Abstract
As a nutritionally essential trace element, selenium (Se) is crucial for fish growth. However, the underlying mechanisms remain unclear. Fish somatic growth relies on the white muscle growth. This study aimed to explore the effects and underlying mechanisms of Se on fish white muscle growth using a juvenile rainbow trout (Oncorhynchus mykiss) model. Fish were fed a basal diet unsupplemented or supplemented with selenium yeast at nutritional dietary Se levels (2 and 4 mg/kg Se, respectively) for 30 days. Results showed that dietary Se supplementation significantly enhanced trout somatic growth. Histological and molecular analysis of trout white muscle tissues at the vent level showed that dietary Se supplementation elevated the total cross-sectional area of white muscle, mean diameter of white muscle fibers, protein content, nuclei number, and DNA content of individual muscle fiber, and suppressed the activities of calpain system and ubiquitin-proteasome pathway. Overall, this study demonstrated that dietary Se within the nutritional range inhibits calpain- and ubiquitin-mediated protein degradation and promotes the fusion of myoblasts into the existed muscle fibers to promote the hypertrophic growth of white muscle, thereby accelerating the somatic growth of rainbow trout. Our results provide a mechanistic insight into the regulatory role of Se in fish growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element for fish [1, 2]. Its deficiency inevitably results in a variety of physiological dysfunctions in fish [3,4,5,6]. In intensive aquaculture systems, fish mainly obtain Se from the artificial feeds [1]. Optimal dietary Se level is crucial for fish to maintain the normal growth performance [1]. However, the underlying mechanisms are still unclear. White muscle, which represents 40–60% of fish body mass, is the largest tissue of fish [7, 8]. Numerous researches reported that white muscle growth makes a great contribution to fish somatic growth [7]. Given that, we proposed a hypothesis whether dietary Se controls fish growth by regulating the white muscle growth.
Fish white muscle derives from the somites formed from the paraxial mesoderm in a stereotypic rostral to caudal progression, and its basic unit is muscle fiber which contains bundles of myofibrils [7]. Muscle formation, including development in embryonic phase and growth in postembryonic phase, is a complex dynamic process involving both the recruitment of new muscle fibers (hyperplasia) and the subsequent enlargement of existing fibers (hypertrophy) [7]. It is well known that two biological processes involve in the hyperplasia and hypertrophy of muscle fibers. Firstly, myoblasts which derive from stem cells undergo proliferation, differentiation, migration, and followed by fusion with each other to form new muscle fibers (hyperplasia), or fuse into the existing muscle fibers to enlarge their size (hypertrophy) [7]. Secondly, protein deposition, resulting from a rate of protein synthesis exceeds protein degradation, in the mature muscle fibers is another important approach for the hypertrophy of muscle fibers [7, 9]. In vertebrates, there is a huge difference in postembryonic growth pattern of skeletal muscle among species. In mammals, the postnatal muscle growth only involves the hypertrophy of muscle fibers while muscle hyperplasia ends soon after birth. Whereas in most fish, except small-size fish species such as zebrafish and medaka, skeletal muscle presents not only a hypertrophic growth but also a persistence of hyperplastic growth throughout the larval, juvenile, and even adult stages [10,11,12].
Rainbow trout (Oncorhynchus mykiss) is a commercially important fish species throughout the world and exhibits an indeterminate postembryonic growth pattern with a persistence of hyperplastic growth in skeletal muscle until around 40% of the maximum body size [13]. Postembryonic growth of trout white muscle has been well studied and proved to be sensitive to dietary nutritional strategies such as feed protein sources [14], dietary lipid content [15], availability of essential amino acids [16], vitamins [17], etc. However, limited attention has been paid to the effects of dietary Se on trout muscle growth.
Our previous study demonstrated that the optimal dietary Se supplemental level (based on Se-enriched yeast, Se-yeast) for rainbow trout growth is 4 mg/kg [18]. Based on the previous result, this study aimed to explore the effects of different supplemental levels of dietary Se (2 and 4 mg/kg as Se-yeast) on the mechanisms of white muscle growth in rainbow trout by using both cellular and molecular approaches.
Materials and Methods
The present experiment was carried out in accordance with the guidelines of The Scientific Ethic Committee of Huazhong Agricultural University, Wuhan, China.
Experimental Diet
Three experimental diets were prepared based on a basal diet unsupplemented or supplemented with 2 and 4 mg/kg Se as Se-yeast (Angel Yeast Co., Ltd., Hubei, China), and the measured total Se contents were 0.75, 2.60, and 4.68 mg/kg, respectively. Feed ingredients and proximate composition of the experimental diets are presented in supplementary Table 1, and diets were prepared as previously described [18].
Experimental Design and Feeding Trial
Juvenile immature rainbow trout (O. mykiss) were obtained from Enshi Guoxi Fishery Development Co., Ltd. (Hubei, China). After being transported to Huazhong Agricultural University, fish were kept in a flow-through rearing system supplied with clean water (0.5 l/min) under artificial photoperiods (12 h/12 h). Fish were fed basal diet for 2 weeks to acclimatize to laboratory conditions. Prior to the trial, 10 fish were randomly selected and the whole fish transverse sections with 0.3-cm width were cut at the vent level for histological characterization. Subsequently, a total of 630 fish (initial body weight and length were 12.92 ± 2.29 g and 90.80 ± 1.75 mm, respectively) were individually weighed and divided into 9 plastic tanks (1000 l), with 70 fish in each tank. Experimental diets were randomly allocated in triplicate tanks. Fish were hand-fed to apparent satiation twice daily (09.00 and 16.00 h) for 30 days. During the experimental period, water temperature and dissolved oxygen were 18 ± 0.5 °C and 8.5 ± 0.2 mg/l, respectively.
