Abstract
Sodium tungstate is an alternative to reduce hyperglycemia for the treatment of diabetes. In previous work, we showed that the administration of sodium tungstate increased the specific activity of salivary amylase in the parotid gland. Here, we investigated the effect of the administration of sodium tungstate on the lipid peroxidation and some antioxidant parameters in the submandibular (SM) and parotid (PA) salivary glands of streptozotocin (STZ)-induced diabetic rats. Thirty-two male Wistar rats were divided into four groups (n = 8, each): control (C), control treated with sodium tungstate (CT), diabetic (D), and diabetic treated with sodium tungstate (CT). Sodium tungstate (2 mg/ml) was administered to the STZ-induced diabetic rats for 15 days. Malondialdehyde (MDA), reduced (GSH) and oxidized (GSSG) glutathione, and blood glucose concentrations were quantified. In addition, superoxide dismutase (SOD) and catalase (CAT) activities were assessed. Results revealed that diabetes caused an increase in MDA concentration in both glands, a reduction in the SOD activity in SM, and an increase in catalase activity in PA glands. Administration of sodium tungstate reduced the blood glucose levels and normalized the SOD activity in the SM and MDA levels in both glands of the STZ-induced diabetic rats. Catalase activity was increased in PA glands of diabetic and tungstate-treated animals (p < 0.05). The GSH/GSSG ratio was increased in SM glands of tungstate-treated animals (p < 0.05). Overall, the reduction of hyperglycemia by sodium tungstate reduced lipid peroxidation and caused alterations in the antioxidant system in the salivary glands of STZ-induced diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia as result of defects in insulin secretion, insulin action, or both [1]. Oxidative stress, a disturbance between pro-oxidants and antioxidants [2, 3], is responsible for several complications in diabetes [4]. The hyperglycemic state in diabetes increases oxidative stress by the overproduction of reactive oxygen species (ROS) [5].
Salivary glands play an important role in the oral cavity homeostasis through the secretion of saliva, a fluid rich in water, protein, and electrolytes [6]. There is evidence of the contribution of ROS molecules to injury in several tissues [7], including salivary glands [8, 9]. Experimental models of diabetes induced by alloxan or streptozotocin have shown the reduction of glandular weight [10] and salivary flow rate [11] as well as alterations in the sialic acid content [12, 13], antioxidant system [8, 9], and carbohydrate metabolism [14,15,16,17]. Besides systemic complications, chronic hyperglycemia was associated with oral manifestations such as low salivary flow rate, xerostomia, taste alterations, sialosis, as well as influence in dental caries, periodontal disease, oral soft lesions, and fungal infections [18].
The exposure of organisms to oxidative stress often leads to an increase in the synthesis of antioxidants and other defense systems [19]. Thus, different antioxidants are necessary to protect the organs against these various events [19]. An antioxidant is “any substance that, when presented at low concentrations compared with those of an oxidizable substrate, significantly delays or prevents oxidation of the substrate” [3]. Superoxide dismutase (SOD) and catalase (CAT) are well-known key enzymes of the antioxidant system [19]. In addition, glutathione plays an important role as a nonenzymatic antioxidant that can eliminate free radicals from the body [19, 20].
Although several antidiabetic drugs are commercially available nowadays, an ideal agent was not found so far [21]. The development of new therapies able to manage glycemia and even to cure diabetes is of great interest [22]. Inorganic compounds are alternatives to reduce hyperglycemia for the treatment of diabetes [23]. Oxyanions derived from vanadium, selenium, molybdenum, and tungstate present effects on energetic metabolism with the advantage to be of oral administration [23, 24].
Sodium tungstate is an inorganic compound with antidiabetic and antioxidant activities [25]. The previous work from our group showed that administration of sodium tungstate reduced the blood glucose concentration after 2 weeks of treatment and increased the specific activity of salivary amylase in the parotid gland of diabetic rats [26]. Here, we aimed to examine the effect of the administration of sodium tungstate on the oxidative stress in the submandibular and parotid salivary glands of streptozotocin-induced diabetic rats. The results of this study were used (1) to test whether significant changes in the blood glucose levels of experimental and control rats were induced by sodium tungstate administration and (2) if the administration of sodium tungstate would have influence on the lipid peroxidation and some antioxidant parameters in the submandibular and parotid salivary glands of the STZ-induced diabetic rats.
Materials and Methods
Animals
Thirty-two adult male Wistar rats with initial body weight of 200 g were used in the present investigation. The protocol of this study was approved by the Animal Ethics Committee of the School of Dentistry of the University of São Paulo (# 003/2014). The care and handling of the animals were conducted following the principles for animal experimentation established by the Brazilian Committee for Animal Experimentation. The animals were kept under constant 12-h light-dark cycle, at a temperature of 24 °C with free access to food (Nuvilab CR-1, Nuvital Nutrientes S/A, Brazil) and water.
