Abstract
Selenium (Se) is one of the most important essential trace elements in livestock production. It is a structural component in at least 25 selenoproteins such as the iodothyronine deiodinases and thioredoxin reductases as selenocysteine at critical positions in the active sites of these enzymes. It is also involved in the synthesis of the thyroid hormone and influences overall body metabolism. Selenium being a component of the glutathione peroxidase enzyme also plays a key role in the antioxidant defense system of animals. Dietary requirements of Se in dairy animals depend on physiological status, endogenous Se content, Se source, and route of administration. Most of the dietary Se is absorbed through the duodenum in ruminants and also some portion through the rumen wall. Inorganic Se salts such as Na-selenate and Na-selenite have shown lower bioavailability than organic and nano-Se. Selenium deficiency has been associated with reproductive disorders such as retained placenta, abortion, early embryonic death, and infertility, together with muscular diseases (like white muscle disease and skeletal and cardiac muscle necrosis). The deficiency of Se can also affect the udder health particularly favoring clinical and subclinical mastitis, along with an increase of milk somatic cell counts in dairy animals. However, excessive Se supplementation (5 to 8 mg/kg DM) can lead to acute toxicity including chronic and acute selenosis. Se is the most vital trace element for the optimum performance of dairy animals. This review focuses to provide insights into the comparative efficacy of different forms of dietary Se (inorganic, organic, and nano-Se) on the health and production of dairy animals and milk Se content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace elements or minerals are classified as micronutrients that have nutritional relevance for livestock generally (ruminants or non-ruminants), and they are considered in their diets [1]. These microelements are divided into essential (Fe, Cu, Co, Se, I, Mn, Zn, and B) and non-essential (toxic elements such as Pb and Cd) elements. The essential microminerals act as co-factors of various metalloenzymes playing key roles in the body metabolism and immune system, which make them vital for animals [2]. Because of the physiological importance of essential trace minerals, their deficiency, due to either low supply or some mineral antagonism, negatively affects the health, reproductive, and productive performances of dairy animals [3].
Selenium (Se) is one of the most important essential trace minerals in livestock production owing to its diverse physiological roles. Se is a structural component of at least 25 selenoproteins as selenocysteine (Se-Cys) in their active catalytic site [4]. The majority of selenoproteins play a vital role in enzymatic redox reactions at the cellular level via Se-Cys, which convene their catalytic or antioxidant capabilities. The key physiological processes which essentially require selenoproteins include DNA synthesis (biosynthesis of dNTPs), scavenging of toxic or signaling peroxides, reduction of oxidized proteins and membranes, redox signaling, metabolism of thyroid hormone, transport, and storage of Se [5]. Three major groups of selenoproteins concerning key physiological functions in mammals include thioredoxin reductases (TrxR), iodothyronine deiodinases, and glutathione peroxidases (GSH-Px) [6]. The thyroxin system (constituting TrxR, thioredoxin (Trx), and NADPH) is a major cellular redox system that effectively regulates many essential cellular processes including DNA synthesis [7, 8], body defense against oxidative stress [9], and integrity of endoplasmic reticulum [10] through reduction of protein disulfides in ribonucleotide reductase, thioredoxin peroxidase, and protein disulfide-isomerase, respectively.
Moreover, this system also regulates the synthesis of the thyroid hormone and mediates the conversion of T4 (thyroxin inactive form) into T3 (active form) leading to influence overall body metabolism and development [11]. Additionally, the T3 hormone regulates the metabolism of the adipose tissue in association with a seleno-dependent hormone. Moreover, the thioredoxin system also regulates the gene expression through mediating transcription factors including NF-ƙs, Ref-1, AP-1, P53, glucocorticoid receptor, and apoptosis-regulating kinase (ASK1). Thus, it indirectly influences key cellular activities like cell cycle, apoptosis, and activation of the immune response [12].
The second most important group of selenoproteins as GSH-Px regulates the concentration of free radicals of reactive oxygen species (ROS) at cellular levels [13, 14]. Owing to the abovementioned physiologically diverse role of selenoproteins, an optimum quantity of Se is required in livestock feed to ensure proper immune functions, production performance, and fertility [15]. Previously, several studies have suggested that the therapeutic level of Se reduces the chances of several diseases, including oxidative stress, muscular dystrophy, cardiovascular disease, cystic fibrosis, and arthritis [16]. Selenium is extensively used as a feed supplement due to its direct connection with immunity and tumorigenesis [17, 18].
Due to the structural role as a co-factor in selenoproteins, Se performs key physiological functions or roles in reproduction, antioxidant defense system, metabolism of thyroid hormone, anti-apoptosis, and muscle functioning and development [19,20,21]. Owing to its major role in thyroid metabolism, optimum levels of Se is also required for body development and metabolism in growing animals [22]. Moreover, major physiological effects of Se are attributed to the functional role of selenoenzymes to mediate oxidative stress for normal cellular homeostasis and functioning. Studies have shown that alleviation of oxidative stress through improving the antioxidant system directly improves the reproductive physiology in different farm animals [23, 24]. So the dietary supplementation of Se is envisaged as crucial for the maintenance of normal reproductive physiology and fertility in dairy animals. The effects of Se supplementation in dairy cattle depend on physiological status (fertility, the incidence of mastitis, etc.) [25, 26], Se status of animals [27], Se type and content, and Se administration types [28]. Generally, two different forms (inorganic and organic) of Se are used, and both have different bioavailabilities in the gastrointestinal tract. Many previous studies have shown that Se yeast has higher bioavailability and is associated with increased milk and blood Se content [29,30,31] with better antioxidant status [32, 33]. As a trace element, Se is used in very minute quantity in animal diets, but issues like low bioavailability, antagonism, and higher excretion from intestine limit its efficacy. Nanotechnology offers the opportunity to mediate these issues as bioavailability can be increased by enhancing the surface area of the Se by making its nanoparticles [34].
Keeping in view the importance of Se in health and production of dairy animals, the present review aims to provide insights into the key physiological role of Se and the potential benefits of its supplementation in dairy animals. Moreover, the comparative efficiency of different forms of Se is discussed with a special focus on nano-selenium (nano-Se).
History of Selenium Discovery
The discovery and research on Se started in August 1817. Selenium was first isolated from Falun pyrite when the Swedish chemist Jacob Berzelius discovered a new element in 1818. Berzelius chose the name selenium (Greek: Selene, moon) for this new element [35]. The first report on the deficiency of Se in animals appeared in 1957 when deficient animals (in Se and vitamin E) showed white muscle disease in ruminants [36]. Other deficiency diseases like skeletal and cardiac muscle necrosis were observed by Smith et al. [37]. In 1973, Rotruck and his team [38] demonstrated the biochemical role of Se as a component of GSH-Px enzyme which functions as an antioxidant to protect cells against oxidative damage. In 1979, the Food and Drugs Administration (FDA) approved Se as a feed additive for dairy cattle, initially at an added level of 0.1 mg/kg of DM [39], and later on (since April 1987), at a concentration of 0.3 mg/kg of DM [40], which is equivalent to 0.7 mg/ewe/day [41]. In 2006, the European Commission authorized the use of Se yeast as a feed additive within the European Union [42]. Today we know that Se, as a trace element, has a wide range of important functions for humans (medicine), animals, and plants (biology) along with various industrial applications (in agriculture and environment).
Sources of Selenium
The major dietary sources of Se include inorganic and organic Se. The most common form of dietary Se includes inorganic Se salts, e.g., Na-selenate (NaSeO4) and Na-selenite (NaSeO3) which is usually provided in mineral premixes, injected or orally given in bolus forms. The chemical form of Se affects the absorption of Se, as sodium selenite (SS) when injected subcutaneously showed more rapid absorbance than barium selenate. Moreover, inorganic salts are considered crucial to achieving optimum levels of Se in dietary supplements [43]. However, inorganic Se sources show poor bioavailability and antagonism with other minerals rendering in poor efficacy. Therefore, organic Se sources such as Se-enriched yeast, e.g., Saccharomyces cerevisiae, which contains Se amino acids (seleno-AA) such as hydroxy-selenomethionine (HMSeBA), selenomethionine (Se-Met), and selenocysteine (Se-Cys) in abundance (Fig. 1) [33, 44, 45]. Selenium is also found in animal fodders, but their Se contents vary with type, texture, and organic matter contents of the soil along with the extent of precipitation in the area. Recently, the nano form of Se is attracting the attention of nutritionists owing to its comparative advantages over conventional sources of Se. Nano-technology utilizes matter at its dimensions from 1 to 100 nm with unique characteristics and novel applications. Such magnificent technologies have the potential to expand the bioavailability measures and other nutritional assessments. Bioavailability can be enhanced by increasing the surface area of respective minerals by making their nanoparticles [34]. It is one of the most innovative technologies to produce materials and different elements with change in structure, texture, and enhanced quality at the molecular level. This technology is contributing significantly in production, processing, storage, transportation, traceability, security, and safety of food [46]. Studies have shown that nano-Se has higher bioavailability, catalytic activity, enhanced adsorbing capability, lower acute toxicity, and no antagonistic effects in the gut [47, 48].
Absorption, Bioavailability, and Metabolism of Selenium in Ruminants
Absorption
Absorption in ruminants is through a rumen wall or in the small intestine. Most of the Se absorption site in both ruminants and non-ruminants cases is the duodenum. However, absorption of Se is different in the rumen and the intestine. Generally, the rumen receives Se in selenite form in the diet, while most of the selenate is reduced to selenite in the rumen before absorption, and part of the selenate bypasses from the rumen to the small intestine and absorbed as selenate. Another proposed mechanism of absorption in the rumen suggests that 30–40% of selenite is converted to low molecular weight insoluble forms of Se [49]. Moreover, 10–15% of the selenite is used to synthesize seleno-AA (predominantly Se-Cys) that is incorporated into the microbial protein by substitution of Se with a sulfur atom in the cysteine residue of the protein. However, 40–60% of the selenite leaves the rumen to the intestine where it is absorbed probably via a passive diffusion [50]. Studies have suggested that the true absorption of Se in the case of dietary inorganic form averages about 50% in dairy cows, goats, and sheep [51, 52]. Generally, the extent of absorption of selenite is quite low in ruminants (only 29%) as compared with monogastric animals and poultry (about 80–90%).
Several in vitro studies have suggested that Se-enriched yeast contains 90% of the Se in the form of Se-Met, and about 55% of which bypasses the rumen. The digestibility of Se from Se-enriched yeast is reported to be about 66%, so considering the digestibility of Se-Met as 80% (as average digestibility of ruminal microbial protein), it is about 30% higher than the true digestibility of Se from selenite. Many studies have reported this fact that absorption and bioavailability of organic Se are substantially higher than those of inorganic Se in ruminants [44, 53,54,55].
Numerous dietary factors affect the absorption of Se in ruminants owing to the antagonistic effects of other nutrients. The diets rich in carbohydrates or some salts such as nitrates, sulfates, calcium, arsenic, vitamin C, mercury, or hydrogen cyanide (clover, flax seeds) negatively influence the digestibility of Se in ruminants [56]. However, dietary proteins, vitamin E, and vitamin A have shown to enhance Se absorption.
Bioavailability
As mentioned above, the availability of Se-Met (organic Se) is greater than that of selenite (inorganic Se). Further, Se-Met is rapidly incorporated into proteins. The inorganic Se is almost exclusively used to produce selenoenzymes, whereas organic Se can be used to produce both selenoenzymes and generally all proteins containing Met (Fig. 2). The only issue with organic forms of Se is their higher costs than inorganic Se sources.