At the end of the feeding trial, trout were fasted for 48 h. To follow growth and feed utilization, the final number, body weight, and body length of fish were measured. Three fish from each tank were randomly selected, anesthetized with tricaine methanesulfonate (100 mg/l water, Western Chemical, Inc., Ferndale, WA, USA) and killed by a sharp blow to the head. Two whole fish transverse sections with 0.3-cm width from the same fish were cut at the vent level for histological characterization and immunofluorescence assay, respectively. Three white muscle pellets (about 100 mg) under the dorsal fin and closed to the vertebra of each fish were collected, snap-frozen in liquid nitrogen, and then stored at − 80 °C for enzymatic activity, mRNA, and protein analysis, respectively. The other dorsal muscles were minced and stored at − 80 °C for the determination of muscle proximate composition and total Se content.
Growth and Feed Utilization Analysis
The following variables were evaluated:
where Nt is the number of fish at the end of the experiment, No is the number of fish at the beginning of the experiment, Wt is the final body weight of fish, Wo is the initial body weight of fish, Tt is the end of the experiment (day), and To is the beginning of the experiment (day).
Histological Characterization
After fixed in paraformaldehyde solution (4%, w/v) and dehydrated with ethanol series, whole fish transverse sections located at the vent level were embedded in paraffin, cut transversely into 10-μm thick sections, stained with hematoxylin and eosin [19], and scanned with a Pannoramic 250 Flash digital microscope (3D HISTECH, Budapest, Hungary). The total cross-sectional area of white muscle, individual cross-sectional area of white muscle fibers, equivalent mean diameter of white fibers, and the total number of white muscle fibers were measured following the method of Alami-Durante et al. [16]. The density of white muscle fibers was calculated according to Wang et al. [19]. The relative contribution of hyperplasia and hypertrophy to white muscle growth throughout the whole feeding trial was calculated as previously reported [20].
Proximate Composition and Total Se Content Analysis
Moisture, crude protein, crude lipid, and ash were analyzed as previously described [21]. Total Se content was measured as described by Fontagné-Dicharry et al. [22].
Extraction of Total RNA and DNA
Total RNA and DNA were extracted from 100-mg muscle samples using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). After extraction, RNA and DNA pellets were resuspended in 40-μL nuclease-free water and the concentrations were determined by NanoDrop ND-1000 (NanoDrop Technologies®, Wilmington, DE, USA). RNA samples were run on a 1.5% agarose gel and visualized on a Gel Doc EQ imaging system (BIO-RAD Laboratories, California, USA). Densitometric measurements of the 28S and 18S ribosomal RNA were performed with Image J software. Relative RNA/DNA ratio and ribosomal RNA (28S and 18S) were counted and normalized to fish fed the basal diet. Relative DNA/muscle fiber was counted as the ratio of DNA content divided by muscle fiber density and normalized to the data in fish fed the basal diet.
Quantitative Real-Time PCR (qPCR)
Complementary DNA synthesis and qPCR were conducted as previously described [18]. Specific primers for target genes are presented in supplementary Table 2. The relative quantification of the target gene was performed using the mathematical model described by Pfaffl [23] and normalized to the geometric mean of the best combination of eukaryotic translation elongation factor 1 alpha (EF1α) and actin beta (β-actin) [24].
Western Blot Analysis
Muscle samples were homogenized and lysated in radio immunoprecipitation assay buffer, which was supplemented with protease inhibitor and phosphatase inhibitor, according to the manufacturer’s instructions (Beyotime Biotechnology, Nanjing, China). Lysates were centrifuged (12,000g, 10 min, 4 °C) and the supernatant was removed for protein quantification by a BCA Protein Assay Kit (P0012S, Beyotime Biotechnology, Nanjing, China). Lysates (20 μg of protein) were separated by SDS-PAGE, transferred to 0.45-μm nitrocellulose membrane (Millipore Co., Bradford, MA, USA) for western blot analysis. Primary antibodies against ribosomal protein S6 (S6, Cat. no. 2217), phosho-S6 (p-S6 Ser235/Ser236, Cat. no. 4856), eukaryotic initiation factor 4E binding protein (4E-BP1, Cat. no. 9452), phosho-4E-BP1 (p-4E-BP1 Thr37/46, Cat. no. 9459), eukaryotic initiation factor 2 α-subunit (eIF2α, Cat. no. 9722), and phospho-eIF2α (p-eIF2α Ser51, Cat. no. 9721) were purchased from Cell Signaling Technology (CST) Inc. which have been previously validated in rainbow trout [25, 26]. For primary antibodies against eukaryotic elongation factor 2 (eEF2, Cat. no. 2332, CST), phospho-eEF2 (p-eEF2 Thr56, Cat. no. 2331, CST), and β-Tubulin (Cat. no. AC008, ABclonal, Wuhan, China), the amino acid sequences were monitored in SIGENAE database (http://www.sigenae.org) to check for a good conservation of the antigen sequence. Subsequently, membranes were washed and incubated with an IRDye Infrared secondary antibody (LI-COR Inc. Biotechnology, Lincoln, NE, USA). Bands were visualized by infrared fluorescence using the Odyssey imaging system (LI-COR Inc. Biotechnology, Lincoln, NE, USA) and were quantified by Odyssey Infrared imaging system software (version 5.2; LI-COR Inc. Biotechnology, Lincoln, NE, USA). Results were normalized to the data in fish fed the basal diet.
Calpain Activity Analysis
Calpain activity of muscle samples was determined using a calpain activity assay kit according to the manufacturer’s instructions (Biovision, Mountain View, CA, USA). Results were normalized to the data in fish fed the basal diet.
