Abstract
Maternal immune activation (MIA) model has been profoundly described as a suitable approach to study the pathophysiological mechanisms of neuropsychiatric disorders, including schizophrenia. Our previous study revealed that prenatal exposure to lipopolysaccharide (LPS) induced working memory impairments in only male offspring. Based on the putative role of prefrontal cortex (PFC) in working memory process, the current study was conducted to examine the long-lasting effect of LPS-induced MIA on several neuroinflammatory mediators in the PFC of adult male pups. We also investigated whether maternal zinc supplementation can alleviate LPS-induced alterations in this region. Pregnant rats received intraperitoneal injections of either LPS (0.5 mg/kg) or saline on gestation days 15/16 and supplemented with ZnSO4 (30 mg/kg) throughout pregnancy. At postnatal day 60, the density of both microglia and astrocyte cells and the expression levels of IL-6, IL-1β, iNOS, TNF-α, NF-κB, and GFAP were evaluated in the PFC of male pups. Although maternal LPS treatment increased microglia and astrocyte density, number of neurons in the PFC of adult offspring remained unchanged. These findings were accompanied by the exacerbated mRNA levels of IL-6, IL-1β, iNOS, TNF-α, NF-κB, and GFAP as well. Conversely, prenatal zinc supplementation alleviated the mentioned alterations induced by LPS. These findings support the idea that the deleterious effects of prenatal LPS exposure could be attenuated by zinc supplementation during pregnancy. It is of interest to suggest early therapeutic intervention as a valuable approach to prevent neurodevelopmental deficits, following maternal infection.
Graphical abstract

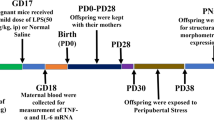
Schematic diagram describing the experimental timeline. On gestation days (GD) 15 and 16, pregnant dams were administered with intraperitoneal injections of either LPS (0.5 mg/kg) or vehicle and supplemented with ZnSO4 (30 mg/kg) throughout pregnancy by gavage. The resulting offspring were submitted to qPCR, immunostaining, and morphological analysis at PND 60. Maternal zinc supplementation alleviated increased expression levels of inflammatory mediators and microglia and astrocyte density induced by LPS in the PFC of treated offspring. PND postnatal day, PFC prefrontal cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a debilitating psychotic disease with a prevalence of 1% in the worldwide population, wherein behavior, cognition, and brain structures are severely affected. Epidemiological and experimental studies have exceedingly suggested the complex interactions of genes and environment, as the main contributing factors in the pathogenesis of this mental disorder [1]. Of environmental factors, gestational infection has been identified to be a potential risk factor causing deleterious impacts on fetal brain development [2]. There is evidence that the cytokine imbalance in the fetal brain, as a consequence of gestational infection, can result in neurodevelopmental disruptions increasing the susceptibility to develop neuropsychiatric diseases such as schizophrenia [3]. Based on this evidence, maternal immune activation (MIA) animal model has become a suitable and widely used model to investigate pharmacotherapeutic options and pathological mechanisms involved in several psychiatric disorders including schizophrenia and autism spectrum disorders (ASD) [4]. In this regard, administration of various immunogenic agents, such as lipopolysaccharide (LPS) and viral mimic polyinosinic-polycytidilic acid (Poly I:C), to pregnant dams have been commonly applied to establish this animal model [5]. As of today, a number of in vivo studies have successfully used various approaches with difference in dose of immunogenic substances, gestational stage, and frequency of injection to mimic maternal infection and evaluate the neural dysfunction and behavioral impairments related to schizophrenia [6,7,8,9]. In a previous study, we showed that prenatal exposure to LPS (0.5 mg/kg, intraperitoneal (i.p.)) at gestation days (GD) 15/16 led to working memory impairments in male but not female offspring [10]. Other animal studies have also demonstrated significant behavioral deficits in offspring born to immune-challenged mothers [6, 8, 9, 11]. Indeed, molecular and cellular mechanisms underlying behavioral manifestations are yet to be fully elucidated. With the development of new techniques, however, several studies have reported the association between prefrontal cortex (PFC) disturbances and working memory impairments in patients with schizophrenia [12,13,14]. For this reason, PFC has become the focus of more investigation in the present study.
A large body of evidence has consistently suggested an association between schizophrenia and neuroinflammation with the focus on the glial cells, including microglia and astrocyte [15,16,17,18]. Activated microglia, as the resident immune cells in the brain, have been identified to be involved in neuropathology of schizophrenia mainly due to the production of free radicals and inflammatory mediators [18, 19]. In support of this, increased microglial activation and proliferation have been observed in the post-mortem tissue samples from schizophrenic patients [20, 21] as well as different brain regions of the offspring from animal models [6, 19]. Likewise, disruption in functionality of astrocytes, the most numerous cells in the brain, is now accepted to have a crucial role in the pathogenesis of this mental disease acting in part by its function in innate immunity [22].
