Abstract
Barbary fig (Opuntia ficus-indica) has attracted great attention in extensive rural agriculture for its potential agronomic, medicinal, and environmental benefits. However, there is only incomplete information about its chemical profile. Energy-dispersive X-ray fluorescence (EDXRF) spectrometry was applied to determine the concentrations of 11 major and trace elements (Br, Cr, Cu, Fe, K, Mn, P, Rb, Sr, Yb, and Zn) in cladodes of O. ficus-indica and the adjacent soil. For analytical accuracy, the standard reference materials CRM–IAEA 336 (Lichen) and CRM–NIST 1646a (Estuarine Sediment) were used. The relationships between the chemical elements were established by Pearson’s correlation coefficient (r) and principal component analysis (PCA). The results show that K, P, Fe, and Mn were the dominant essential elements in O. ficus-indica cladodes; however, Br, Cr, Cu, Rb, Yb, and Zn were present at low concentrations. The cladodes showed high enrichment with K, Sr, and Br (BEF > 1), but the values of this coefficient were below 1 for the remaining elements. The PCA showed that in the O. ficus-indica cladodes, the higher concentrations of Br, K, and Sr were correlated; conversely, the highest contents of Cr, Cu, Fe, Mn, P, Rb, Yb, and Zn were retained in the soil. The present findings enabled us to determine that O. ficus-indica has a high ability to accumulate K, P, Fe, and Mn in its cladodes. Therefore, the data obtained from the analysis of this cactus will be useful for nutritional and medicinal purposes.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prickly pear or Barbary fig (Opuntia ficus-indica) is an important fruit species in the arid and semi-arid zones of North Africa, where it plays a main role in rural agriculture [10, 20]. In these areas, O. ficus-indica is comestible as a fruit for human consumption and as forage for sheep and goats, and is cultivated as an ornamental species. It is used in hedges, as a wind break, and against soil erosion and desertification [14, 21]. Its fruits and oil seeds are used in cosmetics manufacture and for medicinal purposes. Opuntia ficus-indica is a cactus native of and well adapted to the Mediterranean climate, where it is most often grown in shallow, nutrient-poor soils on rocky substrates and is subjected to long periods of drought [15].
Cactus cladodes are rich in dietary fiber (> 43%), carotenoids, polyphenols, vitamin C (ascorbic acid), and some essential elements [30]. The seeds have a high content of linoleic acid (an unsaturated fatty acid) [26]. The natural colors of the fruits are related to the presence of betacyanins and betaxanthins, which have antioxidant properties [29]. The anti-ulcerogenic effect of O. ficus-indica is attributed to the presence in the cladodes of mucilage and pectin (polymers) that inhibit the production of ethanol-induced ulcers and gastritis [6, 16]
Essential trace elements such as Fe, Cu, Mn, and Zn are indispensable for plant growth at very low concentrations; however, other elements such as Rb, Sr, and V are known to stimulate plant development, but their functions are not yet known. These elements are involved in the activation of some enzymes such as dehydrogenases and oxidases, as well as in carbohydrates metabolism and chlorophyll and nucleic acid synthesis [12]. However, substantial concentrations of essential trace elements in the rhizospheric biotope lead to plant toxicity [11].
Analytical techniques employing energetic beams, such as ICPMS, ICPOES, and ICPAES, are widely used for trace element determination in plant material. However, these procedures require acid digestion and pretreatment of the samples prior to measurement, which make them slower and produce residues. In contrast, other analytical tools like EDXRF and PIXE are suitable alternative techniques which have many advantages such as sample preparation simplicity, low cost, and quicker elements quantification [5, 9, 13, 17].
Energy-dispersive X-ray fluorescence (EDXRF) is a non-destructive analytical tool for measurement of macro- and micro-(trace) elements in a wide range of sample types, including pharmaceutical, agricultural, geological, and environmental materials. By using short bursts of high-precision exciting radiation, this technique permits multi-elemental analysis without chemical reagents [18, 23].
In spite of its economic and ecological relevance, there are still not enough data regarding the mineral composition of O. ficus-indica and the interrelationships between the elements present in cladodes and in the soil in which this cactus is grown. In this sense, the purpose of the present study is to determine the main major and trace elements in cladodes of O. ficus-indica and its ability to translocate trace metals from soil to cladodes.
