387-x
Yu H, Jiang N, Yu X, Zhao Z, Zhang X, Xu H (2018) The role of TGFβ receptor 1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 393:73–82. https://doi.org/10.1016/j.tox.2017.11.009
Yang C, Wang Y, Xu H (2017) Fluoride regulate osteoblastic transforming growth factor-beta 1 signaling by mediating recycling of the type I receptor ALK5. PLoS One 12(1):e0170674. https://doi.org/10.1371/journal.pone.0170674
Su P, Tian Y, Yang C, Ma X, Wang X, Pei J, Qian A (2018) Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int J Mol Sci 19(8):2343. https://doi.org/10.3390/ijms19082343
Chen G, Deng C, Li YP (2012) TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8(2):272–288. https://doi.org/10.7150/ijbs.2929
Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, Mishina Y (2018) TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol 10(5):a022202. https://doi.org/10.1101/cshperspect.a022202
Yao Y, Ma Y, Zhong N, Pei J (2019) The inverted U-curve association of fluoride and osteoclast formation in mice. Biol Trace Elem Res 191(2):419–425. https://doi.org/10.1007/s12011-018-1624-3
Wang J, Li G, Li Y, Zhao Y, Manthari RK, Wang J (2020) The effects of fluoride on the gap-junctional intercellular communication of rats’ osteoblast. Biol Trace Elem Res 193(1):195–203. https://doi.org/10.1007/s12011-019-01692-9
Yasui T (2011) Regulation of RANKL-induced osteoclastogenesis by TGF-beta through molecular interaction between Smad3 and Traf6. J Bone Miner Res 26:1447–1456. https://doi.org/10.1002/jbmr.357
Kim KK, Ji C, Chang W et al (2006) Repetitive exposure to TGF-b suppresses TGF-b type I receptor expression by differentiated osteoblasts. Gene 379:175–184. https://doi.org/10.1016/j.gene.2006.05.005
Suzuki E, Ochiai-Shino H, Aoki H, Onodera S, Saito A, Saito A, Azuma T (2014) Akt activation is required for TGF-β1-induced osteoblast differentiation of MC3T3-E1 pre-osteoblasts. PLoS One 9(12):e112566. https://doi.org/10.1371/journal.pone.0112566
Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ (2013) TGF-β induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology 154(10):3745–3452. https://doi.org/10.1210/en.2013-1272
Morikawa M, Derynck R, Miyazono K (2016) TGF-b and the TGF-b family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8:a021873. https://doi.org/10.1101/cshperspect.a021873
Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC (2012) TGF-b regulates sclerostin expression via the ECR5 enhancer. Bone 50:663–669. https://doi.org/10.1016/j.bone.2011.11.016
Funding
This work was supported by grants from the Natural Science Foundation of Jilin Province of China (20180101151JC) and the National Natural Science Foundation of China [81673111].
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, N., Xu, W., Zhang, Z. et al. Role of TGF-β1 in Fluoride-Treated Osteoblasts at Different Stages. Biol Trace Elem Res 200, 740–748 (2022). https://doi.org/10.1007/s12011-021-02686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02686-2