As mentioned earlier, immune dysregulation in the developing brain, as a consequence of gestational infection, results in a long-lasting impact on fetal brain structure and function. There is evidence that maternal immune response, not a specific infectious agent, is responsible for disruption in normal brain development [3]. Although the pro-inflammatory cytokines are considered the main mediators in maternal immune response [23], the secondary consequences of systemic immune activation are also of importance. Interestingly, a recent animal study has suggested a correlation between maternal zinc deprivation and impairments in memory and learning function of offspring [24]. Specifically, LPS exposure during pregnancy has been revealed to induce maternal and fetal hypozincemia and consequently abnormal neurodevelopment with changes in both offspring brain function and structure [25, 26]. However, the mechanism of damage is unclear but may partly be due to the fact that zinc, as an essential trace mineral, is required for the correct functioning of many enzymes and transcription factors involved in brain development and neurogenesis [27]. Thus, fetal zinc deficiency, due to the LPS-mediated maternal hypozincemia, can lead to deleterious and long-lasting consequences in the fetal developing brain [28, 29]. Our previous results showed the preventive effect of maternal zinc supplementation on behavioral deficits in male offspring prenatally exposed to LPS [10]. Added to this, the beneficial effect of maternal zinc supplementation on LPS-induced impairments in cognition, several neurotransmitters, and teratogenicity has been also reported [26, 30, 31]. Although these results are exciting, the exact mechanism by which maternal zinc supplementation alleviates the brain abnormalities induced by LPS in pups is unclear.
Due to the putative role of PFC in working memory [13] and our previous results showing the working memory impairments in only male offspring [10], the current study was conducted to examine the effect of LPS-induced MIA on several inflammatory mediators and microglia and astrocyte density in PFC of adult male offspring. We also investigated whether maternal zinc supplementation can alleviate LPS-induced alterations in this region of brain.
Materials and Methods
Animal
Three-month-old Wistar rats including 24 females and 12 males (animal laboratory of Hormozgan University of Medical Sciences) weighing 250 ± 20 g were housed under controlled humidity (60–70%) and temperature (22 °C) with a 12 h light/dark schedule (light on 7 a.m. to 7 p.m.). Animals had unlimited access to pellet (Pars animal food company, Iran) and tap water.
All animal procedures in this research were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and also approved by the local Institutional Ethics Committee (approval number: IR.HUMS.REC.1397.010). All attempts were made to minimize the number and suffering of animals.
Treatment
Female and male breeders were co-housed in a cage overnight and the first day after detecting spermatozoa in vaginal smears was defined as GD1. The pregnant rats were randomly assigned into the following treatment groups: group 1 (control) and group 2 (LPS); pregnant rats received two consecutive i.p. injections of either saline or LPS (0.5 mg/kg, Escherichia coli O111:B4) [9] dissolved in saline at GD15/16, respectively. Both control and LPS groups were orally gavaged with water (as a vehicle) throughout the pregnancy. Group 3 (LPS + zinc) and group 4 (zinc): pregnant dams were treated with LPS (0.5 mg/kg, i.p.) or saline on GD15/16 and meanwhile were daily supplemented with ZnSO4 (30 mg/kg) [32] by gavage throughout the gestational period. The dose and route of zinc supplementation had no adverse effect on pregnancy outcome.
The offspring born to these dams were weaned and housed based on sex and litter on postnatal day 21 (PND 21) and left undisturbed until experiments at PND 60. In this study, subjects were the male pups from different litters (6 litters per each experimental group) to avoid litter effects. We selected male pups in the present study based on our previous results showing the working memory impairments only in male offspring [10]. Added to this, we used resource equation method for sample size calculation [33]. One male offspring from independent litters were selected randomly for qPCR, immunostaining, and hematoxylin-eosin (H&E) staining.
Tissue Collection
For molecular analysis, six animals from each group (n = 6) were euthanized using CO2 asphyxiation at PND 60. After decapitation, PFC were immediately dissected from the brains, snap-frozen in liquid nitrogen, and finally kept at − 80 °C until further analysis. The dissection of PFC was carried out based on a study by Chiu et al. [34].
For histological analysis, after deep anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg), four animals per each experimental group (n = 4) were transcardially subjected to perfusion using phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA) solution. The brain tissues were removed from the skulls and incubated overnight in 4% PFA. Finally, the brains used for immunostaining were stored in a 30% sucrose dissolved in PBS solution until further use.
Gene Expression Analysis by qPCR
All procedures including RNA extraction, cDNA synthesis, and qPCR analysis were conducted based on MIQE guidelines [35]. Total RNA extraction from PFC tissues was performed using TRI reagent (Sigma, USA), followed by DNase treatment (Fermentas, USA) based on the manufacturer’s instructions. Total RNA was reverse transcribed using one microgram of total RNA and oligo-dT primer with a cDNA synthesis kit (Thermo Ficher, USA) according to the manufacturer’s protocol. For gene expression analysis, cDNA samples were subjected to amplify in triplicate using SYBR Premix Ex Taq II (Takara, Japan) and primers listed in Table 1 in a Mic qPCR system (Australia). qPCR reaction was carried out with a three-step procedure as follows: initiation at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s for a total of 40 cycles. GAPDH expression was used as a reference gene, and relative expression of target genes was determined by the comparative Ct (2-ΔΔCt) method.