Material and Methods
Species Description and Sample Collection
Opuntia ficus-indica (Cactaceae family) is a drought-resistant plant with crassulacean acid metabolism (CAM) [25] (Fig. 1). The green cladodes or “nopalitos” (35–45 cm long and 20–30 cm wide) have a spatulate form and are strongly succulent with abundant small spines. These cladodes can store substantial amounts of water in their parenchymatic tissues. Barbary fig has a superficial root system (max. depth of 30 cm) which extends horizontally to absorb the small amount of moisture provided by light rains in arid climates [33]. The red, purple, or yellow flowers (2–3 cm in length) are hermaphrodites, with 4 attached carpels enclosed in a floral cup and an inferior receptacle (ovary). The fruits (figs or prickly pears) are enclosed in a thick skin, covered with small prickles, and contain up to 270 seeds [28].
Fresh cladodes of O. ficus-indica and their associated soil were collected from the Zaâfrane location of Djelfa province, Algeria (34° 49′ 52″ N latitude, 2° 52′ 38″ E longitude, 852 m elevation). According to the approach described by Nedjimi [22], three parallel transects of 100 m were traced across the plantation of O. ficus-indica with a 100 m interval between the transects. In each transect, at a regular interval (10 m), ten cacti were randomly harvested (5 mature cladodes/cactus) and they were mixed to make up a composite sample.
The soils in the sampling site are typically calcareous in nature (pH = 7.6–8 and EC = 50 dS m−1) with a silt-sand texture and low organic matter content (2–3%). Using a stainless steel sampling auger, three replicate composite soil samples (100 g) were randomly collected at 20–30 cm depth (rooting zone) at the same sites at which the cactus samples were taken. Each of the soil samples consisted of a mixture of 3 subsamples.
Sample Preparation
The plant material was washed with double-distilled water in order to remove dust, desiccated in an oven at 65 °C for 72 h, and subsequently powdered to fine particles (size fraction of 200 μm) using an agate mortar and pestle. The soil samples were oven-dried (65 °C/72 h) and passed through a 2-mm stainless steel mesh sieve. For each sample (plant and soil), 120 mg of powdered material was compressed using a 150-tonne hydraulic press apparatus and made into pellets of 13 mm diameter and 1–2 mm thickness. Triplicates of each sample were made. Two biological reference materials, namely CRM–IAEA 336 (Lichen) and CRM–NIST 1646a (Estuarine sediment), were employed as controls for analytical accuracy.
EDXRF Spectrometry Analysis
A PANalytical X-ray fluorescence spectrometer (Epsilon 3 XL) was applied to measure the trace element concentrations in both cladode and soil samples. The setup consists of an Ag anode X-ray tube operated at 50 kV and 3 mA maximum voltage and a beam current of 1 nA. The X-rays emitted were recorded by an Si(Li) detector SDD with a resolution of 135 keV for the line Mn-Kα (5.9 keV) and an active surface of 5 mm2 provided with a thin beryllium window of 8 mm thickness. The concentrations of the analytes were determined using several removable primary filters consisting of Ti, Ag, Al–50, Al–200, and Cu–300. The spectra were collected with an acquisition time of 1800 s using Epsilon 3 software.
Measurement and Statistical Analysis
The element concentrations (μg g−1) were measured using the following equation [8]:
where Cx and Cs denote the element concentration in the cladode sample and CRM, respectively, ix and is denote the net intensity of the element concentration in the cladode sample and CRM, respectively, and mx and ms are the weight of the sample and CRM, respectively.
Uscore is an important statistical parameter often used to monitor the accuracy of the analytical technique. This parameter was measured using the following equation [32]:
where xlab, σlab, xcert, and σcert denote the laboratory value of the element in the CRM, the uncertainty value of xlab, the certified value, and the uncertainty value of xcert, respectively. The XRF performance is assessed as satisfactory if Uscore ≤ 1 and unsatisfactory for Uscore > 1.
The bioaccumulation element factor (BEF) was calculated, to evaluate the transfer of trace elements from soil to Opuntia cladodes, using the following equation [7, 22]:
where Cplant and Csoil denote the content of the element in the cladode and soil sample, respectively.
Pearson’s correlation coefficient (r) at P < 0.05 was used to identify the correlations among the various element concentrations. Principal component analysis (PCA) was carried out to assess the relationships among the chemical elements in the cladodes and soil. The data were statistically analyzed using the STATISTICA 12.0 software package
Results and Discussion
Accuracy Estimation
The EDXRF methodology was validated using two standard reference materials. The certified and quantified values of IAEA 336 lichen and NIST 1646a estuarine sediment were similar (good reliability and precision). For all chemical elements, the Uscore values of the two CRMs were ≤ 1 (Table 1), indicating the reliability of the analytical technique.