Metabolism
The main utilization of dietary Se is its incorporation into selenoenzymes which constitute the cellular antioxidant system in the cytosol. Metabolic fates of dietary inorganic and organic Se forms differ, as organic Se (including dietary selenocysteine) must first be converted to the inorganic selenide (hydrogen selenide) before the process of forming and incorporating specific selenocysteine into the active site of one of the selenoproteins [57]. Selenate is reduced to selenite, and selenite is reduced via formation of selenodiglutathione to selenide [58, 59]. The putative mechanism of Se metabolism is presented in Fig. 3. Dietary Se sources (organic and inorganic) effectively provide two separate pools of Se in the body. Se-Met (organic) is incorporated into all proteins directly as a substitute for Met (nonspecifically) and reflects its concentration in the diet. While inorganic Se is only incorporated into a specific amino acid (cysteine) in the selenoproteins [60], excess absorbed Se is excreted as trimethylselenonium or exhaled as dimethyl selenide [61]. Studies have reported the putative metabolism and absorption of Se and its potential role in the immune system [62]. Ruminants generally excrete extra Se through urine and feces [63]. The intake of Se in the form of inorganic salts usually is utilized directly into the synthesis of selenoproteins and exhibits a short half-life as the excess amount is directly excreted and not stored in the body [64]. In contrast, Se in its organic Se forms like Se-Met and Se-Cys is efficiently stored in different tissues, thus preventing the deficiency of Se and increasing GSH-Px activity through mediating redox status and oxidative stress [65].
Dietary Requirements of Selenium in Ruminants
Selenium is beneficial within a narrow dose range for all farm animal species [66]. The daily requirements of Se depend on animal species (cows, buffaloes, camels, sheep, and goats), age of the animal (calves, kids, lambs, and adults), physiological stage (lactating or pregnant), and performance trait (meat or milk). Generally, the nutritional requirement of Se is met through animal diets supplemented with mineral salts and different sources and forms of Se [56]. The nutritional requirements of Se for animals are presented in Table 1. Generally, NRC [2] recommends the Se allowance for many livestock species to be about 0.1–0.3 mg/kg DM depending upon their growth performance.
Deficiency and Toxicity of Selenium
The optimum level of dietary Se is required to maintain normal animal physiology; however, an excess or lower dietary Se can lead to toxicity or deficiency, respectively, with adverse consequences. The excessive amounts of Se can be very toxic to animals [69]. Different factors affecting Se toxicity usually include diet, sex, animal species, and chemical form of Se. It is suggested that inorganic Se is three times more toxic than organic Se [58, 70, 71]. In 1930, the first report on Se toxicity was received as hair loss, and lameness was observed in animals that grazed on forage with high Se levels. The signs of Se toxicity usually start to appear at Se levels of 5 to 8 mg/kg DM. The different forms of Se toxicity include chronic selenosis (alkali disease) and acute selenosis (blind staggers) in animals [72]. However, Schöne et al. [73] reported that Se toxicity is not considered as a life threat for animals or humans.
Generally, Se deficiency is observed in both animals and humans, when the dietary intake of Se is less than 0.05 mg/kg DM [74]. Selenium deficiency may affect the reproductive efficiency (retained placenta, abortions, early embryonic death, and infertility), growth performance (white muscle disease), and skeletal and cardiac muscle necrosis (stiffness and lameness) [49, 75]. Deficiency of Se can also affect the udder health, particularly mammary glands health, clinical and subclinical mastitis, and elevated somatic cell counts (SSC) [76]. Forages from Se-deprived soils can cause oxidative stress and pose a serious threat to the immune function of animals [75]. Additionally, the extent of Se toxicity and deficiency in animals vary in different animal species maintained on different forage, soil, and water resources [72, 77, 78]. It is generally accepted that Se deficiencies could be avoided by feeding animals with forages and diets containing sulfur amino acids.
Dietary Supplementation of Se to Improve the Performance of Dairy Animals
As mentioned above, Se plays a significant role in cellular redox and antioxidant defense systems to maintain normal physiology. Ensuring a normal balance of ROS and antioxidant enzymes leads to normal animal physiology resulting in optimum productive and reproductive performance (Fig. 4). The major benefits of Se in dairy animals are summarized as below.
Putative mechanism of action of Se to improve the rumen fermentation. Se has the potential to increase the antioxidant status of ruminal microbiome, subsequently leading to enhanced rumen fermentation and microbial growth. Microbial GSH-Px activity and Se concentration of rumen mucosa were also increased with the provision of dietary Se [79]. Besides, dietary Se supplementation can also stimulate digestive enzyme secretion and increase the GSH-Px activity of duodenal mucosa [80, 81]. Ruminal microbes utilize dietary Se to synthesize protein and cell wall constituents [82]. Fibrolytic bacteria concentration and degradation of structural carbohydrates were increased due to low rumen pH in response to Se supplementation [83]. Microbes in the rumen utilize different nutrients (like ammonia N, carbon-skeleton, and ATP) to synthesize microbial protein [84]. Enhanced microbial protein synthesis in response to Se supplementation may be attributed to the increased total VFA production and rumen bacterial populations together with lower ammonia-N.
Effects on Gestation, Colostrum, and Newborn
Selenium supplementation in dairy animals generally affects thyroid hormone metabolism, redox-active proteins to regulate anti-oxidative status, and immune functions in dairy cows [85, 86]. Several studies have reported the negative relationship between Se concentration in blood and that in milk. The last 4 weeks of the gestation period in cows are very critical regarding Se availability, as Se is transported to the developing fetus via the placenta even when the dietary supply of Se is not optimum [49]. In pregnant female camel, the Se serum concentration was significantly higher post-partum than in the prepartum period [87]. It is attributed to the fact that Se levels (renal) in the fetus remain unchanged as compared with their dams [88].
Dietary supplementation of Se before parturition resulted in twofold higher Se levels (170 vs 87 μg/L) in the colostrum of dairy cows [89], while threefold higher in camels as compared with their unsupplemented counterparts [87]. Moreover, supplementation of organic Se (Se yeast) exhibited higher average Se levels in the colostrum (67 vs 35 μg/L) as compared with SS [28]. However, contrary findings were observed in Holstein cows, as no significant difference in Se contents of colostrum was observed in response to supplementation of both organic and inorganic sources of Se [27].
Selenium is transported from mother to fetus through the placenta, and after parturition, through colostrum and milk in ruminants. However, Se transfer through the placenta is more efficient than that found in milk because less quantity of Se is transferred from the blood into the milk [90, 91]. Hence, it is crucial to maintain adequate Se status in calves. Moreover, a dietary source also affects the extent of Se transfer from the blood to the milk. Many studies have reported better beneficial effects of dietary supplementation and efficiency of organic Se as compared with inorganic Se in calves [91, 92]. Studies have also shown that maternal dietary Se (Se-enriched yeast) resulted in higher birth weight and growth performance in Taihang black goats and male kids [66]. Recently, Żarczyńska et al. [93] reported that suckling dairy calves receiving intramuscular injections of 0.5 mg of Se/mL and 50 mg of vitamin E/mL) showed better Se status and improved passive immunity than control. Similarly, dietary supplementation of a slow-release bolus (consisting of Zn, Se, and Co) in ewes 6 weeks prepartum revealed desirable effects on the antioxidant status of the dam and growth of lambs [94]. It substantially increased serum alkaline phosphatase and GSH-Px activity and plasma concentrations of Zn, Se, and vitamin B12 in ewes and their lambs. Furthermore, ewes receiving Se bolus have shown better birth weights and average daily gain of lambs at weaning [95].
Dietary supplementation of organic Se has shown to improve the immune response of suckling dairy calves [96]. Similarly, feeding of Se-enriched alfalfa hay in beef calves improved immunity against J-5 Escherichia coli bacterin [97]. Moreover, dietary Se also improved serum Se levels (382.7 ± 107.6 vs 176.3 ± 18.0 ng/mL) of female camel calves as compared to their un-supplemented counterparts [87]. Dietary supplementation of HMSeBA in pregnant heifers improved the Se status of both the dam and calf compared with the SS [98]. Moreover, Se content of the maternal diet has shown to enhance testicular development and testosterone synthesis in goats kids through up regulating the testosterone-related genes [99]. Optimum serum Se levels are required to ensure proper immune response leading to normal health and growth performance in animals. For Se-deficient animals, injectable Se preparations can be used to readily establish Se status in calves, but it is usually temporary management, and other Se enriched supplements should be focused to sustain dietary Se intake [49].
Effects on Milk Yield, Composition, and Milk Se Contents
Milk and milk products are considered a good source of micronutrients, including macro- and microminerals, that play important functions to ensure proper growth and health in humans [3]. For example, milk and its products contribute more than 4% of the total dietary Se consumed by humans in Belgium [100]. Owing to the direct association of dietary Se with milk Se contents, the level of Se in milk is considered as an easy and non-invasive way to evaluate herd Se status [49]. The Se contents of fodder/forages/feeds usually depend on the season and agro-ecological conditions which can subsequently influence the Se contents in milk. According to the FDA, bovine milk should contain an average Se level of about 0.14 mg/L [41]. However, milk Se contents of less than 0.12 μmol/L indicate Se deficiency, while milk Se > 0.28 μmol/L shows adequate Se levels. Moreover, milk Se contents between 0.12 and 0.28 μmol/L are considered marginal [101]. Many studies have been carried out in different regions of the world to document Se contents of milk in dairy cows which widely varied such as it was 30 g/kg in Belgium, 60 g/kg in South Korea, and 22 g/kg in Greece [5, 100, 102].
The amount of Se in plasma is three- to fivefold higher than in milk Se. Ivanis and Weiss [52] observed a positive correlation between plasma and milk Se content (y = 0.37x − 4.2, with x = plasma Se (μg/L) and y = milk Se (μg/L) content). Generally, seleno-AA are transported to liver and other tissues such as muscle and the mammary gland through systemic circulation. Mammary gland cells can incorporate Se-Met in place of Met during casein synthesis [92]. Various studies have shown that Se administration has the ability to increase serum albumin, insulin, and total protein contents in dairy cows [103,104,105]. Selenium supplementation can also potentially affect thyroid hormone metabolism and redox-active proteins to regulate anti-oxidative status and immune functions in dairy cows [85, 86]. The effect of dietary supplementation of Se on milk yield and composition has shown variable results. Several studies reported that milk yield and components did not change under a different conditions such as type of animal, parity, age, stage of lactation, and Se source (organic and inorganic) [28, 106,107,108]. Contrarily, Moeini et al. [109] reported that Se supplementation at 0.5 mg/kg DM significantly increased daily milk production during the first 8 weeks of lactation in Holstein cows. Similarly, dietary supplementation of Se and vitamin E increased milk protein, non-fat solids, and lactose contents [25]. Supplementation of HMSeBA in Holstein dairy cows under heat stress conditions tended to increase the milk yield and decrease the fat percentage as compared with SS [110].
Dietary Se has no direct influence on lactogenesis, but it can indirectly affect milk production through diverse pathways particularly under stress conditions. The increase in milk yield in response to Se supplementation may be attributed to enhanced energy availability in lieu of alleviation of oxidative stress and/or maintaining redox homeostasis, because, both eliciting an antioxidant response and repairing free radical-induced damages require energy, which can be saved by maintaining redox balance with appropriate Se plasma levels [111]. In addition, milk yield increase might be attributed to the reduced free radical-induced damage within the mammary gland which can likely enhance milk synthesis [112].