The animals were divided into four groups (n = 8 per group): control (C); control treated with 2 mg/mL of sodium tungstate (CT); diabetic (D); and diabetic treated with 2 mg/mL of sodium tungstate (DT).
Induction of Diabetes
Diabetes was induced in overnight-fasted rats by a single intraperitoneal injection of streptozotocin (60 mg/Kg/BW) dissolved in 0.1-M sodium citrate buffer (pH = 4.5). Animals of the control group received only the vehicle. Seventy-two hours after the injection of streptozotocin, diabetes was confirmed by determination of the blood glucose concentration of the 8-h fasted rats. Only the animals with the initial glycemia higher than 250 mg/dL were considered diabetic.
Animal Treatment
Thirty days after induction of diabetes, sodium tungstate (2 mg/ml) was administered in rats of groups CT and DT for 15 days. The sodium tungstate was dissolved in distilled water and given in place of drinking water. Non-treated control and diabetic animals received only water. Water and water with sodium tungstate were renewed daily.
Sample Collection
After 15 days of treatment, the animals were always sacrificed in the morning (8:00–9:00 a.m.) to minimize circadian rhythm. The parotid (PA) and submandibular (SM) glands were immediately removed, cleaned of adherent tissues, clamped between aluminum tongs previously cooled in dry ice, and stored at −80 °C until the moment of the analysis.
Sample Analysis
Blood glucose was determined by the glucose oxidase-peroxidase method [27]. The salivary gland tissues were homogenized at 10% (w/v) in 10-mM sodium phosphate buffer, pH 7.4, centrifuged at 3020 × g for 10 min. The supernatant was used for the biochemical analysis [26]. Superoxide dismutase (SOD) and catalase activities were determined according to Paoletti [28] and Aebi [29], respectively. Lipid peroxidation was analyzed by measuring the presence of malondialdehyde (MDA) [30]. For the determination of reduced (GSH) and oxidized (GSSG) glutathione levels, a commercial glutathione colorimetric kit (Arbor Assays) was used. Total protein levels were determined by the Folin-phenol reagent method [31].
Normal data distribution was verified through Shapiro-Wilk normality test, and Levene’s test was used to assess for homogeneity of variances. Data were analyzed by analysis of variance (ANOVA) followed by Tukey’s test (α = 0.05). Data are presented as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 7.00 (GraphPad Software, Inc., CA, USA).
Results
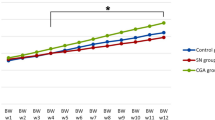
The body weight, glycemia, food consumption, and liquid ingested by non-treated and treated animals are presented in Fig. 1. The initial body weight (Fig. 1a) and glycemia (Fig. 1b) were higher in the diabetic animals compared with the control group (p < 0.05). Diabetic animals also presented higher food (Fig. 1c) and liquid consumption (Fig. 1d) (p < 0.05). Diabetic rats treated with sodium tungstate presented lower blood glucose concentration compared with untreated diabetic (Fig. 1b). These values, however, were still higher than the C group. The administration of sodium tungstate was able to normalize the food (Fig. 1c) and liquid intake (Fig. 1d) of the diabetic group (p < 0.05). The final body weight (Fig. 1a), however, was much lower for diabetic animals treated with sodium tungstate compared with the untreated diabetic and control rats (p < 0.05).
Body weight (a), glycemia (b), food consumption (c), and liquid ingested (d) by control-, diabetic-, and tungstate-treated animals. Each value represents the mean ± SD. Differences between initial and final evaluations of body weight and glycemia were compared using the two-way ANOVA with Tukey’s test. Differences between food consumption and liquid ingested were compared using the one-way ANOVA with Tukey’s test. Differences statistically significant between groups are represented by different letters (p < 0.05). C, control; C + T, control treated with sodium tungstate; D, diabetic; D + T, diabetic treated with sodium tungstate
Figures 2 and 3 show, respectively, the malondialdehyde concentration, superoxide dismutase and catalase activities, reduced and oxidized glutathione levels, and reduced/oxidized glutathione ratio in the submandibular and parotid salivary glands of control and diabetic rats.
Malondialdehyde concentration (a), superoxide dismutase (b) and catalase (c) activities, reduced (d) and oxidized (e) glutathione levels, and reduced/oxidized glutathione ratio (f) in the submandibular glands of control-, diabetic-, and tungstate-treated animals. Each value represents the mean ± SD. Differences between groups were compared using the one-way ANOVA with Tukey’s test. Differences statistically significant between groups are represented by different letters (p < 0.05). C, control; C + T, control treated with sodium tungstate; D, diabetic; D + T, diabetic treated with sodium tungstate
Malondialdehyde concentration (a), superoxide dismutase (b) and catalase (c) activities, reduced (d) and oxidized (e) glutathione levels, and reduced/oxidized glutathione ratio (f) in the parotid glands of control-, diabetic-, and tungstate-treated animals. Each value represents the mean ± SD. Differences between groups were compared using the one-way ANOVA with Tukey’s test. Differences statistically significant between groups are represented by different letters (p < 0.05). C, control; C + T, control treated with sodium tungstate; D, diabetic; D + T, diabetic treated with sodium tungstate
In the submandibular (Fig. 2a) and parotid (Fig. 3a) salivary glands, malondialdehyde concentration was higher in diabetic animals compared with those of the control group (p < 0.05). Control rats treated with sodium tungstate presented higher MDA values compared with C group (p < 0.05) in the SM and PA salivary gland. In both glands, diabetic rats treated with sodium tungstate presented lower MDA levels (p < 0.05). In the submandibular gland (Fig. 2a), however, the levels were still higher than the untreated control rats (p < 0.05).