Elemental Analysis and Bioaccumulation Factor
Eleven trace element concentrations—namely Br, Cr, Cu, Fe, K, Mn, P, Rb, Sr, Yb, and Zn—were determined using EDXRF analysis. The average concentrations are shown in Table 2. Opuntia ficus-indica cladodes were relatively rich in five major and trace elements, namely K, P, Sr, Fe, and Mn, for which the mean values were 32886.94 μg g−1 (K), 251.31 μg g−1 (P), 108.38 μg g−1 (Sr), 44.24 μg g−1 (Fe), and 17.33 μg g−1 (Mn). The elements Rb, Zn, and Br were present in minor amounts (8.68, 7.60, and 5.95 μg g−1, respectively), with Cu, Cr, and Yb present in trace amounts, the mean values being 1.71 μg g−1 (Cu), 1.08 μg g−1 (Cr), and 0.05 μg g−1 (Yb).
The concentrations of chemical elements in the soil adjacent to the plants descended in the following order: P (45000 μg g−1) > K (6630 μg g−1) > Fe (6530 μg g−1) > Mn (142.47 μg g−1) > Sr (62.56 μg g−1) > Zn (17.3 μg g−1) > Rb (15.8 μg g−1) > Cr (15.7 μg g−1) > Cu (7.95 μg g−1) > Br (5.1 μg g−1) > Yb (0.76 μg g−1).
The O. ficus-indica cladodes had a substantial amount of K (32886.94 μg g−1). A similar K value (28985 μg g−1) was determined in young neem (Azadirachta indica) leaves, collected in the Thiruvallur district in Tamil Nadu, southern India, by proton-induced X-ray emission (PIXE) [5]. In the present investigation, the concentration of P in O. ficus-indica cladodes was 251.31 μg g−1. This result is in agreement with de Aragão Tannus et al. [3], who determined trace elements in Maytenus ilifolia from Brazil using ICPOES. The Sr concentration (108.38 μg g−1) obtained in the present study is higher than the values determined in Tinospora cordifolia (20.5 μg g−1) and Moringa oleifera (59.3 μg g−1) by PIXE [9]. The Mn and Fe contents of the Opuntia samples are lower than those found by Kulal et al. [13]. These latter authors determined trace elements concentrations in some medicinal plants (Justica adhatoda, Mesua ferea, and Ocimum sanctum) using the EDXRF technique.
In plant nutrition, K is the most important major element, being involved in stomatal control and acting as an activator of numerous enzymes [1]. It is required for cell division and meristematic differentiation [19]. Phosphorus is an important constituent of the plasma membrane and plays an important role in both energy metabolism (ATP and ADP) and nucleotides and phospholipids synthesis [31]. Manganese is required in oxido-reduction reactions. It is a constituent of certain enzymes, such as pyruvate carboxylase (PC), pyruvate oxidase (POX), and pyruvate dehydrogenase (PDH), and plays an important role in photosynthesis [11]. Iron (Fe) is involved in photosynthesis (the electron transfer system) and in nucleic acids synthesis. It acts as an activator of many enzymes—such as catalase (CAT), peroxidase (POD), and cytochrome oxidase (CYTb, CYTc, and CYTf) [19]. Strontium (Sr) is not an essential trace element for higher plants, but is easily taken up by the root system due to its high similarity to Ca [11].
The BEF results (Table 2) show that K was greatly enriched in Opuntia cladodes (BEF = 4.96), Br and Sr were also enriched, to a lower extent (BEF = 1.17 and 1.73, respectively), and the other elements, having BEF < 1 (Cr, Cu, Fe, Mn, P, Rb, Yb, and Zn), are not expected to bioaccumulate to any significant level in the cladodes.
Principal component analysis (PCA) was performed to assess the interdependences among the 11 elements accumulated in the Opuntia cladodes and adjacent soil. The component loadings show that the first principal component (PC1) explained 87.12% of the total variance (Table 3) and was positively associated with the describing variables K (0.99) and Sr (0.92). In contrast, PC1 was negatively correlated with Cr, Cu, Fe, Mn, P, Rb, Yb, and Zn, for which the loading values were in the range 0.95–0.99. The PC2 accounted for 11.70% of the total variance and was negatively correlated with Br, with a loading value of 0.99 (Table 3).
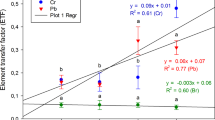
The projection of the data sets on the PC1 and PC2 plane (Fig. 2) indicates that O. ficus-indica cladodes were correlated with higher concentrations of Br, K, and Sr, while the highest contents of Cr, Cu, Fe, Mn, P, Rb, Yb, and Zn were retained in the soil (negatively correlated with PC1).