Immunofluorescence Staining
To measure the astrocyte and microglia density in PFC, immunostaining was carried out as described previously [36]. After transferring the brains in a 30% sucrose solution for 48 h, tissues were frozen in the OCT compound, and then coronal cryosections (6-μm-thick sections) were prepared using a cryostat instrument (MICROM HM 525, Thermo Scientific). After three washes with PBS, sections were permeabilized and blocked with a solution containing 10% normal goat serum (NGS), 0.3% Triton x-100 in PBS for 1 h, followed by overnight incubation with primary antibodies including rabbit anti GFAP (1:400, Z0334, Dako) or rabbit anti-Iba1 (1:500, 019–19,741, Wako) at 4 °C. On the next day, section slides were washed with PBS and incubated in a 1:1000 dilution of a Goat anti-rabbit Alexa Fluor®594 (ab150080) or Goat anti-rabbit Alexa Fluor®488 (ab150077) for 1 h at room temperature. Subsequently, the sections were washed with PBS, stained with 4,6-diamidino-2-phenylindole (DAPI) for 10 min at room temperature and finally visualized with a fluorescence microscope (Nikon E600, Japan). All images were captured at a magnification of 200 with the constant acquisition parameters, including gain, exposure, and intensity. Images from four sections per slide, four slides per animal, and four animals per group were taken. For the quantification of data, the number of Iba1/GFAP positive cells soma were manually counted using Image J software (version 1.42 V, NIH, USA) and averaged from four consecutive sections per slide based on a previous report [37]. The observer was blind to the treatment groups.
Histopathology
For histopathological examination, brain tissues were dehydrated by a series of alcohol and embedded in paraffin. Afterward, 5-μm-thick coronal sections were cut through the PFC region using a microtome. Finally, sections were stained with hematoxylin and eosin (H&E) and evaluated under a microscope (Nikon, Japan) [36]. Images were captured at a magnification of 200. Cell counting was manually performed by an independent and blinded investigator using Image J software (version 1.42 V, NIH, USA) and averaged from four consecutive sections per slide. Four sections from each slide, four slides per each animal, and four animals per each group were evaluated.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software Inc. San Diego, CA, USA). The data were analyzed using two-way analysis of variance (ANOVA), with gestational treatment (saline vs. LPS) and maternal supplementation (vehicle vs. zinc) as between-subject factors. Analyses were followed by Bonferroni post hoc test. The data are expressed as mean ± SEM, and p < 0.05 was considered statistically significant.
Results
Maternal Zinc Supplementation Inhibited Release of Pro-inflammatory Mediators
qPCR technique was applied to investigate the long-lasting consequence of prenatal LPS exposure and possible inhibitory effect of maternal zinc supplementation on the expression of several inflammatory markers in PFC of adult male offspring. For this reason, mRNA expression levels of IL-6, IL-1β, iNOS, TNF-α, NF-κB, and also GFAP as an astrocyte marker was measured in the PFC of pups at PND 60. Statistical analysis by two-way ANOVA revealed a significant effect of gestational treatment for the expression of all measured genes including IL-6 (F (1, 20) = 33.67, p < 0.0001), IL-1β (F (1, 20) = 4.580, p = 0.0449), TNF-α (F (1, 20) = 18.29, p = 0.0004), NF-κB (F (1, 20) = 7.969, p = 0.0105), iNOS (F (1, 20) = 25.02, p < 0.0001), and GFAP (F (1, 20) = 136.9, p < 0.0001). For IL-6 expression, there was a significant effect of maternal zinc supplementation (F (1, 20) = 4.366, p = 0.0496) as well as interaction between maternal zinc supplementation and gestational treatment (F (1, 20) = 12.80, p = 0.0019). As illustrated in Fig. 1a, IL-6 expression strongly increased by at least sixfold (LPS vs control Bonferroni post hoc analysis, p > 0.001) upon prenatally treatment with LPS compared to the control group. Maternal zinc supplementation, however, attenuated this enhancement in LPS + zinc group (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.01) (Fig. 1a). Interestingly, IL-1β and TNF-α showed a similar pattern with significant effect of maternal zinc supplementation (IL-1β (F (1, 20) = 5.955, P = 0.0241), TNF-α (F (1, 20) = 6.050, p = 0.0231)), and no significant interaction between maternal zinc supplementation and gestational treatment (IL-1β (F (1, 20) = 3.001, p = 0.0986), TNF-α (F (1, 20) = 0.9717, p = 0.3360)). Prenatal exposure to LPS led to a significant upregulation in IL-1β (nearly twofold, LPS vs control Bonferroni post hoc analysis, p > 0.05) and TNF-α (twofold, LPS vs control Bonferroni post hoc analysis, p > 0.01) which was inhibited in the offspring prenatally supplemented with zinc (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.05) (Fig. 1b, c). Accordingly, for NF-κB, there was a main effect of maternal zinc supplementation (F (1, 20) = 5.747, p = 0.0264) and significant interaction between maternal zinc supplementation and gestational treatment (F (1, 20) = 6.147, p = 0.0222). NF-κB mRNA was moderately enhanced in the prenatally LPS treated pups (2.5-fold, LPS vs control Bonferroni post hoc analysis, p > 0.01), and this effect was reversed by zinc supplementation in the pups of LPS + Zinc-treated dams (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.01) (Fig. 1d). Whereas statistical analysis showed no main effect of maternal zinc supplementation (F (1, 20) = 3.009, p = 0.0982), a significant interaction (F (1, 20) = 5.310, p = 0.0321) was revealed for iNOS expression. As shown in Fig. 1e, a significant increase was detected for iNOS mRNA (approximately twofold, LPS vs control Bonferroni post hoc analysis, p > 0.001) in the offspring prenatally exposed to LPS related to the control group which returned to control level upon zinc supplementation in the offspring of LPS treated dams (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.05) (Fig. 1e).