Milk is a terminal pool for Se-Met as the mammary gland can extract an immense quantity of Met to produce milk proteins. Regarding milk fat, supplementation of organic Se increased the milk fat percentage (4.15% vs 3.96%) compared with the inorganic form in dairy cows [113]. This increase may be due to the lower enzymatic action of lipases of leukocytes resulting from lower SCC [114]. Neutrophils exhibited better ability against mastitis-causing pathogens when organic Se was supplied to animals [115]. It has also been reported that cows with lower incidence of mastitis exhibit higher milk fat percentage [116]. On the other hand, a recent study reported that supplementation of HMSeBA in cows tended to decrease the milk fat percentage compared with SS [110]. A possible underlying mechanism might be a negative relationship observed between blood Se and milk fat contents for herds at first season of Se supplementation [82], as increased Se might upregulate oxidative reactions within the tricarboxylic cycle and fatty acid [117]. However, Wang et al. [118] did not observe any negative relationship between blood Se and milk fat contents. Possibly, these contrasting results might be due to differences in the composition of the diet, dose of Se, and stage of lactation of dairy cows. However, these putative mechanism and contrary findings require further investigations.
Many studies have reported the effect of Se supplementation on milk Se contents. Generally, it is reported that milk whey contains a higher proportion (56.6%) of Se contents as compared with non-whey milk fat phase (10.1%) in dairy cows [119]. This is mainly attributed to the fact that 55–75% of milk Se is incorporated into casein, while 17–38% is contained in the whey and 7% in milk fat [120]. A survey of Se contents in China revealed average Se contents in milk (0.13–0.44 μg/L), feed samples (0.11–0.38 μg/kg), and drinking water (0.09–0.29 μg/L) of dairy cows [3]. In camel, milk Se contents varied from 39.5 to 482.6 ng/mL [121].
Dietary supplementation of inorganic and organic Se in dairy cows showed an improvement in milk Se contents of up to 32.8 and 41.2%, respectively [32]. Similarly, milk Se contents linearly increased as 0.04, 0.31, 0.52, and 0.78 μg/mL in goats fed in response to 0, 0.5, 2, and 4 mg Se/kg, respectively [66]. Moreover, Bagnicka et al. [107] reported an increase of over 300% in milk Se concentration in response to dietary supplementation of organic Se as compared with that of inorganic Se. Moreover, dietary Se also improved Se levels in the serum (305.9 ± 103.3 vs 109.3 ± 33.1 ng/mL) and camel milk (167.1 ± 97.3 vs 86.4 ± 39.1 ng/mL) as compared with unsupplemented groups [121]. The HMSeBA as an organic Se source has the potential to increase milk and plasma Se content and milk fat percentage with similar milk production parameters compared with SS [33].
Recently, Li et al. [122] reported that feeding HMSeBA (up to 0.5 mg/kg of DM) linearly increased Se content in milk and plasma and milk/plasma Se ratio in dairy cattle. Additionally, milk production, FCM, ECM, protein content, and lactose content of milk also increased in response to supplementation of HMSeBA. A comprehensive review has highlighted the effectiveness of organic Se to improve Se status in dairy animals as compared with SS or selenite [123]. Recently, Barbe et al. [124] evaluated the comparative efficacy of three levels (0.1, 0.2, or 0.3 ppm) of different sources of organic Se including (S2)-2-amino-4-methylselenylbutanoic acid (SM1) or R,S-2-hydroxy-4-methylselenobutanoic acid (SM2) and Saccharomyces cerevisiae R397 (SY) in dairy cattle. They reported that feed intake, milk yield, and milk composition were similar among different sources of organic Se. However, milk Se content was higher in the SY group at a higher level (0.3 ppm), while serum Se and Se bioavailability were higher at 0.3 ppm for all Se sources. In addition, supplementation of coated SS has been shown to increase milk yield, milk fat, and protein contents together with feed efficiency and digestibility of DM and OM in dairy cows [125]. The Se yeast (0.3 mg Se/kg) has shown a quite reasonable increase in milk production (up to 24.8%) than the control group [126]. These findings revealed that dietary supplementation of Se can increase serum and milk Se contents, but effects on milk production of dairy animals are not consistent. Moreover, organic Se is more effective in the enrichment of milk with Se in dairy animals.
Effects on Rumen Microbiome, Rumen Fermentation, and Feed Digestibility
The Se has the potential to increase the antioxidant status of ruminal microbiome subsequently leading to enhanced rumen fermentation and microbial growth. Microbial GSH-Px activity and Se concentration of rumen mucosa were also increased with the provision of dietary Se [79]. Besides, dietary Se supplementation can also stimulate digestive enzyme secretion and increase the GSH-Px activity of duodenal mucosa [80, 81]. Ruminal microbes utilize dietary Se to synthesize protein and cell wall constituents [82]. Fibrolytic bacteria concentration and degradation of structural carbohydrates were increased due to low rumen pH in response to Se supplementation [83]. Microbes in the rumen utilize different nutrients (like ammonia N, carbon-skeleton, and ATP) to synthesize microbial protein [84]. Enhanced microbial protein synthesis in response to Se supplementation may be attributed to the increased total VFA production and rumen bacterial populations together with lower ammonia-N. The putative mechanism of action of Se to modulate rumen microbiome, rumen fermentation, and feed digestion is presented in Fig. 4.
Dietary supplementation of Se has also shown to affect rumen microbiome, rumen fermentation, and feed digestibility (Table 2). Recently, supplementation of HMSeBA has been shown to increase the nutrient digestibly (CP, NDF, and ADF), ruminal propionate concentration, and Se bioavailability compared with SS [127]. A comparison of simple and coated SS showed better DM intake, ADG, and feed efficiency in dairy bulls supplemented with protected SS [17]. Nutrient digestibility (DM, OM, and CP) was also improved in the coated SS group; however, EE, ADF, and NDF digestibilities were similar in both Se groups. An increased ruminal total volatile fatty acids (VFA) and propionate concentration with lower rumen pH, acetate, and acetate to propionate ratio (A:P) concentration were observed in coated SS group. The improvement in nutrient digestibility and animal performance in response to dietary Se supplementation is likely a consequence of the enhanced antioxidant status of ruminal microbes [80]. Moreover, supplementation of coated SS has shown to increase milk production, the ruminal activity of pectinase and α-amylase, concentration of ruminal total VFA, bacteria (Ruminococcus flavefaciens and Butyrivibrio fibrisolvens), protozoa, and fungi population in dairy cows [128]. Similarly, Zhang et al. [125] reported that both SS or coated SS substantially enhanced the activities of cellobiase, carboxymethylcellulase, xylanase, and protease while increasing the population of total bacteria, Ruminococcus flavefaciens, Fibrobacter succinogenes, Ruminococcus amylophilus, and total fungi in dairy cows. Similarly, dietary SS (at 0.3 mg/kg DM) improved xylanase, pectinase, α-amylase, and protease activities as well as total tract digestibility of CP. Notably, populations of total bacteria (R. albus, R. flavefaciens, Fibrobacter succinogenes, and Butyrivibrio fibrisolvens), protozoa, and methanogens as well as urinary total purine derivatives excretion were also enhanced [132]. Lipid microencapsulation of SS enables Se to escape from complete rumen reduction and subsequently more efficiently incorporated into milk than its free form [147].
Similar to protected SS, organic Se has shown more pronounced effects on the promotion of specific microbes in the rumen as compared with SS. Supplementation of Se yeast enhanced the population of rumen protozoa, Ophryoscolex caudatus f. tricoronatus (up to 136%) and Diploplastron population (up to 63%), in sheep as compared with SS. However, relative abundance of Dasytricha ruminantium and Polyplastron multivesiculatum was similar in both groups [131]. Wang et al. [118] also reported an increase in milk yield, fat-corrected milk (4%), ruminal total VFA concentration, and apparent total tract nutrient digestibility in response to Se yeast supplementation in dairy cows. These findings reveal that both protected SS and organic Se can positively influence rumen microbiome, rumen fermentation parameters, and nutrient digestibility leading to overall improvement in performance of dairy animals.
Effects on Blood Metabolites
The blood metabolites are considered as a quick and effective tool to evaluate the health status of dairy herds. The serum is highly correlated to dietary intake of Se within 2 to 6 days after supplementation in diets [148, 149]. The Se in the blood is associated with different proteins including globulins (α and β), lipoproteins (both low- and high-density lipoproteins), and albumin [58]. Ten Se atoms as Se-Cys are included in the selenoprotein-P which is a selenoprotein supplier of Se to different body tissues. Studies have reported that the adequate Se level in the blood of cattle should be above 0.18 μg/mL in whole blood and 0.08 μg/mL in plasma. These concentrations could be achieved through dietary supplementation of Se at 0.3–0.5 mg/kg DM of diet in lactating cows [2, 54, 150].
Dietary supplementation of Se in goats has been shown to significantly affect the serum biochemical profiles in as serum ALT, total protein, globulin, total cholesterol, and glucose of kids improved in response to maternal dietary Se supplementation [66]. Recently, Liu et al. [17] reported that coated SS supplementation in dairy bulls have better blood glucose (29.1 vs 24.1 nmol/L), total protein (73.5 vs 68.5 g/L), and albumin (39.8 vs 34.6 g/L) contents compared with SS and control group; however, blood Se (0.17 μg/mL), insulin (29.4 mIU/L), and total cholesterol levels (8.58 mmol/L) were similar in both Se groups. Contrarily, supplementation of Se-Met in ewe’s diet showed no effect on serum urea, cholesterol, total protein, albumin, A:G ratio, AST, and ALT, while decreasing serum glucose. Moreover, Se supplementation improved serum T3 and T4 levels and their ratio in dairy cows [151]. Similar results regarding serum ALT and AST in camel was observed [28]. Contrarily, higher serum cholesterol and α-tocopherol/cholesterol ratios at calving were observed when dairy cows were fed diet supplemented with organic Se at 48 h compared with the control [97]. Supplementation of Se-Met has been shown to increase serum total protein and albumin concentrations in cows [105]. Moreover, dietary supplementation of Se in male buffalo calves exhibited no effects on serum glucose, total protein, urea, and creatinine contents; however, serum globulins significantly increased while decreasing albumin and A:G ratio [152]. Moreover, the level of different serum enzymes (ALT and AST) and hormones (T3, T4, testosterone, and insulin) did not differ among Se-supplemented and control groups.
Effect on Immune Response and Antioxidant Status
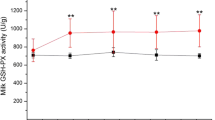
The major effects of Se in dairy animals are attributed to the diverse functions mediated by selenoproteins. Cellular redox system and antioxidant defense of the body necessarily rely on selenoenzymes (GSH-Px, TrxR) and selenoprotein P, so optimum dietary Se is required to ensure proper supply of Se-Cys and Se-Met for the synthesis of selenoproteins. Dietary supplementation of Se has been conceived as a potential strategy to improve the immune response and alleviate metabolic/oxidative burden in dairy animals particularly during stress and disease periods (Table 3). Many studies have reported significant effects of dietary Se sources and their levels on antioxidant and immune status in dairy cattle with a major focus on GSH-Px activity in the blood [32, 62, 167]. Many studies involving dietary supplementation of Se in the diets of calves, heifers, fattening bulls, dairy cows, camels, buffaloes, goats, ewes, kids, and lambs reported increased Se contents and GPx activity in the blood [87, 107, 151, 168]. Recently, Wang et al. [169] revealed a positive correlation of serum Se level with GSH-Px activity and a negative correlation with SCC and Interleukin 6. They showed that the risk of subclinical mastitis increases when serum GSH-Px is less than 148 U/L and Interleukin 6 is less than 451 ng/mL in dairy cattle.