Pearson’s Correlation Coefficients (r)
Pearson’s correlation coefficients (r) calculated for the relationships among the different chemical elements are displayed in Table 4. For the Opuntia cladodes, all the chemical elements were significantly correlated (P < 0.05), except for Cr, which had no correlation with Yb. The same pattern was observed for the adjacent soil, with significant relationships among almost all the chemical elements (P < 0.05). Apart from the pair K-P (r = 0.56, P < 0.05), P was not associated with any of the chemical elements.
Comparison with Other Opuntia spp.
The comparison of our data with those reported for other Opuntia spp. from different regions of the world is shown in Table 5. The O. ficus-indica cladodes in this work generally had concentrations of K and Fe double those of O. dillenii and O. polyacantha, respectively, while their Zn content was very low compared to O. polyacantha. The Cu content of O. ficus-indica was comparable with that of O. macrorhiza but lower than that of O. polyacantha, while its P content was around six times higher than that of O. polyacantha. However, the Algerian species showed higher accumulation of Mn than O. macrorhiza and O. dillenii. These differences in elemental contents among Opuntia spp. could be due to differences in genotypic characters and environmental factors [5, 24].
Conclusion
This work has evaluated the concentrations of some major and trace elements (Br, Cr, Cu, Fe, K, Mn, P, Rb, Sr, Yb, and Zn) in cladodes of O. ficus-indica and their adjacent soil collected from Algerian arid zones. The uptake of these elements from soil and their transfer to cladodes was assessed by calculation of the bioaccumulation element factor (BEF). This showed that (1) O. ficus-indica cladodes were relatively rich in some essential elements such as K, P, Fe, and Mn; (2) the contents in the cladodes declined in the order: K > P > Sr > Fe > Rb > Zn > Br > Cu > Cr > Yb; and (3) this species had a particularly high transfer factor for K, Br, and Sr.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Benlloch-González M, Arquero O, Fournier JM, Barranco D, Benlloch M (2008) K+ starvation inhibits water-stress-induced stomatal closure. J Plant Physiol 165:623–630
Chahdoura H, Morales P, Barreira JCM, Barros L, Fernández-Ruiz V, Ferreira ICFR, Achour L (2015) Dietary fiber, mineral elements profile and macronutrients composition in different edible parts of Opuntia microdasys (Lehm.) Pfeiff and Opuntia macrorhiza (Engelm.). Food Sci Tech 64:446–451
de Aragão TC, de Souza DF, Santana FB, Batista dos Santos DCM, Magalhães HIF, de Souza Dias F, de Freitas Santos Júnior A (2020, 2020) Multielement determination in medicinal plants and herbal medicines containing Cynara scolymus L., Harpagophytum procumbens D.C., and Maytenus ilifolia (Mart.) ex Reiss from Brazil using ICP OES. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02334-1
Díaz Medina EM, Rodríguez Rodríguez EM, Díaz RC (2007) Chemical characterization of Opuntia dillenii and Opuntia ficus indica fruits. Food Chem 103:38–45
Elayaperumal M, Vedachalam Y, Loganathan D, Kumaravelu TA, Anusuya GS, Kennedy J (2020) Ion beam analysis of proton-induced x-ray emission (PIXE) techniques for elemental investigation of young stage Neem leaf of southern India. Tamil Nadu Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02443-x
Galati EM, Monforte MT, Tripodo MM, d’Aquino A, Mondello MR (2001) Antiulcer activity of Opuntia ficus-indica (L.) Mill. (Cactaeceae): ultrastructural study. J Ethnopharmacol 76:1–9
Galinha C, Freitas MC, Pacheco AMG (2010) Enrichment factors and transfer coefficients from soil to rye plants by INAA. J Radioanal Nucl Chem 286:583–589
Gama EM, Nascentes CC, Matos RP, Rodrigues GC, Rodrigues GD (2017) A simple method for the multi-elemental analysis of beer using total reflection X-ray fluorescence. Talanta 174:274–278
Gowrishankar R, Kumar M, Menon V, Divi SM, Saravanan M, Magudapathy P, Panigrahi BK, Nair KGM, Venkataramaniah K (2010) Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera (Moringaceae), and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biol Trace Elem Res 133:357–363
Inglese P, Mondragon C, Nefzaoui A, Sáenz C (2017) Crop ecology, cultivation and uses of cactus pear. FAO, Rome, 225p
Kabata-Pendias A (2011) Trace elements in soils and plants. 4th edition, CRC Press, Boca Raton.
Kathpalia R, Bhatla SC (2018) Plant mineral nutrition. In: Bhatla SC, Lal MA (eds) Plant physiology, development and metabolism. Springer Nature Singapore Pte Ltd. pp: 37–81.