The superoxide dismutase activity in submandibular salivary glands of the non-treated diabetic animals was lower than the C group (p < 0.05). The administration of sodium tungstate normalized the SOD activity in this gland (Fig. 2b). In the PA salivary glands (Fig. 3b), however, no difference in superoxide dismutase activity was observed between C and D groups (p > 0.05). The DT group showed higher SOD activity (p < 0.05).
No differences in catalase activities (Fig. 2c) were observed in the submandibular salivary glands of all groups (p > 0.05), while in the parotid gland (Fig. 3c), it was higher for diabetic and tungstate-treated animals (p < 0.05).
Lower concentrations of reduced glutathione were observed in the submandibular salivary glands (Fig. 2d) of the diabetic animals compared with C group (p < 0.05). The administration of sodium tungstate led to a higher concentration of GSH in the submandibular salivary glands of diabetic animals (p < 0.05) without normalizing it (Fig. 2d). In the parotid salivary glands, GSH concentration was lower (Fig. 3d) in the D group compared with control (p < 0.05). CT group presented lower levels of GSH (p < 0.05), while no difference was observed in the DT group (p > 0.05) (Fig. 3d).
Oxidized glutathione was lower in the SM salivary glands (Fig. 2e) of diabetic animals compared with control (p < 0.05). Rats from the CT group showed lower levels of GSSG (p < 0.05) while it was not observed in the diabetic-treated group (Fig. 2e). In the parotid gland, GSSG was lower in diabetic animals (Fig. 3e). Lower and higher concentrations of GSSG were observed in groups CT and DT, respectively (Fig. 3e).
In the submandibular salivary glands, no difference in GSH/GSSG ratio was observed between control and diabetic animals. This ratio, however, was higher in groups CT and DT (p < 0.05) (Fig. 2f). The GSH/GSSG ratio was higher in the parotid salivary glands of diabetic animals compared with control (p < 0.05). CT and DT groups showed higher and lower GSH/GSSG ratio, respectively (Fig. 3f).
Discussion
In the previous work of our group [8, 9], we evaluated the antioxidant system and the presence of oxidative stress in the submandibular and parotid salivary glands. The STZ-induced diabetic rats showed alterations and disturbances in the enzymatic antioxidant system and oxidative stress mainly in the submandibular salivary glands [8, 9]. The present study demonstrated that reduction in hyperglycemia by the administration of sodium tungstate (2 mg/ml) decreased lipid peroxidation and alterations in the antioxidant system in the salivary glands of the STZ-induced diabetic rats.
The STZ-induced diabetic rats showed increase in plasma glucose concentration and liquid and food intakes and decrease in body weight, which is in agreement with the literature [32, 33]. As observed in other studies, the administration of sodium tungstate to diabetic rats normalized the food consumption and liquid ingestion [24, 26]. The administration of sodium tungstate to diabetic rats normalizes the blood glucose levels in some studies [34, 35]. Controversially, in this study, we show that although sodium tungstate administration reduced the glycemia of the treated diabetic rats, it was not able to bring the glycemic values to the control levels. This corroborates previous works [24, 26]. Interestingly, the body weight of diabetic rats after 15 days of tungstate administration was lower than untreated diabetic and control groups.
Sodium tungstate improves the glycemic state by a direct effect on pancreatic beta-cells and insulinotropic activity and alleviating the activation of the general control of nutrient pathway [22, 36]. In addition, the literature shows sodium tungstate as a suitable and promising agent in the treatment of obesity [37, 38]. Although the mechanism has not been fully described, sodium tungstate was shown to reduce body weight gain and food intake in overweight rats due to its effect on the central nervous system [37, 39] and activation of kinases involved in the leptin signaling pathway [38]. Thus, energy expenditure is increased, and changes in the expression of key genes involved in adipose tissue thermogenesis occur [37].
The chronic hyperglycemia in the diabetic state is directly associated with the production of reactive oxygen species generated in the direct autoxidation process of glucose or impaired antioxidant defenses [40, 41]. In addition, the formation of advanced glycation end products as a result of the production of ROS due to hyperglycemia in diabetes may inactivate the enzymes involved in the antioxidant system of the body [42]. Lipid peroxidation is one of the consequences of increased ROS, which results in binding of ROS to unsaturated fatty acids, thereby modifying their structure and generating malondialdehyde, which is an important marker of oxidative damage [19].