Maternal zinc supplementation ameliorated increased expression of inflammatory mediators in PFC of offspring prenatally exposed to LPS. The expression levels of IL-6 (a), IL-1β (b), TNF-α (c), NF-κB (d), and iNOS (e) were significantly increased in the PFC of rats prenatally exposed to LPS compared to the control. This increment was alleviated by zinc supplementation throughout pregnancy. GAPDH expression level was used as a reference gene. The values are described as mean ± SEM (n = 6), *p < 0.05, **p < 0.01, and ***p < 0.001 compared to control group. #p < 0.05, ##p < 0.01, and ###p < 0.001 compared to LPS group. PND postnatal day, LPS lipopolysaccharide, PFC prefrontal cortex
Notably, a significant main effect of maternal zinc supplementation (F (1, 20) = 58.44, p < 0.0001) as well as interaction (F (1, 20) = 76.60, p < 0.0001) was revealed for GFAP mRNA level. Following prenatal immune challenge, a remarkable upregulation was detected for GFAP expression level by approximately 13-fold (LPS vs control Bonferroni post hoc analysis, p > 0.001) in the offspring compared to the control group, which was found to significantly decrease (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.001) upon maternal zinc supplementation in the offspring of LPS + zinc group (Fig. 2).
Prenatal exposure to LPS increased the expression levels of GFAP in the PFC of offspring at PND 60, which were ameliorated by maternal zinc supplementation. The expression level of GFAP was significantly increased in the PFC of rats prenatally exposed to LPS compared to the control. This increment was alleviated by zinc supplementation throughout pregnancy. GAPDH expression level was used as a reference gene. The values are described as mean ± SEM (n = 6), ***p < 0.001 compared to control group. ###p < 0.001 compared to LPS group. PND postnatal day, LPS lipopolysaccharide, PFC prefrontal cortex
Maternal Zinc Supplementation Attenuated the LPS-Induced Changes in Glial Cells Density
Based on the results of pro-inflammatory genes expression and the previous studies showing the cytokines production as a specific functional aspect of glial activation, the density of both astrocyte and microglia was evaluated by counting the number of GFAP and Iba1 positive cells in the PFC region of the adult offspring, respectively. Statistical analysis by two-way ANOVA showed that maternal LPS treatment led to a marked increase in GFAP and Iba1 positive cells, as supported by a main effect of gestational treatment for GFAP (F (1, 12) = 26.31, P = 0.0002) and Iba1 (F (1, 12) = 13.80, P = 0.0030), and a significant interaction between gestational treatment and maternal zinc supplementation (GFAP (F (1, 12) = 24.85, p = 0.0003), Iba1 (F (1, 12) = 6.670, p = 0.0240)) with no main effect of maternal zinc supplementation (GFAP (F (1, 12) = 2.302, p = 0.1551), Iba1 (F (1, 12) = 3.005, p = 0.1086)). As is evident from Fig. 3b and 4b, LPS treatment during pregnancy caused obvious increase in the number of astrocyte cells labeled by GFAP (LPS vs control Bonferroni post hoc analysis, p > 0.001) as well as microglial density labeled by Iba1 (LPS vs control Bonferroni post hoc analysis, p > 0.01) in the PFC region of prenatally LPS exposed offspring compared to the control group. However, pups born to both LPS and zinc-treated mothers (LPS + zinc) showed a significant reduction in GFAP (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.01) (Fig. 3b) and Iba1 (LPS + zinc vs LPS Bonferroni post hoc analysis, p > 0.05) positive cells in comparison to prenatally immune-challenged offspring (Fig. 4b).
Increased astrocyte density in the PFC of MIA offspring was improved by maternal zinc supplementation. Immunofluorescence images of GFAP-labeled cells, as a marker of astrocyte, in the PFC region of offspring brains at PND 60. Scale bar: 50 μm, magnification: × 200 (a). The quantified histogram of GFAP positive cells exhibited the increased astrocyte density in the PFC of adult offspring born to the LPS treated dams compared to the control. Prenatal zinc supplementation prevented this alteration (b). The values are described as mean ± SEM (n = 4), ***p < 0.001 compared to control group and ##p < 0.01 compared to LPS group. PND postnatal day, LPS lipopolysaccharide, PFC prefrontal cortex, GFAP glial fibrillary acidic protein
Increased microglial density in the PFC of MIA offspring was improved by maternal zinc supplementation. Immunofluorescence images of Iba1-labeled cells, as a marker of microglia, in the PFC region of offspring brains at PND 60. Scale bar: 50 μm, magnification: × 200 (a). The quantified histogram of Iba1 positive cells exhibited the increased microglial density in the PFC of adult offspring born to the LPS treated dams compared to the control. Prenatal zinc supplementation prevented this alteration (b). The values are described as mean ± SEM (n = 4), **p < 0.01 compared to control group and ##p < 0.01 compared to LPS group. PND postnatal day, LPS lipopolysaccharide, PFC prefrontal cortex, Iba1 ionized calcium binding adaptor molecule1
Histology of PFC Region in the Male Offspring from all Experimental Groups
To examine whether changes in inflammatory mediator genes expression, as well as astrocyte and microglial activation, are associated with histological alterations in the PFC region, H&E staining was conducted for the offspring of all experimental groups. As shown in Fig. 5a, the sections of the PFC from the control group exhibited normal neural cells with prominent and clear nuclei. Although the PFC sections of the offspring with prenatal LPS exposure showed neurocytes with hyperchromatic nuclei, it requires further validation for the assessment of neurocyte degeneration to prevent histologic artifact (Fig. 5b). Furthermore, two-way ANOVA analysis showed no significant effects of gestational treatment (F (1, 12) = 0.6726, p = 0.4281), maternal zinc supplementation (F (1, 12) = 0.3784, p = 0.5500), and their interaction (F (1, 12) = 1.686, p = 0.2185) for the number of neuron in the PFC region of offspring from all experimental groups (Fig. 5e).