Kulal C, Padhi RK, Venkatraj K, Satpathy KK, Mallaya SH (2020) Study on trace elements concentration in medicinal plants using EDXRF technique. Biol Trace Elem Res 198:293–302. https://doi.org/10.1007/s12011-020-02037-7
Le Houérou HN (1996) The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. J Arid Environ 33:135–159
Le Houérou HN (2002) Cacti (Opuntia spp.) as a fodder crop for marginal lands in the Mediterranean basin. Acta Hortic 581:21–46
Lee EB, Hyun JE, Li DW, Moon YI (2002) Effects of Opuntia ficus-indica var. Saboten stem on gastric damages in rats. Arch Pharm Res 25:67–70
Prithiviraj B, Manikandan E, Hariharan GN, Nair KGM (2011) Elemental accumulation patterns of the lichen species Physcia tribacoides Nyl., Heterodermia dissecta and Bacidia beckhausii Körber from the Walayar Rf region, Tamil Nadu, India. Int J PIXE 21:133–144
Marguí E, Queralt I, Hidalgo M (2009) Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. Trends Anal Chem 28:362–372
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Murillo-Amador B, Cortés-Avila A, Troyo-Diéguez E, Nieto-Garibay A, Jones HG (2001) Effects of NaCl salinity on growth and production of young cladodes of Opuntia ficus-indica. J Agron Crop Sci 187:269–279
Nefzaoui A, Louhaichi M, Ben Salem H (2014) Cactus as a tool to mitigate drought and to combat desertification. J Arid Land Stud 24(1):121–124
Nedjimi B (2018) Seasonal growth and translocation of some major and trace elements in two Mediterranean grasses (Stipa tenacissima Loefl. ex L. and Lygeum spartum Loefl. ex L.). Acta Oecol 89:43–50
Nedjimi B (2020) Seasonal changes of copper and zinc concentrations in browse saltbush (Atriplex canescens (Pursh) Nutt.) from Algerian arid rangelands. Afr J Range Forage Sci 37(2):143–149
Nedjimi B (2020) Measurement of selected trace elements in Olea europaea L. cv. ‘Sigoise’. J Trace Elem Med Biol https://doi.org/10.1016/j.jtemb.2020.126595.
North GB, Moore TL, Nobel PS (1995) Cladode development for Opuntia ficus-indica (Cactaceae) under current and doubled CO2 concentrations. Am J Bot 82:159–166
Özcan M, Al Juhaimi F (2011) Nutritive value and chemical composition of prickly pear seed (Opuntia ficus-indica L.) growing in Turkey. Int J Food Sci Nutr 62(5):533–536
Phillips KM, Pehrsson PR, Agnewc WW, Scheett AJ, Follett JR, Lukaski HC, Patterson KY (2014) Nutrient composition of selected traditional united states northern plains native American plant foods. J Food Compos Anal 34:136–152
Reyes Agüero JA, Valiente Banue A (2006) Reproductive biology of Opuntia: a review. J Arid Environ 64(4):549–585
Sáenz C, Sepúlveda E, Idalsoaga M (2002) Color extract from purple cactus pear: preparation, characteristics and uses. Cactusnet Newsletter:3–4
Sáenz C, Sepúlveda E, Pak N, Lecaros M (2010) Chemical and physical characterization of cactus cladodes (Opuntia ficus- indica) powder. Ital J Food Sci 22(4):416–422
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Siddique N, Waheed S (2012) Evaluation of laboratory performance using proficiency test exercise results. J Radioanal Nucl Chem 291:817–823
Snyman HA (2006) Root distribution with changes in distance and depth of two-year old cactus pear Opuntia ficus-indica and O. robusta plants. South Afr. Aust J Bot 72:434–441
Acknowledgments
The author is indebted to all staff of the FAP Laboratory for use of its analysis facilities and to B. Beladel for his aid in field sample collection. I gratefully acknowledge the valuable contributions of two anonymous referees in the review process, and Dr. David Walker for his correction of the written English in the manuscript.
Funding
Funding for this research came from the Algerian Ministry of Higher Education and Scientific Research through PRFU Project # D04N01UN170120200003, entitled: “Dosage des éléments traces chez quelques espèces végétales par moyen de spectrométrie à fluorescence X (XRF) : Phytoremediation et implications pour la santé humaine.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nedjimi, B. Determination of Some Major and Trace Elements in Cladodes of Barbary fig (Opuntia ficus-indica Mill.) by X-ray Fluorescence Spectrometry. Biol Trace Elem Res 199, 4353–4359 (2021). https://doi.org/10.1007/s12011-020-02555-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02555-4