Abstract
Mercury chloride (HgCl2) is a compound found in the environment that presents low risk due to low liposolubility. Considering the importance of blood as access rout to the systemic distribution of this toxicant to the organism as well as functions performed by it, this study aimed to investigate the effects of HgCl2 on the peripheral blood of rats, evaluating the oxidative biochemistry, blood count, and morphology of cell populations. For this, 20 adult Wistar male rats were divided into control (n = 10) and exposed (n = 10) groups and received distilled water or HgCl2 at a dose of 0.375 mg/kg for 45 days, respectively, through intragastric gavage. Then, the animals were euthanized and the blood was collected for total mercury (Hg) levels determination, complete blood and reticulocyte count, oxidative biochemistry by Trolox Equivalent Antioxidant Capacity (TEAC), reduced glutathione (GSH) levels, superoxide dismutase activity (SOD), thiobarbituric acid reactive substances (TBARS), and nitric oxide (NO), in blood cells and plasma. Long-term exposure increased total Hg in plasma and blood cells. In blood cells, only TEAC has decreased; in plasma, the HgCl2 increased TBARS and NO levels, followed by a decrease in TEAC and GSH levels. There were no quantitative changes in reticulocytes, erythrocytes, and hemoglobin; however, the number of leukocytes have increased and platelets have decreased. Our results suggest that even in the face of low toxicity when compared with other mercury species, HgCl2 at low doses is able to modulate the systemic redox balance and affect some blood cell populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is considered one of the top ten chemicals of major public health concern [1]. It can be found in three different chemical forms including elemental Hg (Hg0), inorganic mercury compounds [mainly mercury chloride (HgCl2)], and organic mercury compounds (mainly methylmercury) [2]. Human exposure to inorganic mercury is often related to specific working conditions (mining, working environment, handling of mercury salts in the chemical industry and laboratories) [3].
Although for a long time a secondary role has been given to the effects of inorganic mercury when compared with the widely studied toxic effects of methylmercury, it is established that even with its lower liposolubility, inorganic mercury is capable of promoting toxic damages to several biological systems [4,5,6,7], but little is known about the effects of HgCl2 in peripheral blood.
The most common biomarkers of exposure to mercurial species are the levels of Hg found in hair, urine, and blood; however more than the Hg levels found, the effects on peripheral blood may be early predictive signs of Hg-induced toxicity; thus, the concentration of Hg in blood is considered a reliable biomarker of exposure and the biochemical and cellular effects triggered in the blood can serve as important elements of diagnosis of the effects caused by this exposure [7,8,9].
The great importance of evaluating oxidative stress markers in the peripheral blood is that this site is a useful source of biomarkers [10], especially considering that we can evaluate not only the effects of some drug or toxicant over the blood cells, but also correlate the plasma concentration of biomarkers with systemic repercussions. In this way, this study purposed to investigate the effects of a long-term exposure to HgCl2 over peripheral blood of rats and to perform a screening on oxidative biochemistry of blood cells and plasma, blood count and morphology of cell populations, in order to rise a translational appeal of this investigation with the issues raised above.
Materials and Methods
Protocol of Exposure
Twenty Rattus norvegicus (90 days-old, males, 250–300 g) were randomly divided into two groups (n = 10 per group). The animals were kept in collective cages (4 animals per cage), with ad libitum access to food and water, with acclimatized room and 12-h light/dark cycle. The HgCl2 exposed group was intoxicated with a dose of 0.375 mg/kg/day dose of HgCl2 (Sigma-Aldrich, Germany) in distilled water (H2Od) and 10 other animals (control group) received only H2Od, both administered by intragastric gavage over a period of 45 days, according to the procedure previously described by Szász et al. [11] and adapted by our group in others studies [12,13,14,15,16].
Blood Samples Collection
Twenty-four hours after the last exposure, the animals were anesthetized with a solution of ketamine hydrochloride (90 mg/kg) and xylazine hydrochloride (10 mg/kg). After total loss of corneal reflexes, blood was collected by intracardiac puncture. After collection, the samples were placed in heparinized tubes and divided into three aliquots for blood analysis, oxidative stress, and quantification of Hg levels.