Immunofluorescence Assay
Immunofluorescence of whole fish transverse sections at the vent level was performed as previously described [27]. Briefly, muscle cryosections were fixed with 1% paraformaldehyde. After washing, sections were blocked using a PBS solution containing 1% heat-inactivated donkey serum, 1% bovine serum albumin, and 0.025% Tween 20. Sections were incubated with appropriate primary antibodies against forkhead box class O (FoxO) family member transcription factors 1 and 3a at 4 °C overnight, washed with PBS, and then incubated with fluorescent labeled secondary antibodies (Invitrogen Alexa-Fluor). After a 5-min wash with PBS, the sections were incubated with the nuclear stain 4,6-diamidino-2-phenylindole (DAPI), and slides were mounted and sealed using Shandon Immu-Mount (Thermo Scientific, MA, USA) and glass coverslips. Fluorescent images were acquired with a Pannoramic 250 Flash digital microscope (3D HISTECH, Budapest, Hungary).
Statistical Analysis
SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Shapiro-Wilks and Levene’s tests were performed to test the normal distribution and homogeneity of the variances, respectively. One-way analysis of variance (ANOVA) followed by Duncan’s test were carried out for comparing different treatments when normal variables were analyzed. Data with non-normally distribution were subjected to the nonparametric statistical analysis on ranks (Kruskal-Wallis) followed by Dunn-Bonferroni post hoc test. Linear regression analysis was performed to plot dietary Se levels against fish growth and total cross-sectional area of white muscle. Correlations between data were examined using Pearson’s correlation test. For all statistical analysis, the level of significance was set at P < 0.05.
Results
Growth Performance and Feed Utilization
Fish well accepted the experimental diets and no death was observed throughout the 30-day feeding trial (Table 1). Fish final body size (weight and length) and growth rates (WG and SGR) were significantly increased by dietary Se supplementation (Table 1, P < 0.05), and followed the dose responses to the elevated dietary Se levels (final body weight: R2 = 0.93, P < 0.0001; final body length: R2 = 0.75, P = 0.0025; WG: R2 = 0.94, P < 0.0001; SGR: R2 = 0.94, P < 0.0001; supplementary Fig. 1). No significant differences were found in feed intake and feed efficiency among groups (Table 1).
White Muscle Growth
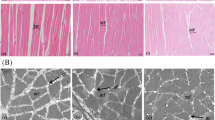
The total cross-sectional area of trout white muscle at the vent level was significantly increased by dietary Se supplementation (P < 0.05) and followed a dose response to dietary Se level (R2 = 0.5226, P < 0.0001; Fig. 1a, b). Additionally, it presented the significantly positive correlations with fish final body size (final body weight, r = 0.7010, P < 0.0001; final body length, r = 0.4976, P = 0.0083; Fig. 1c) and somatic growth rate (WG, r = 0.9320, P = 0.0003; SGR, r = 0.9309, P = 0.0003; Fig. 1d). Transverse sections of white muscle fibers at the vent level are presented in Fig. 1e. Fish fed Se supplemented diets showed higher percentages of large fibers (diameter > 55 μm) and lower percentages of small fibers (diameter < 40 μm) than those fed the basal diet (Fig. 1f, g). The total number of white muscle fibers was only significantly increased by diet supplemented with 4 mg/kg Se (P < 0.05, Fig. 1h), while the significantly larger mean diameter (Fig. 1i) and lower density (Fig. 1j) of white muscle fibers were observed in fish fed diets supplemented with both 2 and 4 mg/kg Se (P < 0.05). Although trout white muscle in all groups presented a high percentage of hyperplastic growth (contributes 53.94–56.56%) throughout the whole feeding trial period, an interesting phenomenon was observed that dietary Se supplementation resulted in a significantly declined percentage of hyperplastic growth and a significantly increased percentage of hypertrophic growth of rainbow trout white muscle (P < 0.05, Fig. 1k).
White muscle growth in juvenile rainbow trout (Oncorhynchus mykiss) fed a basal diet supplemented with or without 2 and 4 mg/kg Se (as Se-yeast) for 30 days. (a) Fish transverse sections at the vent level were stained with hematoxylin and eosin (SC: spinal cord; NC: notochord; WM: white muscle; RM: red muscle; V: vent). (b) Total cross sectional area of white muscle at the vent level (n = 9 fish per treatment). Pearson correlation analysis between the total cross sectional area of white muscle with (c) fish body size and (d) somatic growth rates (n = 3 tanks per treatment). (e) Images of transverse sections of muscle fibers for the analysis of (f) frequency, (g) cumulative frequency, (h) total number, (i) mean diameter and (j) density of white muscle fibers (n = 9 fish per treatment). (k) Relative contribution of hyperplasia and hypertrophy to white muscle growth throughout the feeding trial (n = 9 fish per treatment). Values are means ± SDs. Different superscript letters (a, b, c or A, B, C) indicate significant differences among treatments (P < 0.05)
Proximate Composition and Total se Content of White Muscle
Diets supplemented with 2 and 4 mg/kg Se significantly increased the crude protein content and total Se content of white muscle in juvenile rainbow trout (P < 0.05), while exerted no significant effects on the content of moisture, crude lipid and ash (Table 2).
Protein Synthesis in White Muscle
Factors involve in the processes of protein synthesis including RNA/DNA, quantity of ribosomal RNA (28 S and 16 S), phosphorylation of ribosomal protein S6 on Ser235/236, 4E-BP1 on Thr37/46, eIF2 α on Ser51, and eEF2 on Thr56 exhibited no significant differences among groups (Fig. 2).