In this study, the higher concentrations of malondialdehyde in the submandibular and parotid salivary glands of diabetic animals suggest that both glands are affected, as previously reported [8, 9]. The administration of sodium tungstate exerted a pro-oxidant effect in both SM and PA salivary glands of control rats. Oxidative stress is seen as the major cause of the toxic manifestations of sodium tungstate [43]. Further studies, however, are necessary to uncover the exact mechanism of side effects of tungstate in healthy animals [25]. The normalized and lower MDA levels observed in, respectively, SM and PA salivary glands of the diabetic-treated rats might be due to the improvement of the glycemic state and minor contribution to overall ROS generation [32, 40].
The superoxide dismutase enzyme, found in the nucleus and cytoplasm of the cell, converts by a dismutase reaction, the superoxide anion to hydrogen peroxide [44]. There are conflicting results with regard to SOD status in diabetes. In several tissues, the SOD activity in the diabetic state was shown to be decreased or elevated [45,46,47]. In this study, the superoxide dismutase activity in the SM glands of the diabetic animals was reduced, while no difference was found in the parotid gland. It is known that SM and PA salivary glands react differently in diabetes [8, 9, 48]. In previous studies [8], we reported no difference in the SOD activity in the SM glands in 28 [8] and 30 days [9] after the induction by streptozotocin. In the parotid glands, however, the SOD activity showed to be unaffected [9] or increased [8] compared with nondiabetic animals.
The increased levels or prolonged exposure to ROS causes pathologic changes and modification of DNA, RNA, carbohydrates, proteins, and lipids [49,50,51,52]. The expected increased levels of SOD activity in the diabetic state would be a result of a protective and adaptive mechanism against the oxidative stress [8, 44, 53] as after a mild increment in ROS generation, cells would be able to increase the antioxidant response and overcome oxidative stress [54]. However, in the presence of oxidative damage, cells may no longer be able to protect themselves and even antioxidant enzymes could be degraded [53, 55, 56]. In this study, the MDA levels in the submandibular glands of the diabetic group were higher than the control group suggesting an oxidative damage in this gland. The decreased level of the SOD activity could also be a result of the persistence of the diabetic state in which the free radicals accumulate. After the enzymes scavenge excessive free radicals, the oxidative stress condition will temporary disappear and the cell will cease the production of defense enzymes and drop its activity. This would be investigated by analyzing the expression of the Nrf2 antioxidant transcription factor that activates the production of protective enzymes [57,58,59]. In a recent study of our group [48], SOD activity was reduced in the major salivary glands. In the study, however, the animals were evaluated 30 days after diabetes induction. In addition, the measurement of ROS such as hydrogen peroxide and superoxide anion levels should also be considered in further analysis.
In the submandibular and parotid glands, no difference in SOD activity between control and control-treated animals was observed. The administration of sodium tungstate in the STZ-induced diabetic animals increased SOD activity in both salivary glands. This increase was higher in the parotid gland. In the submandibular gland, however, the tungstate-treated diabetic rats presented normalized values of superoxide dismutase activity compared with control and control-treated animals. Similar results were found in brain tissue in which the superoxide dismutase activity was restored with the administration of sodium tungstate [32].
Catalase is a heme-protein enzyme present in peroxisomes that catalyzes the conversion of hydrogen peroxide to water and oxygen [19]. The activity of this enzyme in diabetic-induced animals is altered in several tissues [60, 61]. In this study, no difference in catalase activity was found in the submandibular salivary glands of the streptozotocin-induced diabetic rats compared with the control group. In the parotid gland, however, this enzyme activity was higher, corroborating previous studies from our group [8, 9]. As also reported in the brain tissue [32], the administration of sodium tungstate to diabetic animals had no effect on both salivary glands, while only in the parotid glands of control-treated animals, catalase activity was increased.
Diabetes causes alterations in carbohydrate metabolism in both submandibular and parotid salivary glands [9, 14,15,16,17]. Despite increase in activity of phosphofructokinase-1 and glycogen synthase as well glycogen and NADP content, a reduction in the hexokinase activity and total ATP and cAMP were reported in the SM salivary gland [9, 14,15,16]. In addition, in the PA gland, total ATP, NAD, and NADP content and cofactors, used mainly in the glycolytic and pentose phosphate pathways, were increased in diabetes [9, 14,15,16]. A reduction of ATP content may lead to a reduction in the activity of several enzymes. The increase in SOD activity in the SM and PA salivary glands of diabetic-treated rats and the higher catalase activity in parotid glands of the control-treated animals observed in this study may be due to the increase on the glycolytic flux [35] and direct enzyme activation or induction of gene expression by sodium tungstate [32]. Moreover, the hyperglycemic state and the tungstate treatment, in which the rats were exposed, might have altered the expression and concentration of several enzymes and cofactors involved in carbohydrate metabolism and the antioxidant system. Thus, a broad experimental approach including different biochemical and biomolecular parameters would be necessary to better address the whole picture of the alterations found in the submandibular and parotid salivary glands in diabetes as well as the effect of sodium tungstate in a dose- and time-dependent manner.