Total Hg Quantification
The aliquots of blood were centrifuged at 3000 rpm for 10 min to measure total Hg levels in plasma and blood cells concentrate. A volume of 1 mL of sample was digested in an acid solution of nitric acid, perchloric acid, and sulfuric acid (1:1:5, v/v) in a hot electric plate (200–300 °C) for 30 min. The analysis of the total Hg was performed with an Atomic Absorption Spectrophotometer (Semi-Automatic Mercury Analyzer - Hg 201). The analytical method for Hg uses cold-to-cold vapor atomic absorption spectrometry, and Hg is converted into elemental Hg vapor to be introduced into an element-quantity absorption cell. Hg concentration was expressed in μg/mL. The limit of detection was 0.001 mg.kg−1.
Oxidative Biochemistry Analyses
Blood aliquots for oxidative biochemistry analyses were centrifuged at 3000 rpm for 10 min. Afterwards, the plasma was collected and placed in microtube at −80 °C. At a ratio of 1: 1 (v/v), saline was added to the cells concentrate followed by centrifugation at 3000 rpm for 10 min (procedure repeated 3 times). After that, the supernatant was discarded, and the blood cell was stored in a microtube under refrigeration at −80 °C until further analysis. At the time of the analyses described below, the plasma and cell concentrate were defrosted in an ice bath, with the adding of saline for cell lysis (1:1, v/v) in the latter.
Evaluation of Trolox Equivalent Antioxidant Capacity (TEAC)
The TEAC level in the samples was measured using a method proposed by Rufino et al. [17]. In this assay, 7 mM 2,2-azinobis, 3-ethylbenzothiazoline, 6-sulfonate (ABTS) was incubated with potassium persulfate (2.45 mM) at room temperature for 16 h to produce ABTS+ radical, which solution was diluted in 95% ethanol (v / v) until absorbance reached 0.70 ± 0.02 at 734 nm. Subsequently, 30 μL of sample or trolox standards (standard curve) were added to 2970 μL of ABTS with absorbance reading after 5 min. The total antioxidant capacity of the samples was calculated by a calibration curve drawn with different concentrations of trolox [18], expressed as μmol/L.
Determination of Reduced Glutathione Content (GSH)
The GSH level measurements were determined by using a modified Ellman’s protocol [19]. This technique is based on GSH ability to reduce 5,5-dithiobis-2nitrobenzoic acid (DTNB) (Sigma-Aldrich) to 5-thio-2-nitrobenzoic acid (TNB), which was quantified in a spectrophotometer (412 nm). For the determination of peripheral blood levels, the red blood cells were hemolyzed in cold distilled water. For this, the samples were deproteinized with 2% trichloroacetic acid and the supernatant collected for analysis after centrifugation at 3000 rpm for 5 min. A 20-μL aliquot of the sample was removed from the red cell suspension and added to a test tube containing 20μL distilled water and 3-mL PBS/EDTA buffer to record the first reading of the sample (T0), and 100μL DTNB was added and after 3 min the second sample reading (T3) was taken to determine the GSH concentration, which was expressed in μg/mL.
Determination of Superoxide Dismutase Activity (SOD)
The SOD activity was determined following the protocol of McCord et al. [20] by inhibiting the reduction of cytochrome-c [21]. This method evaluates the ability of SOD to convert the superoxide (O2−) radical into hydrogen peroxide (H2O2) and oxygen (O2). Activity was determined by the reduction of cytochrome c (0.075 mM) which is mediated by superoxide anions generated by the xanthine/xanthine oxidase system. One unit of SOD was defined as the amount of enzyme needed to inhibit cytochrome c reduction rate by 50%. The reading was performed at a wavelength of 550 nm and is expressed as nmol/mL.
Measurement of Thiobarbituric Acid Reactive Substances (TBARS)
Lipid peroxidation was evaluated by TBARS reaction according to the methodology proposed by Kohn and Liversedge [22], modified by Percário et al. [23]. For this, 1 mL of thiobarbituric acid (TBA) was added to 0.5 mL of sample and incubated for 1 h at 94 °C. After this procedure, the samples were cooled in running water for about 15 min, and then 4 mL of butyl alcohol was added to each sample. Afterwards, the samples were mixed on a vortex shaker to obtain the maximum extraction of TBARS in the organic phase. Finally, the tubes were centrifuged at 2500 rpm for 10 min. A volume of 3 mL of the organic phase is read in a spectrophotometer at 535 nm. TBARS concentration was expressed in μmol/L.
Nitrites Concentration (NO)
Nitrite concentrations were determined by the Granger’s method [24]. Previously, 100 μL of sample was incubated with an equal volume of Griess reagent for 10 min. Absorbance was determined at a wavelength of 550 nm. Afterwards, a standard curve was established with sodium nitrite (NaNO2) solutions and expressed in μM/mL.