Protein synthesis indexes in terms of (a) relative RNA/DNA ratio, (b) relative ribosomal RNA, and phosphorylation (p-) of (c) ribosomal protein S6 (S6) on Ser235/236, (d) eukaryotic translation initiation factor 4E binding protein (4E-BP1) on Thr37/46, (e) eukaryotic translation initiation factor 2 α-subunit (eIF2α) on Ser51, and (f) eukaryotic translation elongation factor 2 (eEF2) on Thr56 in the white muscle of juvenile rainbow trout (Oncorhynchus mykiss) fed a basal diet supplemented with or without 2 and 4 mg/kg Se (as Se-yeast) for 30 days. Values are represented as mean ± SEM (n = 9 fish per treatment)
Protein Degradation in White Muscle
At the transcription level, diets supplemented with both 2 and 4 mg/kg Se significantly decreased the expression of 6 genes related to autophagy-lysosome pathway, 1 gene related to calpain system, and 1 gene related to ubiquitin-proteasome pathway (Fig. 3a, P < 0.05). The autophagy-lysosome pathway-related genes are autophagy-related 4b and 12-like (Atg4b and Atg12l), microtubule-associated light chain 3B (LC3B), gamma-aminobutyric acid type A receptor-associated protein-like 1 (Gabarapl1), and cathepsin D and L (CtsD and CtsL). The calpain system-related gene is calpain 1 (CAPN1). The ubiquitin-proteasome pathway-related gene is muscle atrophy F-box 32 (Fbx32). Additionally, diets supplemented with both 2 and 4 mg/kg Se significantly increased the expression of the long and short isoforms of calpastatin (CAST-L and CAST-S) (Fig. 3a, P < 0.05). The expression of cathepsin B (CtsB) and muscle atrophy F-box 25 (Fbx25) were only declined in fish fed diet supplemented with 4 mg/kg Se compared with the basal diet (Fig. 3a, P < 0.05). At the protein level, white muscle calpain activity (Fig. 3b) was significantly decreased by diet supplementation with 4 mg/kg Se while the abundance of ubiquitinated proteins (Fig. 3d) was significantly declined by diets supplemented with both 2 and 4 mg/kg Se (P < 0.05). However, the LC3-II/LC3-I ratio, a biomarker for activity of autophagy [28], presented no significant difference among groups (Fig. 3c). Immunofluorescence assay showed that dietary Se supplementation exerted no effects on the nuclear localization of FoxO1 (Fig. 3e), while decreased the nuclear localization of FoxO3a (Fig. 3f) in white muscle of juvenile rainbow trout (P < 0.05).
Protein degradation indexes in terms of (a) expression of genes related to autophagy-lysosome pathway, ubiquitin-proteasome pathway and calpain system, (b) LC3-II/LC3-I ratio, (c) relative calpain activity, (d) abundance of ubiquitinated proteins, nuclear localization of (e) FoxO1 and (f) FoxO3a in the white muscle of juvenile rainbow trout (Oncorhynchus mykiss) fed a basal diet supplemented with or without 2 and 4 mg/kg Se (as Se-yeast) for 30 days. Values are represented as mean ± SEM (n = 9 fish per treatment). Different superscript letters indicate significant differences among treatments (P < 0.05). Atg4b, autophagy-related 4b; Atg12l, autophagy-related 12-like; LC3B, microtubule-associated light chain 3B; Gabarapl1, gamma-aminobutyric acid type A receptor-associated protein-like 1; Cts, cathepsin; CAPN 1, catalytic subunits of μ-calpain; CAPN 2, catalytic subunits of m-calpain; CAST-L, calpastatin long isoform; CAST-S, calpastatin short isoform; Fbx, F-box protein; MuRF, muscle RING finger; β-Tubulin, tubulin beta; FoxO, forkhead box class O family member protein
Expression of Genes Involving in Muscle Structure and Myogenesis
The ratio of nuclei/muscle fiber (Fig. 4a) and relative DNA content/muscle fiber (Fig. 4b) were significantly higher in fish fed diet supplemented with 4 mg/kg Se than those fed the basal diet (P < 0.05). Gene expression analysis showed that diets supplemented with 2 and 4 mg/kg Se significantly upregulated the expression of genes coding fast myosin heavy chain (fMyHC), paired-homeobox transcription factor (Pax) 7a and 7b, and myocyte enhancer factor (MEF2c) (P < 0.05). Besides, the expression of gene coding myogenin (MyoG) was significantly increased by diet supplemented with 4 mg/kg Se (Fig. 4c, P < 0.05). No significant differences were observed in the expression of genes coding fast myosin light chain 2 (fMyLC2), proliferative cell nuclear antigen (PCNA), myocyte enhancer factor 2a (MEF2a), myoblast determination protein (MyoD), myogenic regulatory factor 4 (MRF4), myogenic factor 5 (Myf5), and myostatin (MSTN) 1a and 1b among groups (Fig. 4c).
Quantitative data showing the (a) ratio of nuclei/muscle fiber, (b) relative DNA content/muscle fiber, and (c) expression of genes related to muscle structure and myogenesis in the white muscle of juvenile rainbow trout (Oncorhynchus mykiss) fed a basal diet supplemented with or without 2 and 4 mg/kg Se (as Se-yeast) for 30 days. Values are represented as mean ± SEM (n = 9 fish per treatment). Different superscript letters indicate significant differences among treatments (P < 0.05). fMyHC, fast myosin heavy chain; fMyLC2, fast myosin light chain 2; PCNA, proliferative cell nuclear antigen; Pax7, paired-homeobox transcription factor 7; MEF2, myocyte enhancer factor 2; MyoD, myoblast determination protein; MRF4, myogenic regulatory factor 4; Myf5, myogenic factor 5; MyoG, myogenin; MSTN, myostatin
Expression of Selenoprotein Genes in White Muscle
Expression of 11 selenoprotein genes coding glutathione peroxidase (GPX) 1a, 1b2, and 4b; selenoprotein (Sel) K, P, T1-like, T2-like, U, and W-like; thioredoxin reductase 3b (TrxR3b); and methionine sulfoxide reductase B 1A-like (MsrB1Al), which have been proved sensitive to dietary Se level by our previous research [18], have been analyzed. Among these selenoprotein genes, GPX4b, SelK, SelP, SelU, and SelWl were significantly upregulated by dietary Se supplementation (P < 0.05, Fig. 5). Correlation analysis showed that the expression of SelK and SelWl were significantly positively correlated to the WG, SGR, total cross-sectional area of white muscle, relative contribution of hypertrophy and muscle protein content, and negative correlations with the relative contribution of hyperplasia, calpain activity, abundance of ubiquitinated proteins, FoxO3a-labeled nuclei, nuclei/muscle fiber, and relative DNA content/muscle fiber (Table 3).