Glutathione is a tripeptide present in all animal cells and plays an important role as a nonenzymatic antioxidant that can eliminate free radicals from the body [19]. Glutathione participates in the reactions catalyzed by glutathione peroxidase (GPx) and reductase (GR) [19, 53]. It is found in either reduced or oxidized state. The maintenance of optimal GSH/GSSG ratio in the cell is essential for survival [62]. Low levels of GSH are reported in diabetes [63]. In a previous study [9], GSH and GSSG were increased in the SM gland of diabetic animals. In the PA gland, however, GSH and GSH/GSSG ratio was reduced while no difference in GSSG was observed. The differences found between the studies may be due to the different evaluation periods for the determination of GSH and GSSG concentrations.
In this study, the decrease in GSH and GSSG concentrations and GSH/GSSG ratio in the diabetic animals would indicate an impaired glutathione defense [63]. Diabetic patients present glutathione deficiency [64, 65], and the lower glutathione levels may be explained due to the reduced synthesis and increased utilization by non-glycemic mechanisms [64, 65]. In addition, nutritional factors might be considered given that dietary intake of ascorbic acid and α-tocopherol was shown to play a role in oxidative stress in diabetes [48, 66, 67]. In a previous work, we observed a reduction in the serum levels of ascorbic acid (vitamin C) and α-tocopherol (vitamin E) in the diabetic rats, which suggested their importance in the antioxidant system in diabetes [48]. The metabolism of glutathione and the vitamins C and E was reported to be closely related [68, 69]. The higher GSSG levels would indicate lower and higher GPx and GR activities, respectively [19, 53]. This was observed in a previous study [48]. Furthermore, the lower GSSG levels might be a response to oxidative stress and insufficiency to counter the oxidative damage induced by diabetic state in the salivary gland. To confirm this hypothesis, analysis of GPx and GR activities is required.
The increase in GSH/GSSG ratio in control- and diabetic-treated animals would indicate a decrease in oxidative stress [62, 63]. Sodium tungstate presents insulin-like actions [34], and insulin was shown to elevate the GSH/GSSG ratio and decrease intracellular oxidative stress in diabetic patients [70]. Interestingly, in the parotid glands of diabetic-treated animals, a low GSH/GSSG ratio was observed. The submandibular and parotid salivary glands present different antioxidant responses [8, 9, 17] and metabolic profiles [17, 71], and under diabetic state, the glycolytic flux in submandibular is more resilient than in the parotid gland [17]. The effectiveness of catalase, superoxide dismutase, glutathione reductase, and peroxidase depends on the availability of NADPH [72]. Moreover, ATP production via oxidative phosphorylation cannot proceed effectively in the absence of NADPH [73]. Among other mechanisms, the NADPH is generated through the pentose phosphate pathway [74]. The rate-limiting enzyme of the pentose phosphate pathway is glucose-6-phosphate dehydrogenase (G6PDH). In a previous study, we observed a markedly increase in G6PDH activity in the parotid gland of tungstate-treated diabetic animals [75]. In addition, the parotid gland of diabetic rats presented increased amylase and peroxidase activities and sialic acid content, which was hypothesized as a result of an initial behavior of defense [26]. The lower levels of GSH and GSH/GSSG ratio of the diabetic rats indicate that the glutathione defense system of the parotid gland was compromised [76]. This might be also due to the initial response of this gland against oxidative stress and return to its normal function as MDA levels normalized after 15 days of tungstate administration. Moreover, a previous work [26] showed a recovery capacity of this gland after 6 weeks of treatment with sodium tungstate. A further study with a long experimental period would be necessary to confirm or refute this hypothesis.
Conclusion
The reduction in hyperglycemia by the administration of sodium tungstate decreased lipid peroxidation and alterations in the antioxidant system in the salivary glands of streptozotocin-induced diabetic rats.