Complete Blood Count, Reticulocytes Count, and Blood Smear
From the aliquot for blood analysis, 20 μL of each sample was incubated with 20 μL of new methylene blue (1%) and heated at 37 °C for 25 min. Then, extensions with 3 μL of the dye and blood mixture were performed on a microscopy slide to determine the relative frequency of reticulocytes. The slides were photomicrographed by the Leica photomicroscopy system (Leica Microscope DM500 and IC50 HD Camera, Leica Microsystems, Wild AG, Heerbrugg, Sui, SWI).
The remaining fresh blood was destined for quantitative analyses of the cells. The samples were analyzed using semi-automated methodology using the ABX micros 60 counter, in which the following parameters were performed: Erythrocytes number, hemoglobin, Mean Corpuscular Volume (MCV), total leukocytes and platelet count. To visualize possible cellular alterations, such as poikilocytosis, the blood film was performed in all samples, using Giemsa-May Grünwald staining and then, submitted to optical microscopy as a method for the visualization of cellular elements.
Statistical Analyses
After data collection, all the results were tabulated and analyzed by GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA, USA); the data distribution was tested by the Shapiro-Wilk method for verification of normality and then analyzed by the Student t test, assuming p < 0.05. The results of the oxidative biochemistry were expressed as percentage of the control mean ± standard error of the mean (SEM).
Results
Long-Term Exposure of HgCl2 Did Not Cause Changes in Body Mass of Adult Rats
Any significant body mass difference at the beginning (p = 0.912) and at the end (p = 0.313) of the exposure to HgCl2 when compared to control group was not observed (Table 1).
The Exposure to HgCl2 Increases the Total Hg Levels in Blood Cells and Plasma
The total Hg levels in blood cells from exposed group (0.067 ± 0.002 μg/mL) was significantly higher than in control group (0.01 ± 0.0007 μg/mL) (p = 0.003). The same result we found in plasma, in which the animals exposed to HgCl2 had higher levels (0.05 ± 0.003 μg/mL) than control animals (values lower than the detection limit) (p < 0.0001) as observed in Table 2.
Increased Hg Levels Are Associated with Systemic Oxidative Biochemistry Imbalance
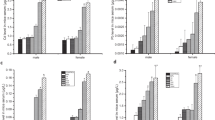
The long-term exposure of HgCl2 did not modulate the TBARS/TEAC ratio in blood cells when comparing both groups (p = 0.62). The same results we found in levels of TBARS that did not show a significant difference (p = 0.83) in exposed group exposed group (102.1 ± 5.61%) in comparison with group control (100 ± 7.62%). In contrast, a decrease on TEAC levels (control group: 100 ± 5.34% vs. exposed group: 74 ± 6.55%; p = 0.01) was observed (Fig. 1a).
Effects of HgCl2 exposure (0.375 mg/kg/day) during 45 days on oxidative biochemistry in levels of antioxidant capacity (TEAC), Lipid peroxidation (TBARS) and the ratio of TEAC/TBARS in red blood cells (a) and plasma (b). Results are expressed as mean ± SEM. Student’s t test, *p < 0.05 statistical difference in relation to the control
A decrease on TEAC levels was observed in plasma of HgCl2-exposed group (89.57 ± 2.05%) in comparison with control group (100 ± 1.52%) (p = 0.003), while TBARS levels had significantly increased in exposed group (control group: 100 ± 6.75%; exposed group: 127.2 ± 2.68%; p = 0.008) (Fig. 1b). This drove to a modulation of TBARS/TEAC ratio in plasma, featuring an oxidative stress state (p = 0.006).
Moreover, animals exposed to HgCl2 for 45 days, did not show significant modifications in GSH levels in blood cells (control group: 100 ± 4.52% vs. exposed group: 103.6 ± 9.28%; p = 0.79). The same result was observed in SOD activity (control group: 100 ± 1.03% vs. exposed group: 102.3 ± 1.53%; p = 0.24) and no concentrations in blood cells (control group: 100 ± 2.94% vs. exposed group: 106.5 ± 2.35%; p = 0.22) (Fig. 2a).