H&E staining of PFC from the resulting offspring in deferent groups. Black and white arrows represent the normal (N) and neurons with hyperchromatic nuclei (H), respectively. The photograph indicates the normal neural cells with prominent and clear nuclei in the PFC from the control group (a). The photograph of the PFC section from offspring born to the LPS treated dams indicates neurocytes with hyperchromatic nuclei (b). No significant changes were observed for the number of neurocytes in the PFC region of offspring from all experimental groups (e). Scale bar: 50 μm, magnification: × 200, (n = 4). LPS lipopolysaccharide, PFC prefrontal cortex
Discussion
In the majority of MIA model studies, the main focus is placed on face validity. However, due to the urgent need for novel therapeutic strategies to alleviate symptoms of neuropsychiatric diseases including schizophrenia, predictive validity is of importance. With this background, in the current study, we examined the protective effect of maternal zinc supplementation on several inflammatory mediators induced by LPS in PFC of adult offspring, which resulted in several main findings. Firstly, prenatal LPS treatment led to the increased density of microglia and astrocyte cells as well as significant upregulation of pro-inflammatory mediators, including IL-6, IL-1β, iNOS, TNF-α, and NF-κB in the PFC region of male offspring at PND 60. Secondly, zinc supplementation was found to effectively alleviate LPS-induced alterations in the PFC region, when given throughout pregnancy.
There is a consensus regarding the involvement of neuroinflammation and cytokine dysregulation in the pathophysiology of schizophrenia, evidenced by the increased levels of inflammatory markers in the serum, CSF, and postmortem brains of schizophrenic patients [38,39,40,41,42]. Most recently, a postmortem study has also reported the increased expression of transcripts related to inflammation in the PFC, hippocampus, and striatum of patients with schizophrenia [17]. In addition, evidence for the immune imbalance in the brain of MIA offspring with specific patterns in various regions has been demonstrated in several studies [3, 5]. In this regard, a long-lasting alteration in several cytokine levels including, IL-6, IL-1α, and IL-10 was reported in the frontal cortex of Poly I:C offspring (20 mg/kg, i.p.) at PND 60 [43]. A more recent study has also demonstrated the remarkable increase in protein levels of IL-6, IL-1β, and TNF-α in the PFC of MIA offspring (Poly I:C, 10 mg/kg, i.v., GD9) at the same age [44]. Similar trends were also observed in our study with a significant upregulation of IL-6, IL-1β, and TNF-α in the PFC region of adult offspring born to the dams treated with LPS. In addition, our study is the first to report an enhanced expression level of iNOS in MIA adult pups. Added to this, we showed that the upregulation of the mentioned mediators was parallel with the increased expression level of NF-κB (p65 subunit), which is linked to the production of pro-inflammatory cytokines [45]. Therefore, these findings provide more evidence for long-lasting changes in brain cytokine levels of offspring exposed to maternal infection.
Alteration in cytokine levels in the brain was further validated by measuring the density of microglia and astrocyte cells. While there is a large body of evidence supporting the cytokine imbalance in the brain of MIA offspring [3, 5], the effect of MIA on microglial and astrocyte activation remains a matter of debate. In the frontal cortex of adult rats (20 mg/kg, i.p.) [43] and mice (4 mg/kg, i.v.) [46] prenatally exposed to Poly I:C, no alteration in microglial activation has been reported. In a recent study by Ding and colleagues [44], however, more activated microglia were detected in the PFC region of the adult pups exposed in utero to Poly I:C (10 mg/kg, i.v.). A similar trend was also observed in our study, whereby the number of Iba1 positive cells was found to be increased in the adult offspring born to LPS challenged mothers. Interestingly, a PET imaging study has reported the microglial activation in the PFC region of adult offspring prenatally exposed to Poly I:C (10 mg/kg, i.v.) in vivo [47]. Our data also identified the increased GFAP positive cells as well as GFAP mRNA levels in the PFC region of LPS-exposed offspring compared to the control. This finding was further supported by the recent study reporting the elevated density of astrocytes in the PFC region of the rats prenatally exposed to Poly I:C (10 mg/kg, i.v.) at PND 60 [44]. Similarly, several studies utilizing LPS MIA model have described significant astrogliosis in the brain of offspring [11, 48]. However, another study showed unchanged astrocytes density in the frontal cortex of Poly I:C (4 mg/kg, i.v.) offspring [46]. Therefore, the prolonged effect of MIA on microglial and astrocytes activation remains controversial, mainly due to the variation in methodology.