References
Classification and diagnosis of diabetes (2015). Diabetes Care 38 Suppl:S8-s16. https://doi.org/10.2337/dc15-S005
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183. https://doi.org/10.1016/j.redox.2015.01.002
Sies H, Cadenas E (1985) Oxidative stress: damage to intact cells and organs. Philos T Roy Soc B 311(1152):617–631. https://doi.org/10.1098/rstb.1985.0168
Ighodaro OM (2018) Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother 108:656–662. https://doi.org/10.1016/j.biopha.2018.09.058
Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, van den Enden M, Kilo C, Tilton RG (1993) Hyperglycemic pseudohypoxia and diabetic complications. Diabetes 42(6):801–813. https://doi.org/10.2337/diab.42.6.801
Pedersen AML, Sorensen CE, Proctor GB, Carpenter GH, Ekstrom J (2018) Salivary secretion in health and disease. J Oral Rehabil 45(9):730–746. https://doi.org/10.1111/joor.12664
Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease. J Biomed Biotechnol 2012:936486–936414. https://doi.org/10.1155/2012/936486
Ibuki FK, Simoes A, Nogueira FN (2010) Antioxidant enzymatic defense in salivary glands of streptozotocin-induced diabetic rats: a temporal study. Cell Biochem Funct 28(6):503–508. https://doi.org/10.1002/cbf.1683
Nogueira FN, Carvalho AM, Yamaguti PM, Nicolau J (2005) Antioxidant parameters and lipid peroxidation in salivary glands of streptozotocin-induced diabetic rats. Clin Chim Acta 353(1–2):133–139
Reuterving CO (1986) Pilocarpine-stimulated salivary flow rate and salivary glucose concentration in alloxan diabetic rats. Influence of severity and duration of diabetes. Acta Physiol Scand 126(4):511–515. https://doi.org/10.1111/j.1748-1716.1986.tb07849.x
Vatta MS, Hope SI, Prendes GM, Bianciotti LG, Elverdin JC, Fernandez BE (2002) Salivary glands and noradrenergic transmission in diabetic rats. Auton Autacoid Pharmacol 22(2):65–71
Romero AC, Ibuki FK, Nogueira FN (2012) Sialic acid reduction in the saliva of streptozotocin induced diabetic rats. Arch Oral Biol 56(9):1189–1193. https://doi.org/10.1016/j.archoralbio.2012.02.016
Nicolau J, Rosa R, Fava-de-Moraes F (1969) The effect of alloxan diabetes upon N-acetylneuraminic acid concentration in the submaxillary glands of rats. Pharmacol Ther Dent 19(2):106–110
Nicolau J, Souza DN, Nogueira FN (2006) Activity, distribution and regulation of phosphofructokinase in salivary gland of rats with streptozotocin-induced diabetes. Braz Oral res 20 (2):108-113. S1806-83242006000200004
Nicolau J, de Matos JA, de Souza DN, Neves LB, Lopes AC (2005) Altered glycogen metabolism in the submandibular and parotid salivary glands of rats with streptozotocin-induced diabetes. J Oral Sci 47(2):111–116
Nogueira FN, Nicolau J (2004) Influence of streptozotocin-induced diabetes on the activity, distribution and isoenzymes of hexokinase of salivary gland of rats. J Physiol Biochem 61(3):421–427. https://doi.org/10.1007/BF03168448
Nogueira FN, Carvalho RA (2017) Metabolic remodeling triggered by salivation and diabetes in major salivary glands. NMR Biomed 30(2). https://doi.org/10.1002/nbm.3683
Manfredi M, McCullough MJ, Vescovi P, Al-Kaarawi ZM, Porter SR (2004) Update on diabetes mellitus and related oral diseases. Oral Dis 10(4):187–200
Halliwell B, Gutteridge JM (1985) Free radicals in biology and medicine
Kosower NS, Kosower EM (1976) The glutathione-glutathione disulfide system. Free radicals in biology, academic press
Bertinat R, Nualart F, Li X, Yanez AJ, Gomis R (2015) Preclinical and clinical studies for sodium tungstate: application in humans. J Clin Cell Immunol 6(1). https://doi.org/10.4172/2155-9899.1000285
Heidari Z, Harati M, Mahmoudzadeh-Sagheb HR, Moudi B (2008) Beta cell protective effects of sodium tungstate in streptozotocin-induced diabetic rats: glycemic control, blockage of oxidative stress and beta cell histochemistry. Iran Biomed J 12(3):143–152
Thompson KH, Chiles J, Yuen VG, Tse J, McNeill JH, Orvig C (2004) Comparison of anti-hyperglycemic effect amongst vanadium, molybdenum and other metal maltol complexes. J Inorg Biochem 98(5):683–690
Barbera A, Gomis RR, Prats N, Rodriguez-Gil JE, Domingo M, Gomis R, Guinovart JJ (2001) Tungstate is an effective antidiabetic agent in streptozotocin-induced diabetic rats: a long-term study. Diabetologia 44(4):507–513. https://doi.org/10.1007/s001250100479
Donmez BO, Ozturk N, Sarikanat M, Oguz N, Sari R, Ozdemir S (2014) Sodium tungstate alleviates biomechanical properties of diabetic rat femur via modulation of oxidative stress. Gen Physiol Biophys 33(4):443–452. https://doi.org/10.4149/gpb_2014020
Leite MF, Nicolau J (2009) Sodium tungstate on some biochemical parameters of the parotid salivary gland of streptozotocin-induced diabetic rats: a short-term study. Biol Trace Elem Res 127(2):154–163. https://doi.org/10.1007/s12011-008-8233-5