The ROS are naturally produced during cellular metabolic processes and are neutralized by the antioxidant defense system of the body. In stressful conditions, ROS overproduction negatively affects animal performance, health, and well-being [170]. The enhanced Se concentration or GSH-Px activity in the plasma/serum of dairy cows during early lactation (as compared with dry and peak lactation) has revealed the importance of Se to alleviate oxidative stress state in early lactation [167]. The comparative effect of coated SS on plasma GSH-Px activity (189 vs 161 U/mL) was substantially higher than that of SS in dairy cattle bulls [17]. Organic Se is considered a potential source for alleviating oxidative stress compared with its inorganic form, as Se-Met has 1.4 times higher blood GSH-Px activity than SS [171]. Recently, Sun et al. [154] reported that organic sources of Se (HMSeBA and Se-Met) resulted in higher GSH-Px activity and mRNA abundance of GPX3 with decreased SOD and ROS activities compared with SS in bovine mammary epithelial cells. However, HMSeBA and Se-Met have similar effects on bovine mammary epithelial cells. Gong and Xiao, [161] have shown a positive effect of the inclusion of Se yeast in the diets of dairy cows as increased plasma Se content and antioxidant status while reduced oxidative stress during early lactation. Improvement in blood parameters (RBC, WBC, and hemoglobin) has also been observed in response to the supplementation of HMSeBA in early lactating cows. However, neutrophils, lymphocytes, and monocytes were unaffected [122]. Higher levels of WBC and RBC indicate improved immunity status of animals.
Many studies reported significant effects of Se supplementation on the immune system of calves [96]. Dietary supplementation of SS and organic Se increased the levels of serum immunoglobulins (Ig) and interleukin (IL)-1 in dairy cows [32]. However, dietary Se sources have shown no effects on the neutrophil function of cows, serum immunoglobulins (IgM and IgG) of growing heifers, and serum IL-1 and IL-2 contents in sheep [54, 55, 172]. These studies provided convincing evidence that dietary supplementation can potentially modulate an immune response at various physiological stages in different animals.
Effect on Reproductive Physiology
Reproductive performance is one of the key determinants of livestock profitability and is significantly affected by dietary trace minerals. Most of the reproductive disorders such as retained placenta, metritis, ovarian cysts, embryonic death during the first month of gestation, and malfunction of reproductive organs are generally caused by microbial infections or metabolic disturbances. The metabolic disorders can be effectively improved by adequately meeting requirements of essential nutrients especially trace minerals in animal diets [173, 174]. The role of Se in animal health is essentially based on maintaining normal physiological functions mediated by Se-Cys containing proteins that effectively constitute the body’s antioxidant defense [175,176,177]. Oxidative stress adversely affects the cellular and tissue redox system leading to poor reproductive functions. Studies have shown that alleviation of oxidative stress through improving the antioxidant system directly improves reproductive physiology in different farm animals [23, 24].
Dietary supplementation of Se in dairy animals has been shown to exhibit variable effects on reproduction (Table 4). However, many studies have provided convincing evidence that stated insufficient Se intake can predispose cows and calves to subclinical diseases, resulting in poor animal performance [11]. Moreover, dietary supplementation of Se can decrease infertility ratio, reduce the incidence of early embryonic mortality, endometritis, and ovarian cysts during the postpartum period [15, 106]. The decrease in the incidence of infertility in response to Se supplementation may be attributed to the reduction of embryonic death during the first month of gestation. Notably, GPx-1 increases in granulosa cells as a consequence of enhanced expression of selenoproteins in large healthy follicles [186]. Se supplementation can improve the success rate to the first service in Se-deficient dairy cows [187]. Moreover, nano-Se has shown an effective potential to promote the development of secondary follicles [188]. Besides, Se can also decrease the incidence of metritis and ovarian cysts during the postpartum period [189]. Studies have reported a substantial decrease in the frequency of retained placenta in dairy cows supplemented with Se [26]. Besides females, deficiency of Se has also been associated with male infertility together with adverse effects on testosterone and spermatozoa synthesis [190]. Furthermore, the morphology of testes, as well as scrotal length and circumference, was improved as a result of Se supplementation [185]. Recently, Lizarraga et al. [184] reported that the treatment of cumulus-oocyte complexes with Se increased cumulus cell viability and hatching rate in cattle. These studies provide practical insights into the potential role of Se in male and female reproductive health and fertility ratio. It is revealed that optimum dietary Se is required to ensure proper reproductive functions and reduce the incidence of calving-related disorders in dairy animals.
Effect on Udder Health
Somatic cell count is considered as a primary indicator of udder health, mastitis, and milk quality in the dairy herd. The milk somatic cells usually constitute leukocytes and mammary epithelial cells [191]. The SSC results during inflammatory response and damage to mammary gland epithelium during mastitis as an immune response to mastitis-causing pathogens consequently leading to a decrease in milk synthesis [192, 193]. The role of Se in the health of dairy cows has been clearly demonstrated during the incidence of mastitis. Many studies conducted during the last two decades have reported a positive association of Se deficiency in dairy cows with a poor immune response (revealed by intracellular neutrophils), higher milk SCC, and lower resistance to clinical and subclinical mastitis during early lactation [108]. In this context, it is suggested that Se deficiency can reduce GPx1 activity, cause lower normal bactericidal capacity of bovine neutrophils, and inhibit lymphocyte (leucocytes in the udder) proliferation leading to the poor immune response in bovines [194]. Dietary supplementation of Se yeast in dairy goats has been shown to reduce the SSC while increasing αS2-casein and bactenecin 7.5 in milk SCC [156]. However, no expression of bactenecin 5, β2-defensin, hepcidin, chemokine 4, tumor necrosis factor α, toll-like receptor 2, cathelicidin-7, and cathelicidin-6 genes was detected in somatic cells.
Stress periods like the post-partum period following parturition result in depletion of antioxidant enzymes leading to compromised immune status owing to the downregulation of mRNA expression of genes related to antioxidant defense mechanisms. This immunosuppression makes the animal more susceptible to oxidative stress and infections [195, 196]. The Se deficiency might mediate a slow influx of polymorphonuclear cells into affected quarters or decrease the ability to kill pathogens causing mastitis [191]. However, optimum Se induces a high production of chemotoxins by macrophages stimulated with opsonized Staphylococcus aureus to mediate infection [108].
The milk SCC and the incidence of clinical mastitis were significantly decreased in response to dietary Se supplementation at 8 weeks of lactation in dairy cows [106, 108, 109]. An intramammary infection (IMI) is common in high-producing dairy cows. An udder quarter is considered to have an IMI at calving when it harbors ≥ 1 CFU/0.05 mL (equivalent to ≥ 20 CFU/mL) of a pathogen like Staphylococci [197]. Staphylococci species, particularly Staphylococcus aureus, are the major etiological agents of ruminant IMI. The Staphylococcus aureus with coagulase negative species (CNS) is the most frequent isolate from subclinical and clinical cases of IMI. Studies have reported that analyses of the quarter samples confirmed coagulase positive species, CNS, bacteria Streptococcus uberis, and Streptococcus agalactiae, which are most often associated with the incidence of subacute and acute mastitis. However, IMI due to non-aureus staphylococci has shown slight elevation in milk SCC after calving, which did not exhibit any adverse effects on subsequent milk production [198]. Non-aureus staphylococci are a heterogeneous group having more than 50 species and subspecies [199]. In dairy herds, clinical IMI may have an incidence at a rate of lower than 5%; however, it can go up to 30–50% in small herds, causing mortality (gangrenous mastitis) or culling of ≥ 70% of the herd [108]. Recently Wang et al. [169] reported that serum Se level was negatively correlated with milk SCC in dairy cattle. In addition, Se has also shown cytoprotective functions as Se-Met attenuated the extended spectrum of β-lactamase E. coli–induced inflammation through activated selenoprotein S-mediated TLR4/NF-κB signaling pathway in bovine mammary epithelial cell and macrophages [200]. However, the mechanism of inhibition of NF-κB expression due to Se-Met is unclear and needs further investigations. The putative role of Se in inflammatory signaling pathways highlights its potential beneficial effects in addition to the antioxidant role, particularly in IMI and udder immune status. Therefore, it is essentially required to manage optimum Se status in dairy herds to sustain the milk performance while ensuring better udder health and lower SCC.
Future Prospects of Nano-Se in Dairy Animals
The transfer of inorganic dietary Se into milk is in a very minute quantity due to its poor bioavailability. Therefore, we need to use better bioavailable Se sources to increase milk Se content to address daily Se requirements of humans [201, 202]. Although organic sources of Se (Se yeast, Se-Met, and HMSeBA) are considered as efficient bioavailable sources compared with inorganic Se, nowadays, nano-Se is getting more attention due to its multiple health benefits compared with inorganic and organic Se sources for use in dairy animals [203]. The comparative advantage and efficacy of nanoparticles stem from their smaller particle size and larger surface area, enhanced mucosal permeability, and higher intestinal absorption as a result of nano-emulsion formation. In addition to enhanced bioavailability, nano-Se has also reduced toxicity and lower antagonism with other minerals compared with other sources of Se [204]. Moreover, higher bioavailability and better utilization of nano-Se shows a desirable effect on body metabolism while reducing their excretion in the environment [205].
The inclusion of nano-Se in sheep diet has been shown to improve the total VFA and propionate concentration while reducing ruminal pH, NH3 nitrogen, and acetate to propionate ratio [130]. Dietary nano-Se has been shown to increase the HDL concentration (37.33 vs 30.37 mg/dL), while decreasing LDL (25.53 vs 32.27 mg/dL) in lambs. Additionally, the concentrations of T4 (1.59 vs 1.23 nmol/L) and GPx (447.39 vs 239.24 kat/Lμ) were also increased [206]. Recently, dietary nano-Se enhanced the antioxidant status in dairy cows by elevating plasma Se and GSH-Px activity together with stimulation of gene expression in the mammary gland of cows compared with SS. The total milk Se concentration and mammary gland mRNA expression levels of GSH-Px 1, 2, and 4; thioredoxin reductase 2 and 3; and selenoproteins W, T, K, and F were also improved with nano-Se [153]. However, milk yield and composition were similar in both groups (SS and nano-Se).
Oral treatment with nano-Se restored plasma Se and serum antioxidant indices in affected deer (P. picticaudata) after treating orally with nano-Se [207]. Oral nano-Se also has been shown to promote growth rate and also in thiobarbituric acid reactive substances, serum Se, copper and zinc levels in neonatal (1 month) lambs [140]. Recently, Shahin et al. [208] reported a positive effect of the inclusion of Se nanoparticles to semen extender for improving the cryotolerance of camel epididymal spermatozoa. They reported that Se nanoparticles could improve the progressive motility, sperm membrane integrity, and vitality and decrease the apoptosis of sperm when frozen and thawed. This positive effect may be a consequence of nanosize leading to increased bioavailability and subsequently reducing undesired liberation of toxic substances (particularly ROS). Nano-Se has also shown potential effects on cryopreservation of sperm, semen purification, and semen preservation processes [209]. Recently, Khalil et al. [210] reported that nano-Se (10 μg/mL) added in semen extender not only improved post-thaw sperm quality of bull, but also enhanced the in vivo fertility rate by reducing apoptosis, lipid peroxidatio,n and sperm damage during cryopreservation. Similarly, nano-Se at 0.5 and 1 μg/mL concentrations in extender exhibited positive effects on motility, acrosome protection, and preservation of sperm membrane integrity [211]. However, there is a need to investigate Se concentrations, combinations of various novel cryoprotectants, and validation of post-thaw sperm fertility [212]. Owing to higher bioavailability and enhanced surface area, nano-Se has most promising future applications to harvest benefits from better antioxidant and immune response in dairy animals. Further investigations are required to explore optimum levels of nano-Se for dietary supplementation at different physiological stages for different dairy species. Moreover, further research work is required to elucidate the putative mechanism of action of nanoparticles of Se particularly regarding modulation of different signaling pathways.