Relative mRNA levels of selenoprotein genes in the white muscle of juvenile rainbow trout (Oncorhynchus mykiss) fed a basal diet supplemented with or without 2 and 4 mg/kg Se (as Se-yeast) for 30 days. Values are represented as mean ± SEM (n = 9 fish per treatment). Different superscript letters indicate significant differences among treatments (P < 0.05). GPX, glutathione peroxidase; MsrB1Al, methionine sulfoxide reductase B 1A-like; Sel, selenoprotein; SelT1l, selenoproteins T1 like; SelT2l, selenoproteins T2 like; SelWl, selenoproteins W like; TrxR3b, thioredoxin reductase 3
Discussion
Optimal dietary Se level is crucial for fish growth [1]. Our previous study reported that rainbow trout reached the maximum growth rate when fed diet supplemented with 4 mg/kg Se as Se-yeast [18]. Based on this result, juvenile rainbow trout in the present study were fed a basal diet unsupplemented or supplemented with gradient levels of Se-yeast within nutritional level (≤ 4 mg/kg Se). Results showed that trout growth was enhanced by diets supplemented with both 2 and 4 mg/kg Se and followed a liner response to the increasing dietary Se level. It is well known that fish somatic growth is largely determined by white muscle growth [7]. Thus, we further investigated the effects of dietary Se on white muscle growth in rainbow trout to attempt to give an insight into the regulatory mechanisms of Se in trout somatic growth.
The increase of total cross-sectional area of white muscle has been regarded as an indicator of fish muscle growth [15, 16]. Our results showed that the total cross-sectional area of trout white muscle at the vent level was increased by diets supplemented with both 2 and 4 mg/kg Se and followed a liner response to the increasing dietary Se level, reflecting an enhancement of nutritional levels of dietary Se on white muscle growth in rainbow trout. It is reported that the total cross-sectional area of fish white muscle increased at almost the same rate as fish somatic growth [29]. In the present study, the significantly positive correlations between the total cross-sectional area of white muscle and trout final body size (weight and length) as well as the somatic growth rate (including WG and SGR) have also been observed in trout fed increasing levels of dietary Se. These results strongly support our hypothesis that the improvement of somatic growth of rainbow trout by dietary Se is contributed to the promoted white muscle growth.
Postembryonic growth of fish muscle involves the hyperplasia and hypertrophy of muscle fibers [7]. Hyperplasia has been proved to be the major contributor (more than 50%) to muscle growth in rainbow trout with the body weight ranges from 0.1 to 200 g [30]. Be in line with the previous report, in the present study, trout white muscle presented a relatively higher percentage (53.94–56.56%) of hyperplastic growth than hypertrophic growth throughout the whole feeding trial. The total number and mean diameter of muscle fibers have been widely used as the indicators for hyperplasia and hypertrophy of muscle fibers, respectively [15, 16]. In the present study, the increased mean diameter of white muscle fibers was observed in trout fed diets supplemented with both 2 and 4 mg/kg Se while the increased total number of white muscle fibers was only observed in trout fed diet supplemented with 4 mg/kg Se. It suggests that, in trout white muscle, hypertrophy of muscle fiber is more sensitive to dietary Se than hyperplasia. This phenomenon could also be proved by the elevated contribution of hypertrophy and the declined contribution of hyperplasia to the growth of white muscle in trout fed diets supplemented with both 2 and 4 mg/kg Se. These results reveal that dietary Se primarily promotes the hypertrophy of muscle fibers to promote white muscle growth in rainbow trout.
Protein is the predominant component of muscle dry biomass; its deposition in muscle has been demonstrated an important approach for the hypertrophy of muscle fibers [7, 9]. It is reported that diets supplemented with 2–4 mg/kg Se from Se-yeast led to an increase of protein content in rainbow trout muscle [21, 31]. Similar to the previous researches, the significantly higher muscle protein content has also been observed in trout fed diets supplemented with 2 and 4 mg/kg Se than those fed the basal diet, suggesting an enhancement of dietary Se on protein deposition in trout white muscle. Protein deposition depends on the balance of protein synthesis and degradation [7, 9]. Thus, protein synthesis and degradation in trout white muscle have been further analyzed.