Bergmeyer H, Bernt E (1974) D-glucose determination with GOD and POD, rapid assay. Methods of enzymatic analysis 3:1211–1212
Paoletti F, Mocali A (1990) Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol 186:209–220. https://doi.org/10.1016/0076-6879(90)86110-h
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-h
Lowry OH, Rosebrough NS, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Nakhaee A, Bokaeian M, Akbarzadeh A, Hashemi M (2010) Sodium tungstate attenuate oxidative stress in brain tissue of streptozotocin-induced diabetic rats. Biol Trace Elem Res 136(2):221–231. https://doi.org/10.1007/s12011-009-8537-0
Akabarzadeh A, Norouzian M, Jamshidi S, Farhangi A, Allahverdi A, Mofidian S (2007) Lamerad B (2007) induction of diabetes by streptozotocine in rats. Indian J Clin Biochem 22(2):60–64. https://doi.org/10.1007/BF02913315
Barbera A, Rodríguez-Gil JE, Guinovart JJ (1994) Insulin-like actions of tungstate in diabetic rats. Normalization of hepatic glucose metabolism. J Biol Chem 269(31):20047–20053
Muñoz MC, Barberà A, Domínguez J, Fernàndez-Alvarez J, Gomis R, Guinovart JJ (2001) Effects of tungstate, a new potential oral antidiabetic agent, in Zucker diabetic fatty rats. Diabetes 50(1):131–138
Rodriguez-Hernandez CJ, Guinovart JJ, Murguia JR (2012) Anti-diabetic and anti-obesity agent sodium tungstate enhances GCN pathway activation through Glc7p inhibition. FEBS Lett 586(3):270–276. https://doi.org/10.1016/j.febslet.2011.12.035
Canals I, Carmona MC, Amigo M, Barbera A, Bortolozzi A, Artigas F, Gomis R (2009) A functional leptin system is essential for sodium tungstate antiobesity action. Endocrinology 150(2):642–650. https://doi.org/10.1210/en.2008-0881
Amigó-Correig M, Barceló-Batllori S, Piquer S, Soty M, Pujadas G, Gasa R, Bortolozzi A, Carmona M, Gomis R (2011) Sodium tungstate regulates food intake and body weight through activation of the hypothalamic leptin pathway. Diabetes Obes Metab 13(3):235–242. https://doi.org/10.1111/j.1463-1326.2010.01339.x
Amigó-Correig M, Barceló-Batllori S, Soria G, Krezymon A, Benani A, Pénicaud L, Tudela R, Planas AM, Fernández E, del Carmen CM (2012) Anti-obesity sodium tungstate treatment triggers axonal and glial plasticity in hypothalamic feeding centers. PLoS One 7(7):e39087. https://doi.org/10.1371/journal.pone.0039087
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48(1):1–9. https://doi.org/10.2337/diabetes.48.1.1
Nakhaee A, Shahabizadeh F, Erfani M (2013) Protein and lipid oxidative damage in healthy students during and after exam stress. Physiol Behav 118:118–121. https://doi.org/10.1016/j.physbeh.2013.05.028
Rellier N, Ruggiero-Lopez D, Lecomte M, Lagarde M, Wiernsperger N (1999) In vitro and in vivo alterations of enzymatic glycosylation in diabetes. Life Sci 64(17):1571–1583. https://doi.org/10.1016/s0024-3205(99)00094-6
Sachdeva S, Flora SJS (2014) Efficacy of some antioxidants supplementation in reducing oxidative stress post sodium tungstate exposure in male Wistar rats. J Trace Elem Med Biol 28(2):233–239. https://doi.org/10.1016/j.jtemb.2014.01.004
Fridovich I (1997) Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem 272(30):18515–18517. https://doi.org/10.1074/jbc.272.30.18515
Rathore N, John S, Kale M, Bhatnagar D (1998) Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol Res 38(4):297–303. https://doi.org/10.1006/phrs.1998.0365
Gunawardena HP, Silva R (2019) Poor Glycaemic control is associated with increased lipid peroxidation and glutathione peroxidase activity in type 2 diabetes patients. Oxid med cell Longev 5;2019:9471697. https://doi.org/10.1155/2019/9471697
Weksler-Zangen S, Yaffe P (2003) Ornoy a (2003) reduced SOD activity and increased neural tube defects in embryos of the sensitive but not of the resistant Cohen diabetic rats cultured under diabetic conditions. Birth Defects Res A Clin Mol Teratol 67(6):429–437. https://doi.org/10.1002/bdra.10043
Ibuki FK, Bergamaschi CT, da Silva PM, Nogueira FN (2020) Effect of vitamin C and E on oxidative stress and antioxidant system in the salivary glands of STZ-induced diabetic rats. Arch Oral Biol 116:104765. https://doi.org/10.1016/j.archoralbio.2020.104765
Nissanka N, Moraes CT (2018) Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett 592(5):728–742. https://doi.org/10.1002/1873-3468.12956
Krylatov AV, Maslov LN, Voronkov NS, Boshchenko AA, Popov SV, Gomez L, Wang H, Jaggi AS, Downey JM (2018) Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr Cardiol Rev 14(4):290–300. https://doi.org/10.2174/1573403x14666180702152436
Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA (2018) Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 9(2):119. https://doi.org/10.1038/s41419-017-0135-z