Effects of HgCl2 exposure (0.375 mg/kg/day) during 45 days on oxidative biochemistry in levels of glutathione (GSH), superoxide dismutase activity (SOD) and nitrite concentrations (NO) in red blood cells (a) and plasma (b). Results are expressed as mean ± SEM. Student’s t test, *p < 0, 05 statistical difference in relation to the control
In plasma, lower GSH levels were observed in exposed group when compared with the control group (control group: 100 ± 5.17% vs. exposed group: 73.98 ± 6.37%; p = 0.01). Moreover, higher levels of NO were found in plasma of exposed group (100 ± 7.71%) when compared with control group (134.3 ± 4.70%) (p = 0.005). In SOD activity, there was no statistical difference in the comparison between the groups (control group: 100 ± 2.14% vs. exposed group: 99.8 ± 3.48%; p = 0.96) (Fig. 2b).
The Long-Term Exposure to HgCl2 Is Associated with Complete Blood Count Changes in Adult Rats
The relative count of reticulocytes in peripheral blood did not suffer any variation after HgCl2 long-term exposure (4.05 ± 0.57%) when compared with control group (4.86 ± 0.68%) (p = 0.381) (Fig. 3).
According to Table 3, the HgCl2 did not cause any change to red blood cell count (p = 0.362), neither over hemoglobin concentration (p = 0.32) and MCV (p = 0.205). The analyses of leukocytes showed an increase in exposed group in comparison with control group (p = 0.009), while the number of platelets was reduced in exposed group (p = 0.040).
The qualitative analyses of the blood smear did not point any apparent cell alteration. Erythrocytes did not show any alterations such as poikilocytosis, hyper/hypochromasia or polychromasia. and anisocytosis (Fig. 4a and b); besides that, lymphocytes and neutrophils did not present atypias as toxic granulations, as observed in Fig. 4c–f.
Effects of HgCl2 (0.375 mg/kg/day) during 45 days on cytopathological analyses of blood smear of adult rats. Erythrocytes in the edge of red triangle of control (a) and exposed (b) groups. Neutrophils from control (c) and exposed (d) group, in the edge of black arrow and lymphocytes from control (E) and exposed (F) group in the edge of red arrow stained with Giemsa-May Grünwald. Scale bar: 20 μm
Discussion
In this study, we showed that long-term exposure to HgCl2 at a dose of 0.375 mg/kg/day was able to cause changes in blood oxidative biochemistry and quantitative changes in leukocytes and platelets in peripheral blood of adult rats. Our results reinforce the understanding that even in the face of its low toxicity when compared with other Hg species, HgCl2 is capable to damage the peripheral blood.
A growing discussion about the safety limits of exposure to Hg, generated by the fact that a long-term exposure at low levels of Hg is able to induce genotoxicity and neurological disorders, mainly in cognition and motor function [12,13,14,15, 25, 26]. The Occupational Safety and Health Administration advocates that the permissible exposure limit for Hg is a ceiling limit of 0.1 mg per cubic meter of air (mg/m3), which is currently enforced as an 8-h time-weighted average [27]. Thus, Szász et al. [11] developed a model of exposure to HgCl2 with a dose 0.8 mg of HgCl2/kg/day of water consumption in rats during treatment with Hg, having a tolerance of animals with different levels of Hg poisoning, with no obvious signs of toxicity. In addition, it was investigated Hg species in commercial fish in the legal Amazon, an endemic area of mercurial exposure, and although inorganic Hg levels, reach 0.3 μg/g of fish muscle, no difference was observed between endemic and non-endemic areas of exposure to Hg [26, 28]. In our work, the analysis of Hg levels revealed the presence of Hg in blood in long-term exposed rats to a low dose (0.375 mg/kg) of HgCl2.
The significant increase of Hg levels found in our study, in blood cells and in plasma, occurred through absorption and distribution into the bloodstream, where inorganic Hg combines with proteins in plasma or enters in red blood cells [29]. Increased levels of total Hg in plasma also indicate a significant route of systemic distribution when orally administered, as observed in other studies in our group in which Hg has accumulated in salivary glands [16] and in brain regions such as the hippocampus [15] and motor cortex [14] and spinal cord [26], triggering oxidative biochemical responses observed in our results. In addition, some clinical evidence points to a possible association between neurological disorders and systemic redox imbalance [29,30,31,32]. Thus, the systemic oxidative stress found in our study suggests a possible association with the functional damage of memory and motor activity mentioned above. The presence of Hg in the blood can promote the activation of mechanisms that cause cell damage as oxidative stress, which can result from an imbalance between antioxidant and pro-oxidant compounds of a cell in damage to biological systems, confirmed in our results obtained through oxidative biochemistry analyses of blood.