Protein synthesis is a complex process of messenger RNA translation on the ribosomes [32]. It can be divided into multiple steps including the translation initiation, elongation, and termination [33]. The activation of translation initiation and elongation (translational activity) is crucial for protein synthesis [32]. In eukaryotic cells, translation initiation is primarily under the control of target of rapamycin complex 1 (TORC1) and eIF2α-eIF2B pathways [34]. TORC1 is activated by a variety of hormones and nutrients, etc., thereby phosphorylating the downstream 4E-BP1 to drive the 5′-cap-dependent translation or phosphorylating ribosomal protein S6 to enhance translation initiation efficiency [34]. eIF2α is phosphorylated by eIF2α kinases under conditions of stress or starvation. Phosphorylation of eIF2α suppresses the catalysis of eIF2B on the conversion of eIF2-GDP to eIF2-GTP, which is crucial for the round of translation initiation [34]. Eukaryotic translation elongation is regulated by various eukaryotic translation elongation factors (eEFs) [34]. Among them, eEF2 mediates the translocation of the ribosome to the next codon during translation [32]. Phosphorylation of eEF2 by its sole kinases dissociates it from ribosomes and inhibits the translation elongation [32]. In the present study, dietary Se supplementation exerted no influences on the phosphorylation of 4E-BP1, S6, eIF2α, and eEF2, indicating no influences of dietary Se on translation initiation and elongation in rainbow trout white muscle. In addition to the translational activity, protein synthesis also relies on the ribosome biogenesis, a determinant of translational capacity [35]. The ratio of RNA/DNA and the abundance of ribosomal RNA are considered accurate indexes of translational capacity and have been used for the evaluation of muscle protein synthesis [30, 36]. In this study, the translational capacity in rainbow trout white muscle was also not affected by dietary Se proved by the similar ratio of RNA/DNA and abundance of ribosomal RNA among groups. Collectively, dietary Se has no effects on protein synthesis in rainbow trout white muscle.
Protein degradation in fish muscle relies on three major proteolytic systems: calpain system, autophagy-lysosome pathway, and ubiquitin-proteasome pathway [7, 9]. Calpain cascade involves CAPN 1, CAPN 2 and their inhibitors, CAST-L, and CAST-S [37]. In the present study, although the expression of CAPN 1 significantly declined in trout fed diets supplemented with both 2 and 4 mg/kg Se, the expression of CAST-L and CAST-S only increased in trout fed diet supplemented with 4 mg/kg Se. The combined effect of the declined expression of CAPN 1 and the elevated expression of CAST-L and CAST-S led to a significantly decreased calpain activity in trout fed diet supplemented with 4 mg/kg Se. Protein degradation through ubiquitin-proteasome pathway relies on the selective attachment of ubiquitin molecules to the protein substrate by E3 ubiquitin ligases. Among these E3 ubiquitin ligases, Fbx32 (also called atrogin-1) and MuRF1 have been studied in depth and shown to play the key roles in the control of skeletal muscle mass [38]. Our results showed that the expression of MuRF1 exhibited no significant difference among groups, whereas, the expression of Fbx32 significantly declined in trout fed diets supplemented with both 2 and 4 mg/kg Se accompanied by a significant decrease of ubiquitinated proteins. Transcription of Fbx32 requires the nuclear localization of the FoxOs [38]. In the present study, the nuclear localization of FoxO3a significantly declined in trout fed diets supplemented with both 2 and 4 mg/kg Se, which could be a reason for the decrease of Fbx32 expression. In autophagy-lysosome pathway, dietary Se significantly downregulated the expression of numerous autophagy-related genes; however, it exerted no significant influences on LC3-II/LC3-I ratio, a biomarker for activity of autophagy [28]. Collectively, these results suggest that dietary Se exerts a negative influence on protein degradation in trout white muscle by suppressing the calpain system and ubiquitin-proteasome pathway.
In addition to the protein deposition, fusion of myoblast into the mature muscle fibers is another important way for the hypertrophy of muscle fibers [7]. Fusion of myoblast into the existed muscle fibers leads to an increased nuclei number of the muscle fiber [7]. In the present study, the elevated nuclei/muscle fiber and DNA content/muscle fiber in white muscle of trout fed diet supplemented with 4 mg/kg Se indicate an acceleration of dietary Se on the fusion of myoblast into the mature muscle fibers. Myoblasts derive from muscle stem cells and undergo proliferation, differentiation, and migration before fusion into the existing muscle fibers [7]. These processes are under the control of a suite of gene products including myogenic regulatory factors (MRFs), Paxs and MEFs, etc. [39]. Our results showed that diets supplemented with 2 and 4 mg/kg Se upregulated the expression of Pax7a, Pax7b, MyoG, and MEF2c. Pax7 has been demonstrated highly expressed in both quiescent and activated muscle satellite cells [39, 40]. The elevated expression of Pax7a and Pax7b might indicate an improvement of dietary Se on the proliferation of muscle satellite cells in trout white muscle. MyoG and MEF2c have been reported crucial for the differentiation of myoblasts [40, 41]. The elevated expression of MyoG and MEF2c in rainbow trout white muscle indicates a positive influence of dietary Se on the differentiation of myoblasts, which could also be evidenced by the upregulated expression of fMyHC, a marker for the sarcomeric assembly in the late stages of myoblast differentiation [40]. The promoted proliferation of muscle satellite cells and differentiation of myoblasts might be the possible explanations for the promoted fusion of myoblast into the mature muscle fibers.
Se performs its biological functions mainly by incorporation into selenoproteins [42]. In the present study, dietary Se supplementation increased the expression of GPX4b, SelK, SelP, SelU, and SelWl in trout white muscle. Correlation analysis showed that the expression of SelK and SelWl were significantly correlated to all the indexes related to trout somatic growth, white muscle growth, white muscle protein deposition, and fusion of myoblasts into the existed muscle fibers. It was similar to our previous observation that expression of SelK and SelWl exhibited the higher and more significant correlations with the rainbow trout growth than other selenoprotein genes [18]. These results reveal that SelK and SelW might be the key selenoproteins involving in the regulation of trout muscle growth. SelW has been proved to highly express in muscle tissues and important to maintain the metabolic stability in muscle [43]. Although limited information is available for the functions of SelK in animal muscle, the aberrant expression of SelK has been reported to lead to the dysfunction in chicken muscles [44] and the apoptosis of chicken myoblasts [45].