Future Implications
Based on our findings, we recommend Se at 0.3–0.5 mg/kg DM (300–500 μg/kg DM) of the diet in dairy cattle. The exact dose of Se supplementation in dairy animals depends on the physiological stage, Se status of animals, type and content of Se, and mode of administration. Dietary supplementation of Se has been shown to improve the ruminal VFA concentration, nutrients digestion, and utilization. Selenoproteins, not only function as antioxidant enzymes, but also influence thyroid hormone metabolism, redox signaling, and regulation of immune responses. Organic and nano-Se had exhibited more bioavailability than inorganic Se sources. Organic Se supplementation in the dairy animal’s diets has shown to increase the whole blood and milk Se contents of dairy cows. Nano-Se is less toxic and more bioavailable and possesses less antagonism with other trace elements in the GI tract. However, nano-Se has shown more promising effects regarding the enrichment of milk and meat Se contents, subsequently leading to enhanced antioxidant status and improved shelf life. Besides, it also opens a horizon for the development of a variety of functional foods for humans with effective health-promoting potential. However, further investigations are warranted to explore the optimum level of supplementation of nano-Se, and its direct comparisons are required at equitable intakes of organic and inorganic Se sources in dairy animals.
References
Fisher GE (2008) Micronutrients and animal nutrition and the link between the application of micronutrients to crops and animal health. Turk J Agric For 32(3):221–233
NRC I (2001) Nutrient requirements of dairy cattle. National Research Council
Zhou X, Qu X, Zhao S, Wang J, Li S, Zheng N (2017) Analysis of 22 elements in milk, feed, and water of dairy cow, goat, and buffalo from different regions of China. Biol Trace Elem Res 176(1):120–129
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Pappa EC, Pappas AC, Surai PF (2006) Selenium content in selected foods from the Greek market and estimation of the daily intake. Sci Total Environ 372(1):100–108
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta (BBA) Gen Subj 1830(5):3289–3303
Holmgren A (1989) Thioredoxin and glutaredoxin systems. J Biol Chem 264(24):13963–13966
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54:237–271
Rhee SG, Chae HZ, Kim K (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38(12):1543–1552
Lundström J, Holmgren A (1990) Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J Biol Chem 265(16):9114–9120
Tórtora-Pérez J (2010) The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res 89(2-3):185–192
Rundlöf A-K, Arnér ES (2004) Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid Redox Signal 6(1):41–52
Sarkar B, Bhattacharjee S, Daware A, Tribedi P, Krishnani K, Minhas P (2015) Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res Lett 10(1):371
Back TG (2013) Investigations of new types of glutathione peroxidase mimetics. In: Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium. ACS Publications, pp 143-162
Qazi IH, Angel C, Yang H, Zoidis E, Pan B, Wu Z, Ming Z, Zeng C-J, Meng Q, Han H (2019) Role of selenium and selenoproteins in male reproductive function: a review of past and present evidences. Antioxidants 8(8):268
Weekley CM, Harris HH (2013) Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev 42(23):8870–8894
Liu Y, Zhang Z, Dai S, Wang Y, Tian X, Zhao J, Wang C, Liu Q, Guo G, Huo W (2020) Effects of sodium selenite and coated sodium selenite addition on performance, ruminal fermentation, nutrient digestibility and hepatic gene expression related to lipid metabolism in dairy bulls. Livest Sci:104062
Masukawa T (1987) Pharmacological and toxicological aspects of inorganic and organic selenium compounds. Org Selenium Tellurium Compd 2:377–392
Arthur JR, Beckett GJ (1999) Thyroid function. Br Med Bull 55(3):658–668
Brown KM, Arthur J (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4(2b):593–599
Rayman MP (2005) Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc 64(4):527–542
Jensen C, Pallauf J (2008) Estimation of the selenium requirement of growing guinea pigs (Cavia porcellus). J Anim Physiol Anim Nutr 92(4):481–491
Marin-Guzman J, Mahan D, Pate J (2000) Effect of dietary selenium and vitamin E on spermatogenic development in boars. J Anim Sci 78(6):1537–1543
Kendall N, McMullen S, Green A, Rodway R (2000) The effect of a zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim Reprod Sci 62(4):277–283
Juan Eulogio GL, Jorge Alberto SO, Vazquez Hugo C, Nunez Antonio C, Alejandro C-I, Quiroz Juan M (2012) Effects of the selenium and vitamin E in the production, physicochemical composition and somatic cell count in milk of Ayrshire cows. J Anim Vet Adv 11(5):119–123
Spears JW, Weiss WP (2008) Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J 176(1):70–76
Karkoodi K, Chamani M, Beheshti M, Mirghaffari SS, Azarfar A (2012) Effect of organic zinc, manganese, copper, and selenium chelates on colostrum production and reproductive and lameness indices in adequately supplemented Holstein cows. Biol Trace Elem Res 146(1):42–46
Salman S, Dinse D, Khol-Parisini A, Schafft H, Lahrssen-Wiederholt M, Schreiner M, Scharek-Tedin L, Zentek J (2013) Colostrum and milk selenium, antioxidative capacity and immune status of dairy cows fed sodium selenite or selenium yeast. Arch Anim Nutr 67(1):48–61
Briens M, Mercier Y, Rouffineau F, Mercerand F, Geraert P-A (2014) 2-Hydroxy-4-methylselenobutanoic acid induces additional tissue selenium enrichment in broiler chickens compared with other selenium sources. Poult Sci 93(1):85–93
Givens DI, Allison R, Cottrill B, Blake JS (2004) Enhancing the selenium content of bovine milk through alteration of the form and concentration of selenium in the diet of the dairy cow. J Sci Food Agric 84(8):811–817
Walker G, Dunshea F, Heard J, Stockdale C, Doyle P (2010) Output of selenium in milk, urine, and feces is proportional to selenium intake in dairy cows fed a total mixed ration supplemented with selenium yeast. J Dairy Sci 93(10):4644–4650
Gong J, Ni L, Wang D, Shi B, Yan S (2014) Effect of dietary organic selenium on milk selenium concentration and antioxidant and immune status in midlactation dairy cows. Livest Sci 170:84–90
Sun P, Wang J, Liu W, Bu D, Liu S, Zhang K (2017) Hydroxy-selenomethionine: a novel organic selenium source that improves antioxidant status and selenium concentrations in milk and plasma of mid-lactation dairy cows. J Dairy Sci 100(12):9602–9610
Hassan S, Hassan F-u, Rehman MS-u (2020) Nano-particles of trace minerals in poultry nutrition: Potential applications and future prospects. Biol Trace Elem Res 195(2):591–612
Berzelius JJ (1826) On selenium crystals and the preparation of selenium. Ann Phys 7:242–243
Muth O, Oldfield J, Remmert L, Schubert J (1958) Effects of selenium and vitamin E on white muscle disease. Science 128(3331):1090–1090
Smith HA, Jones TC, Hunt RD (1972) Veterinary pathology-4
Rotruck JT, Pope AL, Ganther HE, Swanson A, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590
FDA F Drug Administration (1979) In: Conference on mycotoxins in animal feeds and grains related to animal health. Rockville
FDA U (1987) General principles of validation, Rockville, MD. Center for Drug Evaluation and Research (CDER)
Food U, Administration D (2009) Qualified health claim petition-selenium and a reduced risk of site-specific cancers (FDA-2008-Q-0323). Silver Spring: US Food and Drug Administration
Authority EFS (2006) Opinion of the panel on additives and products or substances used in animal feed (FEEDAP) on the safety and efficacy of the product Sel-Plex 2000 as a feed additive according to Regulation (EC) No 1831/2003. EFSA J 4(5):348
Revilla-Vázquez A, Ramírez-Bribiesca E, López-Arellano R, Hernández-Calva LM, Tórtora-Pérez J, García-García E, Cruz Monterrosa RG (2008) Suplemento de selenio con bolos intrarruminales de selenito de sodio en ovinos. Agrociencia 42(6):629–635
Schrauzer GN (2003) The nutritional significance, metabolism and toxicology of selenomethionine. Adv Food Nutr Res 47:73–112
Stewart W, Bobe G, Pirelli G, Mosher W, Hall J (2012) Organic and inorganic selenium: III. Ewe and progeny performance. J Anim Sci 90(12):4536–4543
Mahmoud UT (2012) Silver nanoparticles in poultry production. J Adv Vet Res 2(4):303–306
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42(10):1524–1533
Zhang J, Wang X, Xu T (2008) Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci 101(1):22–31
Mehdi Y, Dufrasne I (2016) Selenium in cattle: a review. Molecules 21(4):545
Serra A, Nakamura K, Matsui T, Harumoto T, Fujihara T (1994) Inorganic selenium for sheep I. Selenium balance and selenium levels in the different ruminal fluid fractions. Asian Australas J Anim Sci 7(1):83–89
Koenig K, Rode L, Cohen R, Buckley W (1997) Effects of diet and chemical form of selenium on selenium metabolism in sheep. J Anim Sci 75(3):817–827
Ivancic J Jr, Weiss W (2001) Effect of dietary sulfur and selenium concentrations on selenium balance of lactating Holstein cows. J Dairy Sci 84(1):225–232
Weiss WP (2003) Selenium nutrition of dairy cows: comparing responses to organic and inorganic selenium forms. In: Nutritional Biotechnology in the Feed and Food Industries. Proceedings of Alltech’s 19th Annual Symposium. Nottingham University Press. Nottingham. pp 333-343
Weiss WP, Hogan JS (2005) Effect of selenium source on selenium status, neutrophil function, and response to intramammary endotoxin challenge of dairy cows. J Dairy Sci 88(12):4366–4374
Qin S, Gao J, Huang K (2007) Effects of different selenium sources on tissue selenium concentrations, blood GSH-Px activities and plasma interleukin levels in finishing lambs. Biol Trace Elem Res 116(1):91–102
Kessler J (1993) Carence en sélénium chez les ruminants: Mesures prophylactiques. Rev Suisse Agric 25:21–26
Daniels LA (1996) Selenium metabolism and bioavailability. Biol Trace Elem Res 54(3):185–199
Schrauzer GN (2000) Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr 130(7):1653–1656
Foster L, Sumar S (1997) Selenium in health and disease: a review. Crit Rev Food Sci Nutr 37(3):211–228
Burk RF (1991) Molecular biology of selenium with implications for its metabolism 1. FASEB J 5(9):2274–2279
Janghorbani M, Rockway S, Mooers CS, Roberts EM, Ting BT, Sitrin MD (1990) Effect of chronic selenite supplementation on selenium excretion and organ accumulation in rats. J Nutr 120(3):274–279