Exposure to inorganic Hg may be associated with hematological damage, such as anemia, lymphocytosis and lymphopenia, neutrophilia, and basophilia [33]. In the literature, there is a hypothesis that mercury could be responsible for hematological damage through direct bone marrow toxicity, immunity/hypersensitivity, apoptosis, inflammatory reaction, hemolysis, blood loss, increased calcium in the cytoplasm, and increased erythropoietin level [33,34,35]. A recent study evaluated the effects of HgCl2, in vitro, on blood cells [36]. However, there is a lack of evidence from extensive and detailed research on the components of oxidative biochemistry, with a differential analysis of plasma as a distribution fluid.
Oxidative damage caused by low doses of Hg in the blood may be associated with mitochondrial disorders and changes in factors regulating oxidative stress in the body, such as nuclear factor erythroid 2-related factor 2 (Nrf2). In this regard, some authors relate that the Hg2+ can lead to the formation of pores in the mitochondrial membrane, as well as increasing its permeability and replacing Fe2+ of the iron-sulfur protein in the mitochondrial complexes, interrupting the respiratory chain and leading to overproduction of Reactive Oxygen Species (ROS) that can induce DNA damage, protein carbonylation, and lipid peroxidation [37,38,39,40].
In order to prevent oxidative stress, caused by the imbalance of excessive ROS, antioxidant factors, enzymatic and non-enzymatic systems, found in the blood and cells, are often consumed frequently to reduce oxidative damage caused by macromolecules of the body. The analysis of the antioxidant capacity can provide some insight into the general biological antioxidant health, and it is generally maintained by multiple antioxidant enzymes, including the SOD isoforms, GSH system, and CAT [40,41,42]. In this sense, SOD and CAT can lead the detoxification of ROS in cells, since SOD catalyzes the dismutation reaction of the anion superoxide forming H2O2, which is then degraded by CAT forming H2O and O2 molecules, thereby preventing the formation of hydroxyl radicals and prevent lipid peroxidation (LPO); and GSH system also is considered a potent inhibitor of LPO by inhibition of the mitochondrial pathway of apoptosis induced by ROS [40,41,42,43,44].
Meanwhile, LPO and oxidative stress can be monitored by malondialdehyde (MDA), which is produced for degradation of membrane lipid by free radicals and is one of the most significant oxidative stress markers; in addition, the LPO is plausibly the most extensively investigated process induced by free radicals and, therefore, considered an excellent index of oxidative stress [45]. On the other hand, the non-enzymatic systems, such as ceruloplasmin, transferrin, non-protein thiols, vitamins C and E, and uric acid can lead the compensatory capacity against external stimuli in plasma [33, 41, 42, 46]. Our results showed that the antioxidant capacity, in blood cells, using the TEAC method, was reduced, although no increase in MDA has been observed. In plasma, the TEAC and MDA levels were decreased and significantly increased, respectively. These findings may suggest that plasma is a more sensitive fluid to be used as a systematic marker of the induction of oxidative stress by Hg in the body.
As previously commented, endogenous antioxidant molecules, such as glutathione and SOD enzyme, are the main antioxidant defense system [20, 41, 42, 47]. In our study, after exposure to HgCl2, there were no changes in SOD levels in blood cells or plasma. In other studies, our group showed that chronic exposure to inorganic Hg has also led to the toxicity of various tissues, including salivary glands and hippocampus, with an increase in pro-oxidant factors, such as nitrites and MDA, and decreased total antioxidant capacity (ACAP) [12,13,14,15,16]. Therefore, the induction of this oxidative stress caused by Hg in the blood and in different tissues may be associated to Nrf2-Kelch-like ECH-associated protein 1 (Keap1) signal pathway activation, leading to Nrf2 separates from Keap1 in the cytoplasm, then Nrf2 is transferred to the cell nucleus and finally modulate antioxidant enzymes gene expressions [48, 49].
Regarding GSH, it serves to remove free radicals and therefore limit the useful life of the oxidative signal; thus GSH plays a significant role in the regulation of intracellular detoxification [49]. Within this context, GSH is undoubtedly linked to the signaling function, particularly interactions with nitric oxide (NO) [50]. In this scenario, there were no significant changes in GSH levels and nitrite concentration in blood cells. When analyzing these plasma oxidative parameters, there was a significant decrease in GSH percentages, although there was an increase in NO concentration.