Liao Z, Chua D, Tan NS (2019) Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer 30 18(1):65. https://doi.org/10.1186/s12943-019-0961-y
Halliwell B, Gutteridge JM (1986) Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys 246(2):501–514. https://doi.org/10.1016/0003-9861(86)90305-x
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70(5):257–265. https://doi.org/10.1111/j.1753-4887.2012.00476.x
Stvolinskii S, Fedorova T, Yuneva M (2003) Boldyrev a (2003) protective effect of carnosine on cu, Zn-superoxide dismutase during impaired oxidative metabolism in the brain in vivo. Bull Exp Biol Med 135(2):130–132. https://doi.org/10.1023/a:1023855428130
Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies K (1990) Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem 265(20):11919–11927
He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE (2011) Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis 21(4):277–285. https://doi.org/10.1016/j.numecd.2009.12.008
Negi G, Kumar A, Joshi RP, Sharma SS (2011) Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun 29 408(1):1–5. https://doi.org/10.1016/j.bbrc.2011.03.087
Jiménez-Osorio AS, Picazo A, González-Reyes S, Barrera-Oviedo D, Rodríguez-Arellano ME, Pedraza-Chaverri J (2014) Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci 15(11):20290–20305. https://doi.org/10.3390/ijms151120290
Aragno M, Tamagno E, Gatto V, Brignardello E, Parola S, Danni O, Boccuzzi G (1999) Dehydroepiandrosterone protects tissues of streptozotocin-treated rats against oxidative stress. Free Rad Biol Med 26(11–12):1467–1474. https://doi.org/10.1016/s0891-5849(99)00012-x
Maritim AC, Moore BH, Sanders RA, Watkins JB III (1999) Effects of melatonin on oxidative stress in streptozotocin-induced diabetic rats. Int J Toxicol 18(3):161–166. https://doi.org/10.1080/109158199225440
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57(3–4):145–155. https://doi.org/10.1016/s0753-3322(03)00043-x
Maritim AC, Sanders RA, Watkins JB 3rd (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17(1):24–38. https://doi.org/10.1002/jbt.10058
Lutchmansingh FK, Hsu JW (2018) Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS One 13(6):e0198626. https://doi.org/10.1371/journal.pone.0198626 eCollection 2018
Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F (2011) Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34(1):162–167. https://doi.org/10.2337/dc10-1006
Ahmad M, Khan MA, Khan AS (2003) Naturally occurring antioxidant vitamin levels in patients with type-II diabetes mellitus. J Ayub Med Coll Abbottabad 15(1):54–57
Pazdro R, Burgess JR (2010) The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev 131(4):276–286. https://doi.org/10.1016/j.mad.2010.03.005
van Haaften RI, Haenen GR, Evelo CT, Bast A (2003) Effect of vitamin E on glutathione-dependent enzymes. Drug Metab Rev 35(2–3):215–253. https://doi.org/10.1081/dmr-120024086
Meister A (1994) Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem 269(13):9397–9400
Bravi MC, Armiento A, Laurenti O, Cassone-Faldetta M, De Luca O, Moretti A, De Mattia G (2006) Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism 55(5):691–695. https://doi.org/10.1016/j.metabol.2006.01.003
Nicolau J, Sassaki KT (1976) Metabolism of carbohydrate in the major salivary glands of rats. Arch Oral Biol 21(11):659–661. https://doi.org/10.1016/0003-9969(76)90140-0
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54(4):287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Jo S-H, Son M-K, Koh H-J, Lee S-M, Song I-H, Kim Y-O, Lee Y-S, Jeong K-S, Kim WB, Park J-W (2001) Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem 276(19):16168–16176. https://doi.org/10.1074/jbc.M010120200
Schowen RL (1993) Principles of biochemistry 2nd ed.(Lehninger, Albert L.; Nelson, David L.; cox, Michael M.). ACS publications
Leite MF (2006) Estudo temporal do efeito da administração de tungstato de sódio sobre alguns parâmetros de glândulas salivares e saliva de ratas diabéticas. Universidade de São Paulo
Pastore A, Federici G, Bertini E, Piemonte F (2003) Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333(1):19–39. https://doi.org/10.1016/s0009-8981(03)00200-6
Funding
This work was supported by CAPES (Projects 001 and 88881.062183/2014-01) and FAPESP (2013/18609-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, D.N., de Souza, E.M.N., da Silva Pedrosa, M. et al. Effect of Tungstate Administration on the Lipid Peroxidation and Antioxidant Parameters in Salivary Glands of STZ-Induced Diabetic Rats. Biol Trace Elem Res 199, 1525–1533 (2021). https://doi.org/10.1007/s12011-020-02273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02273-x