Sordillo L (2016) Nutritional strategies to optimize dairy cattle immunity. J Dairy Sci 99(6):4967–4982
Leng L, Boldižárova K, Faix Š, Kováč G (2000) The urinary excretion of selenium in sheep treated with a vasopressin analogue. Vet Res 31(5):499–505
Levander OA, DeLoach DP, Morris VC, Moser PB (1983) Platelet glutathione peroxidase activity as an index of selenium status in rats. J Nutr 113(1):55–63
Dalto DB, Roy M, Audet I, Palin M-F, Guay F, Lapointe J, Matte JJ (2015) Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidative stress induced by oestrus in pubertal pig. J Trace Elem Med Biol 32:21–29
Shi L, Ren Y, Zhang C, Yue W, Lei F (2017) Effects of maternal dietary selenium (Se-enriched yeast) on growth performance, antioxidant status and haemato-biochemical parameters of their male kids in Taihang black goats. Anim Feed Sci Technol 231:67–75
Rutigliano HM (2006) Effects of source of supplemental Se and method of presynchronization on health, immune responses, reproductive efficiency, uterine health, lactation performance of high producing dairy cows. University of California, Davis
Zhang L, Liu X, Liu J, An X, Zhou Z, Cao B, Song Y (2018) Supplemented organic and inorganic selenium affects milk performance and selenium concentration in milk and tissues in the Guanzhong dairy goat. Biol Trace Elem Res 183(2):254–260
Lemly AD (2002) Symptoms and implications of selenium toxicity in fish: the Belews Lake case example. Aquat Toxicol 57(1-2):39–49
Arthur JR, McKenzie RC, Beckett GJ (2003) Selenium in the immune system. J Nutr 133(5):1457S–1459S
Lyons M, Papazyan T, Surai P (2007) Selenium in food chain and animal nutrition: lessons from nature-review. Asian Australas J Anim Sci 20(7):1135–1155
Zarczynska K, Sobiech P, Radwinska J, Rekawek W (2013) Effects of selenium on animal health. J Elem 18(2)
Schöne F, Steinhöfel O, Weigel K, Bergmann H, Herzog E, Dunkel S, Kirmse R, Leiterer M (2013) Selenium in feedstuffs and rations for dairy cows including a view of the food chain up to the consumer. J Verbr Lebensm 8(4):271–280
Claude J (2002) Introduction à la nutrition des animaux domestiques. Tec & Doc/EM Inter: Paris
Huo B, Wu T, Song C, Shen X (2020) Studies of selenium deficiency in the Wumeng semi-fine wool sheep. Biol Trace Elem Res 194(1):152–158
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Malevu TD, Sochor J, Baron M, Melcova M, Zidkova J (2017) A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int J Mol Sci 18(10):2209
Khanal DR, Knight AP (2010) Selenium: its role in livestock health and productivity. J Agric Environ 11:101–106
Terry N, Zayed A, De Souza M, Tarun A (2000) Selenium in higher plants. Annu Rev Plant Biol 51(1):401–432
Hidiroglou M, Heaney DP, Jenkins K (1968) Metabolism of inorganic selenium in rumen bacteria. Can J Physiol Pharmacol 46(2):229–232
Čobanová K, Faix Š, Plachá I, Mihaliková K, Váradyová Z, Kišidayová S, Grešáková Ľ (2017) Effects of different dietary selenium sources on antioxidant status and blood phagocytic activity in sheep. Biol Trace Elem Res 175(2):339–346
McDonald P, Edwards R, Greenhalgh J, Morgan C, Sinclair L, Wilkinson R (2011) Animal Nutrition 7th edition England UK. Pearson Education Limited
Fraser A, Ryan T, Sproule R, Clark R, Anderson D, Pederson E (1987) The effect of selenium supplementation on milk production in dairy cattle. In: Proc NZ Soc Anim Prod. pp 61-64
Wales W, Kolver E, Thorne P, Egan A (2004) Diurnal variation in ruminal pH on the digestibility of highly digestible perennial ryegrass during continuous culture fermentation. J Dairy Sci 87(6):1864–1871
Reynolds C, Kristensen NB (2008) Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis. J Anim Sci 86(suppl_14):E293–E305
McKenzie RC, Arthur JR, Beckett GJ (2002) Selenium and the regulation of cell signaling, growth, and survival: molecular and mechanistic aspects. Antioxid Redox Signal 4(2):339–351
Salman S, Khol-Parisini A, Schafft H, Lahrssen-Wiederholt M, Hulan H, Dinse D, Zentek J (2009) The role of dietary selenium in bovine mammary gland health and immune function. Anim Health Res Rev 10(1):21–34
Seboussi R, Faye B, Alhadrami G, Askar M, Ibrahim W, Mahjoub B, Hassan K, Moustafa T, Elkhouly A (2010) Selenium distribution in camel blood and organs after different level of dietary selenium supplementation. Biol Trace Elem Res 133(1):34–50
House WA, Bell AW (1994) Sulfur and selenium accretion in the gravid uterus during late gestation in Holstein cows. J Dairy Sci 77(7):1860–1869
Rowntree J, Hill G, Hawkins D, Link J, Rincker M, Bednar G, Kreft R Jr (2004) Effect of Se on selenoprotein activity and thyroid hormone metabolism in beef and dairy cows and calves. J Anim Sci 82(10):2995–3005
Enjalbert F, Lebreton P, Salat O, Schelcher F (1999) Effects of pre-or postpartum selenium supplementation on selenium status in beef cows and their calves. J Anim Sci 77(1):223–229
Pehrson B, Ortman K, Madjid N, Trafikowska U (1999) The influence of dietary selenium as selenium yeast or sodium selenite on the concentration of selenium in the milk of suckler cows and on the selenium status of their calves. J Anim Sci 77(12):3371–3376
Muñiz-Naveiro Ó, Domínguez-González R, Bermejo-Barrera A, Cocho de Juan JA, Fraga Bermúdez JM, Goris Pereiras A, López Santamariña A, Martínez Lede I, Valledor Puente J, Fernández-Couto Gómez L (2005) Selenium content and distribution in cow’s milk supplemented with two dietary selenium sources. J Agric Food Chem 53(25):9817–9822
Żarczyńska K, Samardžija M, Sobiech P (2019) Influence of selenium administration to dry cows on selected biochemical and immune parameters of their offspring. Reprod Domest Anim 54(9):1284–1290
Aliarabi H, Fadayifar A, Alimohamady R, Dezfoulian AH (2019) The effect of maternal supplementation of zinc, selenium, and cobalt as slow-release ruminal bolus in late pregnancy on some blood metabolites and performance of ewes and their lambs. Biol Trace Elem Res 187(2):403–410
Zarbalizadeh-Saed A, Seifdavati J, Abdi-Benemar H, Salem AZ, Barbabosa-Pliego A, Camacho-Diaz LM, Fadayifar A, Seyed-Sharifi R (2019) Effect of slow-release pellets of selenium and iodine on performance and some blood metabolites of pregnant Moghani ewes and their lambs. Biol Trace Elem Res:1–11
Salles MSV, Zanetti MA, Junior LCR, Salles FA, Azzolini AECS, Soares EM, Faccioli LH, Valim YML (2014) Performance and immune response of suckling calves fed organic selenium. Anim Feed Sci Technol 188:28–35
Hall JA, Bobe G, Vorachek WR, Gorman ME, Mosher WD, Pirelli GJ (2013) Effects of feeding selenium-enriched alfalfa hay on immunity and health of weaned beef calves. Biol Trace Elem Res 156(1-3):96–110
Juniper DT, Rymer C, Briens M (2019) Bioefficacy of hydroxy-selenomethionine as a selenium supplement in pregnant dairy heifers and on the selenium status of their calves. J Dairy Sci 102(8):7000–7010
Shi L, Song R, Yao X, Duan Y, Ren Y, Zhang C, Yue W, Lei F (2018) Effects of maternal dietary selenium (Se-enriched yeast) on testis development, testosterone level and testicular steroidogenesis-related gene expression of their male kids in Taihang Black Goats. Theriogenology 114:95–102
Waegeneers N, Thiry C, De Temmerman L, Ruttens A (2013) Predicted dietary intake of selenium by the general adult population in Belgium. Food Addit Contam Part A 30(2):278–285
Wichtel JJ, Keefe GP, Van Leeuwen JA, Spangler E, McNiven MA, Ogilvie TH (2004) The selenium status of dairy herds in Prince Edward Island. Can Vet J 45(2):124
Choi Y, Kim J, Lee H-S, Kim C-i, Hwang IK, Park HK, Oh C-H (2009) Selenium content in representative Korean foods. J Food Compos Anal 22(2):117–122
Zahrazadeh M, Riasi A, Farhangfar H, Mahyari SA (2018) Effects of close-up body condition score and selenium-vitamin E injection on lactation performance, blood metabolites, and oxidative status in high-producing dairy cows. J Dairy Sci 101(11):10495–10504
Ibrahim E (2017) Effect of parenteral supplementation of vitamin E plus selenium on nutrient digestibility, productive performance and some serum biochemical indicators of lambs. Egypt J Sheep Goats Sci 12(1):1–12
Khalili M, Chamani M, Amanlou H, Nikkhah A, Sadeghi AA (2019) Effects of different sources of selenium supplementation on antioxidant indices, biochemical parameters, thyroid hormones and Se status in transition cows. Acta Sci Anim Sci 41:44392
Machado V, Bicalho M, Pereira R, Caixeta L, Knauer W, Oikonomou G, Gilbert R, Bicalho R (2013) Effect of an injectable trace mineral supplement containing selenium, copper, zinc, and manganese on the health and production of lactating Holstein cows. Vet J 197(2):451–456
Bagnicka E, Kościuczuk EM, Jarczak J, Jóźwik A, Strzałkowska N, Słoniewska D, Krzyżewski J (2017) The effect of inorganic and organic selenium added to diets on milk yield, milk chemical and mineral composition and the blood serum metabolic profile of dairy cows. Anim Sci Paper Rep 35(1):17–33
Vasil M, Zigo F, Elečko J, Zigová M, Farkašová Z (2017) Effect of peroral supplementation with selenium and vitamin E during late pregnancy on udder health and milk quality in dairy cows. Potravinárstvo Slovak J Food Sci 11(1):535–538
Moeini M, Karami H, Mikaeili E (2009) Effect of selenium and vitamin E supplementation during the late pregnancy on reproductive indices and milk production in heifers. Anim Reprod Sci 114(1-3):109–114
Sun L, Gao S, Wang K, Xu J, Sanz-Fernandez M, Baumgard L, Bu D (2019) Effects of source on bioavailability of selenium, antioxidant status, and performance in lactating dairy cows during oxidative stress-inducing conditions. J Dairy Sci 102(1):311–319
Pedernera M, Celi P, García SC, Salvin HE, Barchia I, Fulkerson WJ (2010) Effect of diet, energy balance and milk production on oxidative stress in early-lactating dairy cows grazing pasture. Vet J 186(3):352–357
Miller R, Paape M, Fulton L, Schutz MM (1993) The relationship of milk somatic cell count to milk yields for Holstein heifers after first calving. J Dairy Sci 76(3):728–733
Oltramari CE, Pinheiro MG, de Miranda MS, Arcaro JR, Castelani L, Toledo LM, Ambrósio LA, Leme PR, Manella MQ, Júnior IA (2014) Selenium sources in the diet of dairy cows and their effects on milk production and quality, on udder health and on physiological indicators of heat stress. Ital J Anim Sci 13(1):2921
Juniper DT, Phipps RH, Jones AK, Bertin G (2006) Selenium supplementation of lactating dairy cows: effect on selenium concentration in blood, milk, urine, and feces. J Dairy Sci 89(9):3544–3551