As mentioned above, many MIA studies provide support for the involvement of microglia and astrocyte cells in mediating long-lasting immune dysregulation in the brains of offspring [5, 44, 48]. It is well documented that these cells both structurally and functionally incorporate in neural connectivity and significantly influence synaptic function [49]. Accordingly, dysregulation of microglial and astrocyte activation induced by MIA can lead to disturbance in neural functions and connectivity, which eventually might result in the development of psychiatric disorders later in life [18, 49]. In support of this, several studies have reported a significant association between abnormal neural connectivity and different cognitive impairments in schizophrenia [14, 50]. In particular, disturbed connectivity of brain areas involved in working memory networks, including PFC, has been consistently demonstrated in schizophrenic patients [51, 52]. Based on these findings, our results reinforce the idea that the disturbed neural connectivity induced by long-lasting alterations in microglial and astrocyte activation in the PFC region might be a potential mechanism behind working memory impairment observed in adult male offspring [10]. To investigate this hypothesis further, we performed H&E staining in the PFC region of offspring in all experimental groups. Our results showed that prenatal immune challenge using LPS did not significantly change the number of neurons in the PFC of adult pups, which further raises the possibility that the disruption in neural connectivity [14] rather than a reduction in neuronal density might be involved in the psychopathology of schizophrenia.
Zinc, the concentration of which is the highest in the brain, is potentially involved in approximately every cellular mechanism such as gene expression, immune response, cell growth, apoptosis, and in particular normal brain functions [53]. The prominent role of zinc dyshomeostasis in the pathogenesis of several neurological diseases has been consistently reported in the literature [53]. In particular, a reduced level of zinc in subjects suffering from schizophrenia has been confirmed in several human studies [54,55,56]. Interestingly, maternal infection has been found to be associated with fetal zinc deficiency and subsequent neurodevelopmental abnormalities in the progeny [25, 28]. However, the underlying mechanism is unclear, but it might be due to the fact that zinc is an important prerequisite for the function of essential proteins involving in neurodevelopment [27]. More importantly, in our previous study, we showed the therapeutic effect of prenatal zinc supplementation to prevent LPS-induced behavioral impairments [10]. In light of this evidence, here we found that dietary zinc supplementation during pregnancy can prevent LPS-related alterations in pro-inflammatory mediators, including IL-6, IL-1β, iNOS, TNF-α, and NF-κB. Indeed, a potential influence of zinc on immune system is undeniable, as is supported by numerous human studies demonstrating the association between zinc deficiency and infectious disease [45, 57, 58]. In this regard, there is some evidence that zinc acts as a negative regulator of NF-κB pathway in part by inhibition of IKKβ, which subsequently suppresses LPS-induced activation of NF-κB [59]. NF-κB signaling pathway is a major pathway in response to inflammation, which triggers the expression of pro-inflammatory mediators including IL-6, IL-1β, TNF-α, and iNOS [57, 60]. Therefore, a decreased expression level of NF-κB in the PFC region of the offspring (from LPS + zinc group) and subsequent reduction in mRNA level of IL-6, IL-1β, TNF-α, and iNOS might be a possible mechanism by which zinc could alleviate LPS-induced impairments.
Our results also revealed that oral zinc supplementation throughout pregnancy ameliorated increased microglial and astrocyte density in the PFC region of offspring born to LPS-challenged mothers. Similar protection was also found in another MIA model study, in which zinc supplementation during pregnancy prevented LPS-induced astrogliosis in the cortex and hippocampus of fetal brains [28]. Based on these findings, it can be concluded that maternal zinc supplementation alleviates glial activation induced by prenatal LPS exposure in offspring. As mentioned earlier, prenatal exposure to LPS leads to maternal and fetal hypozincemia [29]. Given that zinc is an essential component in the immune system as well as numerous biological processes involved in neurodevelopment, zinc deficiency may result in deleterious consequences in the fetal brain [27]. Maternal zinc supplementation, on the other hand, inhibits these abnormalities possibly by limiting the reduction in maternal plasma zinc induced by LPS [28, 29]. The protective effect of zinc supplementation can also be explained by the antioxidant property of this trace element. With this characteristic, zinc might reduce maternal immune response and oxidative stress induced by LPS and consequently moderate its influence on the developing brain of fetus [61].
Finally, some limitations of the present study should be acknowledged. First, the MIA animal model does not reproduce all neurobehavioral and neuropathological features of a specific psychiatric disorder. Second, our results showed the increased activation of microglia and astrocyte cells with unchanged neuron number in the PFC of offspring prenatally exposed to LPS. Given the putative role of glial cells in maintenance of neuron function, structure, and connectivity, the assessment of neural connectivity and synaptic function in this region would be of interest. Third, this study only focused on the PFC region and several inflammatory markers. It would be interesting to evaluate more brain regions as well as other markers relevant to neuroinflammation.
Conclusion
Taken together, our results show that maternal LPS treatment on GD15/16 leads to significant changes in the PFC region of adult offspring, evident by the enhanced production of pro-inflammatory markers and increased microglia and astrocyte density, which supports the long-lasting influence of MIA on the brain of the next generation. These prominent and chronic alterations might be one of the possible mechanisms behind working memory impairments in adult male offspring. Moreover, prenatal zinc treatment was found to prevent these LPS-mediated impairments. Our study provides further support for the pivotal role of zinc for normal brain development. It is of interest to suggest early therapeutic intervention as a valuable approach to prevent neuroinflammation in offspring, following maternal infection.
Data Availability
The data that support the findings of this study can be accessed upon reasonable request from the corresponding author.