When entering the bloodstream, Hg binds to morphological elements, especially erythrocytes, changing blood parameters [51]. Our results did not show significant changes in the number of erythrocytes, in the concentration of hemoglobin, and in the MCV. This result may be associated with the findings of oxidative biochemistry, in which we did not observe the presence of oxidative stress, showing that only the TEAC parameter was altered. However, there was a significant increase in the number of leukocytes that may derive from the pro-inflammatory effect of Hg on blood and tissues. In this condition, pro-inflammatory cytokines are released, such as Interleukine 1β, Interleukine 6, and Tumor necrosis factor α, which act in the immune response against Hg toxicity expressed mainly through the generation of ROS [52]. Thus, the oxidative stress generated by Hg contributes to the increase in the inflammatory response [53], promoting the recruitment of leukocytes into the bloodstream and intensifying the immune response [52].
In addition, our results show a reduction in the number of platelets in the blood plasma; this may be associated with the integrating mechanisms of inflammation through platelet aggregation that reduces the number of platelets circulating in the blood [54]. Considering that platelets can assist in the recruitment of leukocytes to sites of inflammation, the result of this cellular interaction contributes to hemostasis and immune response through an inflammatory or infectious process through the binding of P-selectin, present in platelets, with the PSGL receptor-1 present in leukocytes [55].
Reticulocytes, which are immature red blood cells that form a network or granules of reticules, showed no significant changes between groups. This data corroborates the absence of changes in mature red blood cells. This information may be associated because the Hg kinetics in the blood is relatively fast because this biomarker has the limitation of being only useful for a short period of time after acute exposure or in the case of chronic exposure [56].
The toxic effect of Hg at the molecular level is mainly due to the formation of stable Hg complexes with sulfur present in the thiol and selenium (Se) groups of proteins and other molecules of greater biological significance, such as glutathione and sulfhydryl proteins, which can contribute to its toxicity. Once bound to GSH, Hg can let the cell circulate in serum or lymph and be deposited in other organs or tissues [57]. Thus, this process culminates in the inactivation of enzymes, structural proteins, and processes of transport or alteration of the permeability of the cell membrane [58]. The absence of cellular and oxidative damage in blood cells may be associated with the active expression of eNOS in red blood cells, contributing to the constitutive production of nitrite homeostasis [59]. In addition, the elevation of NO from the endothelium to the vascular system and red blood cells can alter the deformation capacity of erythrocytes in response to mechanical forces [60].
Conclusion
Our evidences allow us to point that even lower liposoluble species of Hg are able to affect the systemic redox system and impact blood cells like platelets and leukocytes, through increasing of pro-oxidant factors in plasma and reducing antioxidant capacity in the blood. Moreover, previous central disorders associated with HgCl2 now may be related to this systemic redox imbalance.
References
Organization, W.H. (2008) Guidance for identifying populations at risk from mercury exposure
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012:1–10
Franco JL, Braga Hde C, Nunes AK, Ribas CM, Stringari J, Silva AP, Garcia Pomblum SC, Moro AM, Bohrer D, Santos AR, Dafre AL, Farina M (2007) Lactational exposure to inorganic mercury: evidence of neurotoxic effects. Neurotoxicol Teratol 29(3):360–367
Azimi S, Moghaddam M (2013) Effect of mercury pollution on the urban environment and human health. Environ Ecol Res 1(1):12–20
Park J-D, Zheng W (2012) Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health 45(6):344
Takahashi T, Shimohata T (2019) Vascular dysfunction induced by mercury exposure. Int J Mol Sci 20(10):2435
Omanwar S, Ravi K, Fahim M (2011) Persistence of EDHF pathway and impairment of the nitric oxide pathway after chronic mercury chloride exposure in rats: mechanisms of endothelial dysfunction. Hum Exp Toxicol 30(11):1777–1784