Ibeagha A, Ibeagha-Awemu E, Mehrzad J, Baurhoo B, Kgwatalala P, Zhao X (2009) The effect of selenium sources and supplementation on neutrophil functions in dairy cows. Animal 3(7):1037–1043
Duncan S, Christen G, Penfield M (1991) Rancid flavor of milk: relationship of acid degree value, free fatty acids, and sensory perception. J Food Sci 56(2):394–397
Pehrson B (1993) Selenium in nutrition with special reference to the biopotency of organic and inorganic selenium compounds. In: Proceedings the 9th Alltech Symposium, Biotechnology in the Feed Industry (ed. PT Lyons). pp 71-89
Wang C, Liu Q, Yang W, Dong Q, Yang X, He D, Zhang P, Dong K, Huang Y (2009) Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest Sci 126(1-3):239–244
Muniz-Naveiro O, Domínguez-González R, Bermejo-Barrera A, Cocho JA, Fraga JM, Bermejo-Barrera P (2005) Determination of total selenium and selenium distribution in the milk phases in commercial cow’s milk by HG-AAS. Anal Bioanal Chem 381(6):1145–1151
Van Dael P, Vlaemynck G, Van Renterghem R, Deelstra H (1991) Selenium content of cow’s milk and its distribution in protein fractions. Z Lebensm Unters Forsch 192(5):422–426
Seboussi R, Faye B, Askar M, Hassan K, Alhadrami G (2009) Effect of selenium supplementation on blood status and milk, urine, and fecal excretion in pregnant and lactating camel. Biol Trace Elem Res 128(1):45–61
Li Y, Liu J, Xiong J, Wang Y, Zhang W, Wang D (2019) Effect of hydroxyselenomethionine on lactation performance, blood profiles, and transfer efficiency in early-lactating dairy cows. J Dairy Sci 102(7):6167–6173
Surai PF, Kochish II, Fisinin VI, Juniper DT (2019) Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals 9(7):462
Barbé F, Chevaux E, Castex M, Elcoso G, Bach A (2020) Comparison of selenium bioavailability in milk and serum in dairy cows fed different sources of organic selenium. Anim Prod Sci 60(2):269–276
Zhang Z, Wang C, Du H, Liu Q, Guo G, Huo W, Zhang J, Zhang Y, Pei C, Zhang S (2020) Effects of sodium selenite and coated sodium selenite on lactation performance, total tract nutrient digestion and rumen fermentation in Holstein dairy cows. Animal:1–9
Ullah H, Khan RU, Mobashar M, Ahmad S, Sajid A, Khan NU, Usman T, Khattak I, Khan H (2019) Effect of yeast-based selenium on blood progesterone, metabolites and milk yield in Achai dairy cows. Ital J Anim Sci 18(1):1445–1450
Wei J, Wang J, Liu W, Zhang K, Sun P (2019) Effects of different selenium supplements on rumen fermentation and apparent nutrient and selenium digestibility of mid-lactation dairy cows. J Dairy Sci 102(4):3131–3135
Du HS, Wang C, Wu ZZ, Zhang GW, Liu Q, Guo G, Huo WJ, Zhang YL, Pei CX, Zhang SL (2019) Effects of rumen-protected folic acid and rumen-protected sodium selenite supplementation on lactation performance, nutrient digestion, ruminal fermentation and blood metabolites in dairy cows. J Sci Food Agric 99(13):5826–5833
Del Razo-Rodriguez O, Ramirez-Bribiesca J, Lopez-Arellano R, Revilla-Vazquez A, Gonzalez-Munoz S, Cobos-Peralta M, Hernandez-Calva L, McDowell L (2013) Effects of dietary level of selenium and grain on digestive metabolism in lambs. Czech J Anim Sci 58(6):253–261
Shi L, Xun W, Yue W, Zhang C, Ren Y, Shi L, Wang Q, Yang R, Lei F (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin Res 96(1):49–52
Mihaliková K, Boldižárová K, Faix Š, Kišidayová S (2005) The effects of organic selenium supplementation on the rumen ciliate population in sheep. Folia Microbiol 50(4):353–356
Zhang G, Wang C, Du H, Wu Z, Liu Q, Guo G, Huo W, Zhang J, Zhang Y, Pei C (2020) Effects of folic acid and sodium selenite on growth performance, nutrient digestion, ruminal fermentation and urinary excretion of purine derivatives in Holstein dairy calves. Livest Sci 231:103884
Ramadan S, Mahboub HDH, Helal MAY, Sallam MA (2018) Effect of vitamin E and selenium on performance and productivity of goats. Int J Chem Biomed Sci 4(2):16–22
Shahid AB, Malhi M, Soomro SA, Shah MG, Kalhoro NH, Kaka A, Mal R, Soomro MA, Samo SP, Sanjrani MN (2020) Influence of dietary selenium yeast supplementation on fermentation pattern, papillae morphology and antioxidant status in rumen of goat. Pakistan J Zool 52(2):565
Silva J, Rodriguez F, Trettel M, Abal R, Lima C, Yoshikawa C, Zanetti M (2020) Performance, carcass characteristics and meat quality of Nellore cattle supplemented with supranutritional doses of sodium selenite or selenium-enriched yeast. Animal 14(1):215–222
Dhari EA, Kassim WY (2019) Effect of adding selenium with or without vitamin E and combination of them on some of productive and physiological characteristics of Awassi lambs. Basrah J Agric Sci 32(2):115–125
Ibrahim E, Mohamed M (2018) Effect of different dietary selenium sources supplementation on nutrient digestibility, productive performance and some serum biochemical indices in sheep. Egypt J Nutr Feeds 21(1):53–64
Wang Z, Tan Y, Cui X, Chang S, Xiao X, Yan T, Wang H, Hou F (2019) Effect of different levels of selenium yeast on the antioxidant status, nutrient digestibility, selenium balances and nitrogen metabolism of Tibetan sheep in the Qinghai-Tibetan Plateau. Small Rumin Res 180:63–69
Yaghmaie P, Ramin A, Asri-Rezaei S, Zamani A (2017) Evaluation of glutathion peroxidase activity, trace minerals and weight gain following administration of selenium compounds in lambs. In: Veterinary Research Forum. vol 2. Faculty of Veterinary Medicine, Urmia University, Urmia, p 133
Kojouri G, Arbabi F, Mohebbi A (2019) The effects of selenium nanoparticles (SeNPs) on oxidant and antioxidant activities and neonatal lamb weight gain pattern. Comp Clin Pathol:1–6
de Paiva Ferreira AV, Cominotte A, Ladeira MM, Casagrande DR, Teixeira PD, van Cleef E, Ezequiel J, Castagnino P, Neto ORM (2020) Feedlot diets with soybean oil, selenium and vitamin E alters rumen metabolism and fatty acids content in steers. Anim Feed Sci Technol 260:114362
Rashnoo M, Rahmati Z, Azarfar A, Fadayifar A (2020) The effects of maternal supplementation of selenium and iodine via slow-release blouses in late pregnancy on milk production of goats and performance of their kids. Ital J Anim Sci 19(1):502–513
Saba FE, Saleh A, Al Moafy A (2019) Effect of supplementation with different types of selenium on lactation performance and some blood parameters of Farafra and Saidi ewes and performance of their lambs. Egypt J Sheep Goats Sci 14(2):19–30
Faixová Z, Piešová E, Maková Z, Čobanová K, Faix Š (2016) Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on ruminal enzyme activities and blood chemistry in sheep. Acta Vet Brno 85(2):185–194
Bao K, Wang X, Wang K, Yang Y, Li G (2019) Effects of dietary supplementation with selenium and vitamin E on growth performance, nutrient apparent digestibility and blood parameters in female Sika deer (Cervus nippon). Biol Trace Elem Res:1–7
Rossi CS, Compiani R, Baldi G, Muraro M, Marden J, Rossi R, Pastorelli G, Corino C, Dell’Orto V (2017) Organic selenium supplementation improves growth parameters, immune and antioxidant status of newly received beef cattle. J Anim Feed Sci 26(2):100–108
Grilli E, Gallo A, Fustini M, Fantinati P, Piva A (2013) Microencapsulated sodium selenite supplementation in dairy cows: effects on selenium status. Animal 7(12):1944–1949
Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, Patterson KY, Holden JM, Morris JS (1996) Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 7:384–390
Ellis R, Herdt T, Stowe H (1997) Physical, hematologic, biochemical, and immunologic effects of supranutritional supplementation with dietary selenium in Holstein cows. Am J Vet Res 58(7):760–764
Dargatz D, Ross P (1996) Blood selenium concentrations in cows and heifers on 253 cow-calf operations in 18 states. J Anim Sci 74(12):2891–2895
Mousaie A, Valizadeh R, Naserian AA, Heidarpour M, Mehrjerdi HK (2014) Impacts of feeding selenium-methionine and chromium-methionine on performance, serum components, antioxidant status, and physiological responses to transportation stress of baluchi ewe lambs. Biol Trace Elem Res 162(1-3):113–123
Mudgal V, Garg AK, Dass RS, Varshney VP (2008) Effect of selenium and copper supplementation on blood metabolic profile in male buffalo (Bubalus bubalis) calves. Biol Trace Elem Res 121(1):31–38
Han L, Pang K, Fu T, Phillips CJ, Gao T (2020) Nano-selenium supplementation increases selenoprotein (Sel) gene expression profiles and milk selenium concentration in lactating dairy cows. Biol Trace Elem Res:1–7
Sun L, Wang F, Wu Z, Ma L, Baumrucker C, Bu D (2020) Comparison of selenium source in preventing oxidative stress in bovine mammary epithelial cells. Animals 10(5):842
Novoselec J, Šperanda M, Klir Ž, Mioč B, Steiner Z, Antunović Z (2017) Blood biochemical indicators and concentration of thyroid hormones in heavily pregnant and lactating ewes depending on selenium supplementation. Acta Vet Brno 86(4):353–363
Reczyńska D, Witek B, Jarczak J, Czopowicz M, Mickiewicz M, Kaba J, Zwierzchowski L, Bagnicka E (2019) The impact of organic vs. inorganic selenium on dairy goat productivity and expression of selected genes in milk somatic cells. J Dairy Res 86(1):48–54
De K, Sahoo A, Shekhawat I, Kumawat P, Kumar D, Naqvi S (2017) Effect of selenium-yeast feeding on amelioration of simulated heat stress and reproductive performance in Malpura ewe under semi-arid tropical environment. Indian J Anim Sci 87(2):163–167
Abbasi B, Malhi M, Ali Siyal F, Arain M, Bhutto Z, Soomro S, Rui R (2018) Influence of dietary selenium yeast on fermentation pattern mucosal growth and glutathione peroxidase (gsh-px) activity in colon of goat. J Dairy Vet Anim Res 6:253–259
Suganthi R, Ghosh J, Malik P, Awachat V, Krishnamoorthy P, Pal D, Nongkhlaw S (2019) Effect of dietary organic selenium (Se) on immune response, hepatic antioxidant status, selenoprotein gene expression and meat oxidative stability in lambs. J Anim Feed Sci 28(2):138–148
Jaaf S, Batty B, Krueger A, Estill CT, Bionaz M (2020) Selenium biofortified alfalfa hay fed in low quantities improves selenium status and glutathione peroxidase activity in transition dairy cows and their calves. J Dairy Res:1–7
Gong J, Xiao M (2018) Effect of organic selenium supplementation on selenium status, oxidative stress, and antioxidant status in selenium-adequate dairy cows during the periparturient period. Biol Trace Elem Res 186(2):430–440