Abbreviations
- LPS:
-
Lipopolysaccharide
- PND:
-
Postnatal day
- PFC:
-
Prefrontal cortex
- GD:
-
Gestation day
- MIA:
-
Maternal immune activation
- CNS:
-
Central nervous system
- GFAP:
-
Glial fibrillary acidic protein
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin 6
- NF-κB:
-
Nuclear factor kappa B
- iNOS:
-
Inducible nitric oxide synthase
- iba1:
-
Ionized calcium binding adaptor molecule 1
- DAPI:
-
4′,6-diamidino2-phenylindole
- MT:
-
Maternal metallothionein
- i.p.:
-
Intraperitoneal
- qPCR:
-
Quantitative PCR
- H&E:
-
Hematoxylin-eosin
- ANOVA:
-
Analysis of variance
- ELISA:
-
Enzyme-linked immunosorbent assay
References
McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW (2003) The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med 35(2):86–93
Lang UE, Puls I, Müller DJ, Strutz-Seebohm N, Gallinat J (2007) Molecular mechanisms of schizophrenia. Cell Physiol Biochem 20(6):687–702
Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES (2018) Maternal immune activation in neurodevelopmental disorders. Dev Dyn 247(4):588–619
Prata J, Santos SG, Almeida MI, Coelho R, Barbosa MA (2017) Bridging autism spectrum disorders and schizophrenia through inflammation and biomarkers-pre-clinical and clinical investigations. J Neuroinflammation 14(1):179
Bergdolt L, Dunaevsky A (2019) Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol 175:1–19
Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossío LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184
Santos-Toscano R, Borcel É, Ucha M, Orihuel J, Capellán R, Roura-Martínez D, Ambrosio E, Higuera-Matas A (2016) Unaltered cocaine self-administration in the prenatal LPS rat model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 69:38–48
Waterhouse U, Roper VE, Brennan KA, Ellenbroek BA (2016) Nicotine ameliorates schizophrenia-like cognitive deficits induced by maternal LPS exposure: a study in rats. Dis Model Mech 9(10):1159–1167
Wischhof L, Irrsack E, Osorio C, Koch M (2015) Prenatal LPS-exposure–a neurodevelopmental rat model of schizophrenia–differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuro-Psychopharmacol Biol Psychiatry 57:17–30
Alizadeh F, Davoodian N, Kazemi H, Ghasemi-Kasman M, Shaerzadeh F (2020) Prenatal zinc supplementation attenuates lipopolysaccharide-induced behavioral impairments in maternal immune activation model. Behav Brain Res 377:112247
O’Loughlin E, Pakan JM, Yilmazer-Hanke D, McDermott KW (2017) Acute in utero exposure to lipopolysaccharide induces inflammation in the pre-and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J Neuroinflammation 14(1):212
Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL (2000) Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 48(2):99–109
Perlstein WM, Carter CS, Noll DC, Cohen JD (2001) Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatr 158(7):1105–1113
Ruiz S, Birbaumer N, Sitaram R (2013) Abnormal neural connectivity in schizophrenia and fMRI-brain-computer interface as a potential therapeutic approach. Front Psychiatry 4:17
Bernstein H-G, Steiner J, Guest PC, Dobrowolny H, Bogerts B (2015) Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res 161(1):4–18
De Keyser J, Mostert JP, Koch MW (2008) Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci 267(1–2):3–16
Lanz TA, Reinhart V, Sheehan MJ, Rizzo SJS, Bove SE, James LC, Volfson D, Lewis DA, Kleiman RJ (2019) Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: a comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl Psychiatry 9(1):1–13
Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, Kanba S (2013) Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:115–121
Juckel G, Manitz MP, Brüne M, Friebe A, Heneka MT, Wolf RJ (2011) Microglial activation in a neuroinflammational animal model of schizophrenia—a pilot study. Schizophr Res 131(1–3):96–100
Radewicz K, Garey LJ, Gentleman SM, Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 59(2):137–150
Schnieder TP, Dwork AJ (2011) Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry 69(2):134–139
Matute C, Melone M, Vallejo-Illarramendi A, Conti F (2005) Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia 49(3):451–455
Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD (2015) The poly (I: C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther 149:213–226
Yu X, Chen W, Wei Z, Ren T, Yang X, Yu X (2016) Effects of maternal mild zinc deficiency and different ways of zinc supplementation for offspring on learning and memory. Food Nutr Res 60(1):29467
Carey LC, Berbée PL, Coyle P, Philcox JC, Rofe AM (2003) Zinc treatment prevents lipopolysaccharide-induced teratogenicity in mice. Birth Defects Res A: Clin Molec Teratol 67(4):240–245
Kirsten TB, Chaves-Kirsten GP, Bernardes S, Scavone C, Sarkis JE, Bernardi MM, Felicio LF (2015) Lipopolysaccharide exposure induces maternal hypozincemia, and prenatal zinc treatment prevents autistic-like behaviors and disturbances in the striatal dopaminergic and mTOR systems of offspring. PLoS One 10(7):e0134565
Levenson CW, Morris D (2011) Zinc and neurogenesis: making new neurons from development to adulthood. Adv Nutr 2(2):96–100
Chua JS, Cowley CJ, Manavis J, Rofe AM, Coyle P (2012) Prenatal exposure to lipopolysaccharide results in neurodevelopmental damage that is ameliorated by zinc in mice. Brain Behav Immun 26(2):326–336
Coyle P, Tran N, Fung JN, Summers BL, Rofe AM (2009) Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res 197(1):210–218
Sharma N, Arora P, Nehru B (2017) Prenatal zinc supplementation to lipopolysaccharide infected female rats prevents neurochemical, behavioral and biochemical deficits produced in infants. Neuroimmunol Neuroinflamm 4(4):33–45