Conclusion
This study illustrated that dietary Se within the nutritional range promotes the hypertrophic growth of trout white muscle by upregulating the expression of selenoproteins, in particular SelK and SelW, which (1) inhibit the calpain system and ubiquitin-proteasome pathway-mediated protein degradation and (2) promote the fusion of myoblasts into existed muscle fibers in trout muscle. Finally, the enhanced hypertrophic growth of white muscle leads to the improvement of trout somatic growth. The present results reveal a possible regulatory mechanism of dietary Se on fish growth, and could help to better understand the physiological functions of Se in fish.
References
Khan KU, Zuberi A, Fernandes JBK, Ullah I, Sarwar H (2017) An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol Biochem 43:1689–1705. https://doi.org/10.1007/s10695-017-0402-z
Prabhu PAJ, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquacult 8:172–219. https://doi.org/10.1111/raq.12090
Lin YH, Shiau SY (2005) Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 250:356–363. https://doi.org/10.1016/j.aquaculture.2005.03.022
Bell JG, Cowey CB, Adron JW, Pirie BJS (1987) Some effects of selenium deficiency on enzyme-activities and indexes of tissue peroxidation in Atlantic salmon parr (Salmo-salar). Aquaculture 65(1):43–54. https://doi.org/10.1016/0044-8486(87)90269-9
Gao XJ, Tang B, Liang HH, Yi L, Wei ZG (2019) Selenium deficiency induced an inflammatory response by the HSP60-TLR2-MAPKs signalling pathway in the liver of carp. Fish Shellfish Immunol 87:688–694. https://doi.org/10.1016/j.fsi.2019.02.017
Zheng L, Feng L, Jiang WD, Wu P, Tang L, Kuang SY, Zeng YY, Zhou XQ, Liu Y (2018) Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 77:53–70. https://doi.org/10.1016/j.fsi.2018.03.024
Valente LMP, Moutou KA, Conceicao LEC, Engrola S, Fernandes JMO, Johnston IA (2013) What determines growth potential and juvenile quality of farmed fish species? Rev Aquacult 5:S168–S193. https://doi.org/10.1111/raq.12020
Seiliez I, Dias K, Cleveland BM (2014) Contribution of the autophagy-lysosomal and ubiquitin-proteasomal proteolytic systems to total proteolysis in rainbow trout (Oncorhynchus mykiss) myotubes. Am J Physiol Regul Integr Comp Physiol 307:R1330–R1337. https://doi.org/10.1152/ajpregu.00370.2014
Johnston IA, Bower NI, Macqueen DJ (2011) Growth and the regulation of myotomal muscle mass in teleost fish. J Exp Biol 214:1617–1628. https://doi.org/10.1242/jeb.038620
Johnston I, Strugnell G, McCracken ML, Johnstone R (1999) Muscle growth and development in normal-sex-ratio and all-female diploid and triploid Atlantic salmon. J Exp Biol 202:1991–2016
Mommsen TP (2001) Paradigms of growth in fish. Comp Biochem Phys B 129:207–219. https://doi.org/10.1016/s1096-4959(01)00312-8
Rowlerson A, Veggetti A (2001) Cellular mechanisms of post-embryonic muscle growth in aquaculture species. Fish Physiol 18:103–140. https://doi.org/10.1016/S1546-5098(01)18006-4
Weatherley A, Gill H, Lobo A (1988) Recruitment and maximal diameter of axial muscle fibres in teleosts and their relationship to somatic growth and ultimate size. J Fish Biol 33:851–859
Alami-Durante H, Medale F, Cluzeaud M, Kaushik SJ (2010) Skeletal muscle growth dynamics and expression of related genes in white and red muscles of rainbow trout fed diets with graded levels of a mixture of plant protein sources as substitutes for fishmeal. Aquaculture 303:50–58. https://doi.org/10.1016/j.aquaculture.2010.03.012
Alami-Durante H, Cluzeaud M, Duval C, Maunas P, Girod-David V, Médale F (2014) Early decrease in dietary protein:energy ratio by fat addition and ontogenetic changes in muscle growth mechanisms of rainbow trout: short- and long-term effects. Br J Nutr 112:674–687. https://doi.org/10.1017/s0007114514001391
Alami-Durante H, Bazin D, Cluzeaud M, Fontagné-Dicharry S, Kaushik S, Geurden I (2018) Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 483:273–285. https://doi.org/10.1016/j.aquaculture.2017.10.030
Alami-Durante H, Cluzeaud M, Bazin D, Mazurais D, Zambonino-Infante JL (2011) Dietary cholecalciferol regulates the recruitment and growth of skeletal muscle fibers and the expressions of myogenic regulatory factors and the myosin heavy chain in European sea bass larvae. J Nutr 141:2146–2151. https://doi.org/10.3945/jn.111.146118
Wang L, Zhang XZ, Wu L, Liu Q, Zhang DF, Yin JJ (2018) Expression of selenoprotein genes in muscle is crucial for the growth of rainbow trout (Oncorhynchus mykiss) fed diets supplemented with selenium yeast. Aquaculture 492:82–90. https://doi.org/10.1016/j.aquaculture.2018.03.054
Wang L, Chen CY, Liu WJ, Xia H, Li J, Zhang XZ (2017) Effects of toxic cyanobacteria and ammonia on flesh quality of blunt snout bream (Megalobrama amblycephala). J Sci Food Agric 97:1200–1206. https://doi.org/10.1002/jsfa.7850
Valente L, Rocha E, Gomes E, Silva M, Oliveira M, Monteiro R, Fauconneau B (1999) Growth dynamics of white and red muscle fibres in fast-and slow-growing strains of rainbow trout. J Fish Biol 55:675–691
Wang L, Wu L, Liu Q, Zhang DF, Yin JJ, Xu Z, Zhang X (2018) Improvement of flesh quality in rainbow trout (Oncorhynchus mykiss) fed supranutritional dietary selenium yeast is associated with the inhibited muscle protein degradation. Aquac Nutr 24:1351–1360. https://doi.org/10.1111/anu.12672