Erdoğan S, Karadaş F, Yılmaz A, Karaca S (2017) The effect of organic selenium in feeding of ewes in late pregnancy on selenium transfer to progeny. Rev Bras Zootec 46(2):147–155
Nedelkov K, Chen X, Martins C, Melgar A, Harper M, Räisänen S, Oh J, Felix T, Wall E, Hristov A (2020) Alternative selenium supplement for sheep. Anim Feed Sci Technol 261:114390
Saadi A, Dalir-Naghadeh B, Asri-Rezaei S, Anassori E (2020) Platelet selenium indices as useful diagnostic surrogate for assessment of selenium status in lambs: an experimental comparative study on the efficacy of sodium selenite vs. selenium nanoparticles. Biol Trace Elem Res 194(2):401–409
Kachuee R, Abdi-Benemar H, Mansoori Y, Sánchez-Aparicio P, Seifdavati J, Elghandour MM, Guillén RJ, Salem AZ (2019) Effects of sodium selenite, L-selenomethionine, and selenium nanoparticles during late pregnancy on selenium, zinc, copper, and iron concentrations in Khalkhali Goats and their kids. Biol Trace Elem Res 191(2):389–402
Biazus AH, Cazarotto CJ, Machado G, Bottari NB, Alves MS, Morsch VM, Schetinger MR, Leal ML, Fernandes NF, Moresco RN (2019) Diphenyl diselenide subcutaneous supplementation of dairy sheep: effects on oxidant and antioxidant status, inflammatory response and milk composition. Anim Prod Sci 59(3):461–470
Gong J, Xiao M (2016) Selenium and antioxidant status in dairy cows at different stages of lactation. Biol Trace Elem Res 171(1):89–93
Chorfi Y, Girard V, Fournier A, Couture Y (2011) Effect of subcutaneous selenium injection and supplementary selenium source on blood selenium and glutathione peroxidase in feedlot heifers. Can Vet J 52(10):1089
Wang D, Jia D, He R, Lian S, Wang J, Wu R (2020) Association between serum selenium level and subclinical mastitis in dairy cattle. Biol Trace Elem Res 198:1–8
Chauhan SS, Celi P, Ponnampalam EN, Leury BJ, Liu F, Dunshea FR (2014) Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/milk) quality: role of vitamin E and selenium. Anim Prod Sci 54(10):1525–1536
Malbe M, Klaassen M, Fang W, Myllys V, Vikerpuur M, Nyholm K, Sankari S, Suoranta K, Sandholm M (1995) Comparisons of selenite and selenium yeast feed supplements on Se-incorporation, mastitis and leucocyte function in Se-deficient dairy cows. J Veterinary Med Ser A 42(1-10):111–121
Kincaid R, Cronrath D (2001) Source of dietary selenium on tissue retention and mobilization of selenium in growing heifers. J Anim Sci 79(Suppl 1):87(Abs)
Kafilzadeh F, Kheirmanesh H, Karami Shabankareh H, Targhibi MR, Maleki E, Ebrahimi M, Yong Meng G (2014) Comparing the effect of oral supplementation of vitamin E, injective vitamin e and selenium or both during late pregnancy on production and reproductive performance and immune function of dairy cows and calves. Sci World J 2014:1–5
Horký P (2015) Effect of selenium on its content in milk and performance of dairy cows in ecological farming. Potravinarstvo Slovak J Food Sci 9(1):324–329
Fairweather-Tait SJ, Collings R, Hurst R (2010) Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr 91(5):1484S–1491S
Meyer U, Heerdegen K, Schenkel H, Dänicke S, Flachowsky G (2014) Influence of various selenium sources on selenium concentration in the milk of dairy cows. J Verbr Lebensm 9(2):101–109
Qazi IH, Angel C, Yang H, Pan B, Zoidis E, Zeng C-J, Han H, Zhou G-B (2018) Selenium, selenoproteins, and female reproduction: a review. Molecules 23(12):3053
Palani ZM, Ahmed KA, Saeed EA, Hama KM, Rasheed AM, Hamid BS (2019) Effect of selenium or zinc feed supplementation on some physiological characters in blood of Kurdi male lambs. J Anim Poult Prod 10(4):95–98
Farahavar A, Rostami Z, Alipour D, Ahmadi A (2020) The effect of pre-breeding vitamin E and selenium injection on reproductive performance, antioxidant status, and progesterone concentration in estrus-synchronized Mehraban ewes. Trop Anim Health Prod:1–8
Awawdeh M, Eljarah A, Ababneh M (2019) Multiple injections of vitamin E and selenium improved the reproductive performance of estrus-synchronized Awassi ewes. Trop Anim Health Prod 51(6):1421–1426
Díaz-Sánchez VM, Rodríguez-Patiño G, Álvarez-Ávila G, Ramírez-Bribiesca JE, Silva-Mendoza R, Revilla-Vazquez AL, López-Arellano R, Tórtora-Pérez JL (2020) Evaluation of intraruminal boluses dosed with sulfamethazine and selenium in goat kids naturally infected with Eimeria spp. J Appl Anim Res 48(1):244–251
Ghorbani A, Moeini MM, Souri M, Hajarian H (2018) Influences of dietary selenium, zinc and their combination on semen characteristics and testosterone concentration in mature rams during breeding season. J Appl Anim Res 46(1):813–819
El-Hafez A, Solouma G, Kassab A, Ali A (2016) Some reproductive performance of male lambs and feeding values of rations as affected by supplementation of different selenium sources. Egypt J Nutr Feeds 19(1):103–114
Lizarraga RM, Anchordoquy JM, Galarza EM, Farnetano NA, Carranza-Martin A, Furnus CC, Mattioli GA, Anchordoquy JP (2019) Sodium selenite improves in vitro maturation of Bos primigenius taurus oocytes. Biol Trace Elem Res:1–10
Marai IFM, El-Darawany A-H, Ismail E, Abdel-Hafez MAM (2009) Reproductive and physiological traits of Egyptian Suffolk rams as affected by selenium dietary supplementation and housing heat radiation effects during winter of the sub-tropical environment of Egypt. Arch Anim Breeding 52(4):402–409
Ceko M, Hummitzsch K, Hatzirodos N, Bonner W, Aitken J, Russell D, Lane M, Rodgers R, Harris H (2015) Correction: X-Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 7(1):188–188
Kommisrud E, Østerås O, Vatn T (2005) Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet Scand 46(4):229–240
Wu X, Yao J, Yang Z, Yue W, Ren Y, Zhang C, Liu X, Wang H, Zhao X, Yuan S (2011) Improved fetal hair follicle development by maternal supplement of selenium at nano size (Nano-Se). Livest Sci 142(1-3):270–275
Wilde D (2006) Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim Reprod Sci 96(3-4):240–249
Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz M, Iqbal Z (2014) Role of selenium in male reproduction—A review. Anim Reprod Sci 146(1-2):55–62
Smith KL, Hogan J, Weiss W (1997) Dietary vitamin E and selenium affect mastitis and milk quality. J Anim Sci 75(6):1659–1665
Oliver S, Calvinho L (1995) Influence of inflammation on mammary gland metabolism and milk composition. J Anim Sci 73(suppl_2):18–33
Rajala-Schultz P, Gröhn Y, McCulloch C, Guard C (1999) Effects of clinical mastitis on milk yield in dairy cows. J Dairy Sci 82(6):1213–1220
Cao Y-Z, Maddox JF, Mastro AM, Scholz RW, Hildenbrandt G, Reddy CC (1992) Selenium deficiency alters the lipoxygenase pathway and mitogenic response in bovine lymphocytes. J Nutr 122(11):2121–2127
Burton JL, Erskine RJ (2003) Immunity and mastitis some new ideas for an old disease. Vet Clin Food Anim Pract 19(1):1–45
Colitti M, Stefanon B (2006) Effect of natural antioxidants on superoxide dismutase and glutathione peroxidase mRNA expression in leukocytes from periparturient dairy cows. Vet Res Commun 30(1):19–27
Ceballos-Marquez A, Barkema H, Stryhn H, Dohoo I, Keefe G, Wichtel J (2010) Milk selenium concentration and its association with udder health in Atlantic Canadian dairy herds. J Dairy Sci 93(10):4700–4709
Valckenier D, Piepers S, De Visscher A, Bruckmaier R, De Vliegher S (2019) Effect of intramammary infection with non-aureus staphylococci in early lactation in dairy heifers on quarter somatic cell count and quarter milk yield during the first 4 months of lactation. J Dairy Sci 102(7):6442–6453
Vanderhaeghen W, Piepers S, Leroy F, Van Coillie E, Haesebrouck F, De Vliegher S (2015) Identification, typing, ecology and epidemiology of coagulase negative staphylococci associated with ruminants. Vet J 203(1):44–51
Zhuang C, Liu G, Barkema HW, Zhou M, Xu S, Liu Y, Kastelic JP, Gao J, Han B (2020) Selenomethionine suppressed TLR4/NF-κB pathway by activating selenoprotein S to alleviate ESBL Escherichia coli-induced inflammation in bovine mammary epithelial cells and macrophages. Front Microbiol 11:1461
Knowles S, Grace N, Knight T, McNabb W, Lee J (2004) Adding nutritional value to meatand milk from pasture-fed livestock. N Z Vet J 52(6):342–351
Ceballos A, Sánchez J, Stryhn H, Montgomery J, Barkema H, Wichtel J (2009) Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J Dairy Sci 92(1):324–342
Pieszka M, Bederska-Łojewska D, Szczurek P, Pieszka M (2019) The Membrane Interactions of Nano-Silica and Its Potential Application in Animal Nutrition. Animals 9(12):1041
Gopi M, Beulah P, Dhinesh Kumar R, Shanmathy M, Prabakar G (2017) Role of nanoparticles in animal and poultry nutrition: modes of action and applications in formulating feed additives and food processing
Surai PF, Kochish II, Velichko OA (2017) Nano-Se assimilation and action in poultry and other monogastric animals: is gut microbiota an answer? Nanoscale Res Lett 12(1):1–7
Ghaderzadeh S, Mirzaei Aghjegheshlagh F, Nikbin S, Navidshad B (2019) Correlation effects of nano selenium and conjugated linoleic acid on the performance, lipid metabolism and immune system of male Moghani lambs. Iran J Appl Anim Sci 9(3):443–451
Shen X, Min X, Zhang S, Song C, Xiong K (2020) Effect of heavy metal contamination in the environment on antioxidant function in Wumeng semi-fine wool sheep in Southwest China. Biol Trace Elem Res:1–10
Shahin MA, Khalil WA, Saadeldin IM, Swelum AA-A, El-Harairy MA (2020) Comparison between the effects of adding vitamins, trace elements, and nanoparticles to shotor extender on the cryopreservation of dromedary camel epididymal spermatozoa. Animals 10(1):78
Hashem NM, Gonzalez-Bulnes A (2020) State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals 10(5):840
Khalil WA, El-Harairy MA, Zeidan AE, Hassan MA (2019) Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 126:121–127
Hozyen HF, El Shamy AA Screening of genotoxicity and oxidative stress effect of selenium nanoparticles on ram spermatozoa
Peris-Frau P, Soler AJ, Iniesta-Cuerda M, Martín-Maestro A, Sánchez-Ajofrín I, Medina-Chávez DA, Fernández-Santos MR, García-Álvarez O, Maroto-Morales A, Montoro V (2020) Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int J Mol Sci 21(8):2781
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arshad, M.A., Ebeid, H.M. & Hassan, Fu. Revisiting the Effects of Different Dietary Sources of Selenium on the Health and Performance of Dairy Animals: a Review. Biol Trace Elem Res 199, 3319–3337 (2021). https://doi.org/10.1007/s12011-020-02480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02480-6