Branco V et al (2017) Biomarkers of mercury toxicity: past, present, and future trends. J Toxicol Environ Health B 20(3):119–154
Berglund M et al (2005) Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health 4(1):1–11
Miranda GHN et al (2018) Chronic exposure to sodium fluoride triggers oxidative biochemistry misbalance in mice: effects on peripheral blood circulation. Oxidative Med Cell Longev 2018
Szász A, Barna B, Gajda Z, Galbács G, Kirsch-Volders M, Szente M (2002) Effects of continuous low-dose exposure to organic and inorganic mercury during development on epileptogenicity in rats. Neurotoxicology 23(2):197–206
Teixeira FB et al (2014) Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int J Environ Res Public Health 11(9):9171–9185
Teixeira FB, Leão LKR, Bittencourt LO, Aragão WAB, Nascimento PC, Luz DA, Braga DV, Silva MCF, Oliveira KRM, Herculano AM, Maia CSF, Lima RR (2019) Neurochemical dysfunction in motor cortex and hippocampus impairs the behavioral performance of rats chronically exposed to inorganic mercury. J Trace Elem Med Biol 52:143–150
Teixeira F et al (2018) Exposure to inorganic mercury causes oxidative stress, cell death, and functional deficits in the motor cortex. Front Mol Neurosci 11:125
Aragão WAB et al (2018) Hippocampal dysfunction provoked by mercury chloride exposure: evaluation of cognitive impairment, oxidative stress, tissue injury and nature of cell death. Oxidative Med Cell Longev 2018
Aragao W et al (2017) Chronic exposure to inorganic mercury induces biochemical and morphological changes in the salivary glands of rats. Metallomics 9(9):1271–1278
Rufino M, Alves R, Britoetal E (2007) Determination of the total antioxidant activity in fruits by the capture of free radical DPPH. Press Release Embrapa 127:1–4
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Flohe L (1984) [10] Superoxide dismutase assays, in Methods in enzymology. Elsevier, pp 93–104
Kohn HI, Liversedge M (1944) On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J Pharmacol Exp Ther 82(3):292–300
Percário S (1994) Dosagem das LDLs modificadas através da peroxidação lipídica: correlação com o risco aterogênico. An Méd Hosp Fac Ciênc Méd Santa Casa São Paulo 13(49–52):7–9
Granger DL et al (1999) Measuring nitric oxide production in human clinical studies, in Methods in enzymology. Elsevier, pp 49–61
Nevado JB et al (2010) Mercury in the Tapajós River basin, Brazilian Amazon: a review. Environ Int 36(6):593–608
Corrêa MG et al (2020) Spinal cord neurodegeneration after inorganic mercury long-term exposure in adult rats: ultrastructural, proteomic and biochemical damages associated with reduced neuronal density. Ecotoxicol Environ Saf 191:110159
Administration., O.S.a.H.a (2012) Protecting workers from mercury exposure while crushing and recycling fluorescent bulbs. US Department of Labor, Occupational Safety and Health Administration, Washington Available from: https://www.osha.gov/Publications/mercuryexposure_fluorescentbulbs_factsheet.html. 15th Sept 2020
Martín-Doimeadios RR et al (2014) Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ Sci Pollut Res 21(12):7466–7479
Aschner M, Eberle NB, Miller K, Kimelberg HK (1990) Interactions of methylmercury with rat primary astrocyte cultures: inhibition of rubidium and glutamate uptake and induction of swelling. Brain Res 530(2):245–250
Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, Feki AE (2009) Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol 28(1):81–89
Bailey DM, Brugniaux JV, Filipponi T, Marley CJ, Stacey B, Soria R, Rimoldi SF, Cerny D, Rexhaj E, Pratali L, Salmòn CS, Murillo Jáuregui C, Villena M, Smirl JD, Ogoh S, Pietri S, Scherrer U, Sartori C (2019) Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J Physiol 597(2):611–629
Baierle M et al (2015) Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxidative Med Cell Longev 2015
Lauwerys R, Bonnier C, Evrard P, Gennart JP, Bernard A (1987) Prenatal and early postnatal intoxication by inorganic mercury resulting from the maternal use of mercury containing soap. Hum Toxicol 6(3):253–256
Winship KA (1985) Toxicity of mercury and its inorganic salts. Adverse Drug React Acute Poisoning Rev 4(3):129–160
Shenker B, Rooney C, Vitale L, Shapiro IM (1992) Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. I. Suppression of T-cell activation. Immunopharmacol Immunotoxicol 14(3):539–553
Ahmad S, Mahmood R (2019) Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ Sci Pollut Res 26(6):5645–5657
Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q, Zhang X, Zhang Z (2018) Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of AKT/Nrf2 and NF-κB pathways. Food Chem Toxicol 113:296–302
Moneim AEA (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis 30(4):935–942
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38(4):769–789
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Schalcher TR, Borges RS, Coleman MD, Batista Júnior J, Salgado CG, Vieira JL, Romão PR, Oliveira FR, Monteiro MC (2014) Clinical oxidative stress during leprosy multidrug therapy: impact of dapsone oxidation. PLoS One 9(1):e85712
Albuquerque RV, Malcher NS, Amado LL, Coleman MD, Dos Santos DC, Borges RS, Valente SA, Valente VC, Monteiro MC (2015) In vitro protective effect and antioxidant mechanism of resveratrol induced by Dapsone hydroxylamine in human cells. PLoS One 10(8):e0134768
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int 2014:1–26
Kumar A et al (2018) Biochemical and molecular targets of heavy metals and their actions, in Biomedical applications of metals. Springer, pp 297–319
Çubukçu HC, Yurtdaş M, Durak ZE, Aytaç B, Güneş HN, Çokal BG, Yoldaş TK, Durak İ (2016) Oxidative and nitrosative stress in serum of patients with Parkinson’s disease. Neurol Sci 37(11):1793–1798
Chiang P-L, Chen HL, Lu CH, Chen YS, Chou KH, Hsu TW, Chen MH, Tsai NW, Li SH, Lin WC (2018) Interaction of systemic oxidative stress and mesial temporal network degeneration in Parkinson’s disease with and without cognitive impairment. J Neuroinflammation 15(1):281
Kumar A, Pandey R, Sharma B (2020) Modulation of superoxide dismutase activity by mercury, lead, and arsenic. Biol Trace Elem Res 1–8
Zhang LJ, Li Y, Chen P, Li XM, Chen YG, Hang YY, Gong WJ (2017) A study of genotoxicity and oxidative stress induced by mercuric chloride in the marine polychaete Perinereis aibuhitensis. Environ Toxicol Pharmacol 56:361–365
Ma Y, Zheng YX, Dong XY, Zou XT (2018) Effect of mercury chloride on oxidative stress and nuclear factor erythroid 2-related factor 2 signalling molecule in liver and kidney of laying hens. J Anim Physiol Anim Nutr 102(5):1199–1209
Feng P, Wei J, Zhang Z (2011) Intervention of selenium on chronic fluorosis-induced injury of blood antioxidant capacity in rats. Biol Trace Elem Res 144(1–3):1024–1031
Dhouib IB, Annabi A, Doghri R, Rejeb I, Dallagi Y, Bdiri Y, Lasram MM, Elgaaied A, Marrakchi R, Fazaa S, Gati A (2017) Neuroprotective effects of curcumin against acetamiprid-induced neurotoxicity and oxidative stress in the developing male rat cerebellum: biochemical, histological, and behavioral changes. Environ Sci Pollut Res 24(35):27515–27524
Milnerowicz H, Ściskalska M, Dul M (2015) Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J Trace Elem Med Biol 29:1–10
Penta KL, Fairweather D, Shirley DL, Rose NR, Silbergeld EK, Nyland JF (2015) Low-dose mercury heightens early innate response to coxsackievirus infection in female mice. Inflamm Res 64(1):31–40
Ho-Tin-Noé B, Boulaftali Y, Camerer E (2018) Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood 131(3):277–288
Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pörnbacher M, Lauber K, Krombach F, Reichel CA (2016) Platelets guide leukocytes to their sites of extravasation. PLoS Biol 14(5):e1002459
Diaz-Vivancos P, de Simone A, Kiddle G, Foyer CH (2015) Glutathione–linking cell proliferation to oxidative stress. Free Radic Biol Med 89:1154–1164
Lorscheider F, Vimy M (2000) Mercury and idiopathic dilated cardiomyopathy. J Am Coll Cardiol 35(3):819–820
Hansen JM, Zhang H, Jones DP (2006) Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med 40(1):138–145
Alissa EM, Ferns GA (2011) Heavy metal poisoning and cardiovascular disease. J Toxicol 2011:1–21
Satoh H (2000) Occupational and environmental toxicology of mercury and its compounds. Ind Health 38(2):153–164
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 supported by the Brazilian National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The animals in this study were supplied by the Central Animal Hospital of the Federal University of Pará under the protocol of the Ethics Committee for the Use of Animals n° 9,228,050,418.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos Chemelo, V., Bittencourt, L.O., Aragão, W.A.B. et al. Long-Term Exposure to Inorganic Mercury Leads to Oxidative Stress in Peripheral Blood of Adult Rats. Biol Trace Elem Res 199, 2992–3000 (2021). https://doi.org/10.1007/s12011-020-02411-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02411-5