Fontagné-Dicharry S, Godin S, Liu HK, Prabhu PAJ, Bouyssière B, Bueno M, Tacon P, Médale F, Kaushik SJ (2015) Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br J Nutr 113:1876–1887. https://doi.org/10.1017/s0007114515001300
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. https://doi.org/10.1093/nar/29.9.e45
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. https://doi.org/10.1186/gb-2002-3-7-research0034
Seiliez I, Gabillard J-C, Riflade M, Sadoul B, Dias K, Avérous J, Tesseraud S, Skiba S, Panserat S (2012) Amino acids downregulate the expression of several autophagy-related genes in rainbow trout myoblasts. Autophagy 8:364–375. https://doi.org/10.4161/auto.8.3.18863
Belghit I, Skiba-Cassy S, Geurden I, Dias K, Surget A, Kaushik S, Panserat S, Seiliez I (2014) Dietary methionine availability affects the main factors involved in muscle protein turnover in rainbow trout (Oncorhynchus mykiss). Br J Nutr 112:493–503. https://doi.org/10.1017/s0007114514001226
Zhang Q, Vashisht AA, O'Rourke J, Corbel SY, Moran R, Romero A, Miraglia L, Zhang J, Durrant E, Schmedt C, Sampath SC (2017) The microprotein Minion controls cell fusion and muscle formation. Nat Commun 8. https://doi.org/10.1038/ncomms15664
Park HYL, Kim JH, Park CK (2012) Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis 3:e290. https://doi.org/10.1038/cddis.2012.26
AlamiDurante H, Fauconneau B, Rouel M, Escaffre AM, Bergot P (1997) Growth and multiplication of white skeletal muscle fibres in carp larvae in relation to somatic growth rate. J Fish Biol 50:1285–1302. https://doi.org/10.1006/jfbi.1997.0388
Valente LMP, Gomes EFS, Fauconneau B (1998) Biochemical growth characterization of fast and slow-growing rainbow trout strains: effect of cell proliferation and size. Fish Physiol Biochem 18:213–224. https://doi.org/10.1023/a:1007774929535
Hunt AO, Berkoz M, Ozkan F, Yalin S, Ercen Z, Erdogan E, Gunduz SG (2011) Effects of organic selenium on growth, muscle composition, and antioxidant system in rainbow trout. Isr J Aquacult-Bamid 63
Sivan G, Stein OE (2008) Regulation of mRNA translation during cellular division. Cell Cycle 7:741–744. https://doi.org/10.4161/cc.7.6.5596
Pestova TV, Hellen CUT (2000) The structure and function of initiation factors in eukaryotic protein synthesis. Cell Mol Life Sci 57:651–674. https://doi.org/10.1007/pl00000726
Goodman CA, Mayhew DL, Hornberger TA (2011) Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 23:1896–1906. https://doi.org/10.1016/j.cellsig.2011.07.013
Figueiredo VC, McCarthy JJ (2019) Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology 34:30–42. https://doi.org/10.1152/physiol.00034.2018
Bennett AH, O'Donohue MF, Gundry SR, Chan AT, Widrick J, Drapers I, Chakraborty A, Zhou Y, Zon LI, Gleizes PE, Beggs AH, Gupta VA (2018) RNA helicase, DDX27 regulates skeletal muscle growth and regeneration by modulation of translational processes. PLoS Genet 14:e1007226. https://doi.org/10.1371/journal.pgen.1007226
Goll DE, Thompson VF, Li HQ, Wei W, Cong JY (2003) The calpain system. Physiol Rev 83:731–801. https://doi.org/10.1152/physrev.00029.2002
Sanchez AMJ, Candau RB, Bernardi H (2014) FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci 71:1657–1671. https://doi.org/10.1007/s00018-013-1513-z
Buckingham M, Rigby PWJ (2014) Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev Cell 28:225–238. https://doi.org/10.1016/j.devcel.2013.12.020
Zammit PS (2017) Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 72:19–32. https://doi.org/10.1016/j.semcdb.2017.11.011
Liu N, Nelson BR, Bezprozvannaya S, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN (2014) Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc Natl Acad Sci U S A 111:4109–4114. https://doi.org/10.1073/pnas.1401732111
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727. https://doi.org/10.1074/jbc.R800045200
Whanger PD (2009) Selenoprotein expression and function-Selenoprotein W. Biochim Biophys Acta 1790:1448–1452. https://doi.org/10.1016/j.bbagen.2009.05.010
Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Lu XG, Le XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141:1605–1610. https://doi.org/10.3945/jn.111.145722
Fan RF, Cao CY, Chen MH, Shi QX, Xu SW (2018) Gga-let-7f-3p promotes apoptosis in selenium deficiency-induced skeletal muscle by targeting selenoprotein K. Metallomics 10:941–952. https://doi.org/10.1039/c8mt00083b
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (grant number: 2662019FW013); the Da Bei Nong Group Promoted Project for Young Scholar of HZAU (grant number: 2017DBN018) and the Fundamental Research Funds for the Central Universities (grant number 2662015PY024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study was approved by The Scientific Ethic Committee of Huazhong Agricultural University (no. HZAUFI-2018-017).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 731 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Zhang, D., Li, S. et al. Dietary Selenium Promotes Somatic Growth of Rainbow Trout (Oncorhynchus mykiss) by Accelerating the Hypertrophic Growth of White Muscle. Biol Trace Elem Res 199, 2000–2011 (2021). https://doi.org/10.1007/s12011-020-02282-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02282-w