Chua JS, Rofe AM, Coyle P (2006) Dietary zinc supplementation ameliorates LPS-induced teratogenicity in mice. Pediatr Res 59(3):355–358
Moazedi A, Ghotbeddin Z, Parham G (2007) Comparison of the effects of dose-dependent zinc chloride on short-term and long-term memory in young male rats. Pak J Biol Sci 10(16):2704–2708
Charan J, Kantharia N (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4(4):303–306
Chiu K, Lau WM, Lau HT, So K-F, Chang RC-C (2007) Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J Visual Exp 7:269
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL (2009) The MIQE guidelines: minimum information for publication of quantitative Real-Time PCR experiments. Clin Chem 55(4):611–622
Naeimi R, Safarpour F, Hashemian M, Tashakorian H, Ahmadian SR, Ashrafpour M, Ghasemi-Kasman M (2018) Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci Lett 674:1–10
Akbari A, Khalili-Fomeshi M, Ashrafpour M, Moghadamnia AA, Ghasemi-Kasman M (2018) Adenosine A2A receptor blockade attenuates spatial memory deficit and extent of demyelination areas in lyolecithin-induced demyelination model. Life Sci 205:63–72
Kirkpatrick B, Miller BJ (2013) Inflammation and schizophrenia. Schizophr Bull 39(6):1174–1179
Luo Y, He H, Zhang J, Ou Y, Fan N (2019) Changes in serum TNF-α, IL-18, and IL-6 concentrations in patients with chronic schizophrenia at admission and at discharge. Compr Psychiatry 90:82–87
Mazza MG, Lucchi S, Rossetti A, Clerici M (2020) Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in nonaffective psychosis: A meta-analysis and systematic review. World J Biol Psychiatry 21(5):326–338
Miller BJ, Culpepper N, Rapaport MH (2014) C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses 7(4):223–230
Wang AK, Miller BJ (2018) Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44(1):75–83
Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013) Maternal immune activation causes age-and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68
Ding S, Hu Y, Luo B, Cai Y, Hao K, Yang Y, Zhang Y, Wang X, Ding M, Zhang H (2019) Age-related changes in neuroinflammation and prepulse inhibition in offspring of rats treated with poly I: C in early gestation. Behav Brain Funct 15(1):3
Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 25(1):11–24
Paylor JW, Lins BR, Greba Q, Moen N, De Moraes RS, Howland JG, Winship IR (2016) Developmental disruption of perineuronal nets in the medial prefrontal cortex after maternal immune activation. Sci Rep 6:37580
Li X, Tian X, Lv L, Hei G, Huang X, Fan X, Zhang J, Zhang J, Pang L, Song X (2018) Microglia activation in the offspring of prenatal poly I: C exposed rats: a PET imaging and immunohistochemistry study. Gen Psychiatry 31(1):e000006
Hao L, Hao X, Li S, Li X (2010) Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience 166(3):763–770
Fields RD, Woo DH, Basser PJ (2015) Glial regulation of the neuronal connectome through local and long-distant communication. Neuron 86(2):374–386
Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, Suzuki M (2012) Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Res Neuroimaging 202(3):233–238
Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P (2003) Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage 19(3):751–763
Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM (2005) Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain 128(9):2097–2108
Portbury SD, Adlard PA (2017) Zinc signal in brain diseases. Int J Mol Sci 18(12):2506
Cai L, Chen T, Yang J, Zhou K, Yan X, Chen W, Sun L, Li L, Qin S, Wang P (2015) Serum trace element differences between schizophrenia patients and controls in the Han Chinese population. Sci Rep 5:15013
Cao B, Yan L, Ma J, Jin M, Park C, Nozari Y, Kazmierczak OP, Zuckerman H, Lee Y, Pan Z (2019) Comparison of serum essential trace metals between patients with schizophrenia and healthy controls. J Trace Elem Med Biol 51:79–85
Joe P, Petrilli M, Malaspina D, Weissman J (2018) Zinc in schizophrenia: a meta-analysis. Gen Hosp Psychiatry 53:19–24
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651
Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ (2007) Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 85(3):837–844
von Bülow V, Dubben S, Engelhardt G, Hebel S, Plümäkers B, Heine H, Rink L, Haase H (2007) Zinc-dependent suppression of TNF-α production is mediated by protein kinase A-induced inhibition of Raf-1, IκB kinase β, and NF-κB. J Immunol 179(6):4180–4186
Ghosh S, Hayden MS (2012) Celebrating 25 years of NF-κB research. Immunol Rev 246(1):5–13
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1:14
Funding
This study was supported by a grant [grant no. 960329] from the Research Vice-Chancellor of Hormozgan University for Medical Science (HUMS).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: Nahid Davoodian and Fatemeh Shaerzadeh
Data collection: Ronak Mousaviyan, Faezeh Alizadeh, Nahid Davoodian, and Haniyeh Kazemi
Data analysis: Nahid Davoodian, Maryam Ghasemi-Kasman, and Seyed Abdollah Mousavi
Manuscript preparation: Nahid Davoodian and Maryam Ghasemi-Kasman
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interest.
Ethics Approval
All animal procedures in this research were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and also approved by the local Institutional Ethics Committee (approval number: IR.HUMS.REC.1397.010).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mousaviyan, R., Davoodian, N., Alizadeh, F. et al. Zinc Supplementation During Pregnancy Alleviates Lipopolysaccharide-Induced Glial Activation and Inflammatory Markers Expression in a Rat Model of Maternal Immune Activation. Biol Trace Elem Res 199, 4193–4204 (2021). https://doi.org/10.1007/s12011-020-02553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02553-6