Abstract
In this work a simple and inexpensive method to assess the concentration ratio of the labile and mineral-bound microelements of the bone tissue was developed. The approach is based on the separation of the components of bone tissue by their selective solubility with the subsequent determination of microelements with atomic absorption spectrometry. The total concentrations of Mg, Zn, Fe, Sr, Al, Cu, and Mn and the concentrations of these elements in aqueous solutions with pH 6.5, 10, and 12 after their ultrasonically activated interaction with the powder of dried bone were determined. Two quite different bone samples were analyzed: a cortical fragment of the femur of a mature healthy cow and the spongy part of a human femoral head affected by osteoporosis. Some common and individual features of the both type of bones in regard to the total concentrations and fractional distribution of microelements are discussed. The obtained concentrations of the “soluble” fractions of microelements were critically analyzed taking into account the possible reactions leading to new insoluble phases’ formation in alkaline solutions. Based on the data obtained, the ability of elements to form labile fractions in the bone tissue could be arranged in the following descending series: Mg ≥ Zn > Al > Fe > Mn > Cu > Sr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent investigations [1,2,3] and earlier researches [4,5,6] show that the principal mineral phase of the mammals’ bone tissue is a poorly crystalline carbonate-substituted apatite, which also is calcium-deficient due to vacancies and substitutions in the lattice, and can be represented by the simplified formula: Ca10–x□x [(PO4)6–x(CO3)x](OH)2–x□x, where □ are vacancies.
Bone tissue contains several essential microelements that support its critical physiological and biochemical functions. Some of them affect the crystal-chemical characteristics of the mineral part of the bone. Alkaline and alkaline-earth metals (Na, Mg, K) are especially important in biogenic apatite. In the bone mineral, these elements are found in relatively large concentrations (Na ~ 1.0 wt%, Mg ~ 0.2–0.6 wt%, K ~ 0.07 wt%; [7]). They are often called macroelements (or major dopants) in contrast with minor or microelements, the content of which in the mineral of the normal bone tissue does not exceed a few hundredth or thousandth parts of percent. The main microelements of the bone are iron, zinc, strontium, lead, aluminum, copper, and some others.
The accumulation of biologically significant ions in the bone mineral is functionally determined, since, in addition to its mechanical function, the bone serves as a reservoir of elements circulating in biological fluids. In addition, a bone may accumulate (partially inactivating) some undesirable elements which get into an organism from environment. This is possible due to the tolerance of the apatite structure to isomorphous substitutions, and it allows forming satisfactorily functional mineral component of the skeletal tissues under conditions of wide fluctuations of environmental factors and diet.
In several works [1, 8], the ultrastructural organization of bone mineral is described as apatite nanocrystals surrounded by a relatively labile, but structured, hydrate shell containing various cations and anions. The bone microelements are not only incorporated in bioapatite and/or adsorbed on the crystallite surface but also might be localized in organic components (mainly type I collagen) as well as in biological fluids and cellular elements of the tissue. Therefore, it is important to know not only the total concentrations of macro- and microelements in mineralized tissues but also the distribution of elements in the different parts of a tissue.
Commonly, the elemental composition of mineralized tissues is investigated by electron probe and proton probe X-ray spectral analysis (EDX and PIXE, respectively) as well as X-ray fluorescence (XRF) analysis. These methods allow estimating the content of basic elements and major impurities with concentration not lower than 0.01 wt%. These techniques, however, have relatively high detection limit. Atomic absorption spectrometry (AAS) and elemental analysis mass spectrometry including inductively coupled plasma technique (ICP-MS) have much lower detection limit, but both these methods cannot give information on the localization and distribution of the elements in the samples.
In recent years, new cutting-edge techniques based on X-ray absorption spectroscopy (XAS) has been developed, namely, the analysis of X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) [9,10,11,12,13,14] that provide information on the local environment of ions. However, the availability of these techniques is significantly limited by the restricted access to the sources of high brightness (synchrotron) X-rays. Another drawback is that only relative differences between the elements’ contents could be measured, since still there is no suitable reference material for the calibration of XAS experiment, which would have allowed obtaining the absolute concentrations of trace elements in bone or other mineralized biological tissues [12].
There are XAS data about the distribution of Sr2+ between bone apatite, hydrated environment, and collagen [9]. The evidence of the preferential localization of Pb in the Ca2+ positions of the apatite lattice was obtained using the μ-XANES method [12], as well as the information on the chemical status of Zn2+ in bones, cartilage, and pathological calcifications [13, 14]. When applied to mineralized tissues, XAS methods are often used in combination with X-ray fluorescence microanalysis (μXRF), X-ray microdiffraction (μXRD), and other complementary approaches [13].
Hence, it seems reasonable to supplement the aforesaid approaches with AAS as an accessible and common technique for the quantitative determination of microelements in combination with simple methods for the separation of mineralized tissue components. In this case, the key stage of the analysis is the preparation of the material. The purpose of this study was to develop an approach to determine the fractional distribution of the labile and mineral-bound microelements in bone tissue using a chemical separation combined with AAS.
Materials and Methods
Sample Selection and Preparation
In bone samples of various origins and biological functions, essential differences in the concentration and location of microelements can be observed [15,16,17,18]. In this study, we investigated two extreme cases. The samples were prepared from (1) the cortical fragment (mid-diaphysis) of the femur of a mature healthy cow and (2) the spongy part of a human femoral head (from a patient after hip replacement surgery, male, age 64) affected by osteoporosis (throughout the work “healthy cortical bone” and “osteoporosis spongy bone,” respectively). The healthy cortical bone specimen (femur of a mature cow) was obtained from a local butcher (Lanzhou city, Gansu Province, China) immediately after slaughter. The animal passed a preslaughter veterinary control, and the bone has been taken after standard laboratory examination of carcass meat after slaughter. The human hip joint affected by osteoporosis was obtained from the Medical Institute of Sumy State University (Ukraine), where anatomical analysis, histomorphology, and medical morphometry of it have been performed. The research was approved by the Ethics Committee of the Medical Institute of Sumy State University (protocol #1/1, April 8, 2019).
Preliminary sample preparation included mechanical cleaning, drying in air at 120 °C for 4 h with slow cooling in a muffle furnace to room temperature. The spongy bone before drying was splintered and then washed with chloroform-methanol 2:1 mixture to remove lipids. After that, the bone samples (2–3 g) were carefully ground in a porcelain mortar to get homogenous fine powder. To determine the total concentrations of elements by AAS, 0.015 g of the powder was transferred to a polypropylene tube, where 0.5 ml of concentrated (56%) nitric acid was added, and after a sample was completely dissolved, the sample volume was adjusted to 10 ml. The completeness of the dissolution of the sample was confirmed by the absence of the Tyndall effect in the resulting solution.
Sample preparation for measuring the concentrations of elements in solutions with different pH values (6.5, 10, and 12) included the following procedures: (1) preparing the solutions with pH 12: NaOH, NH4OH, and dilution of the prepared solutions to pH 10 in separate polyethylene tubes (10 ml); (2) putting 0.1 g of bone powder into test tubes (5 × 2 pcs.); (3) filling the tubes with 5 ml of various solutions; (4) ultrasound treatment of the samples (in the tubes) for 10 min at 22 kHz, ~ 20 W/cm2; and (5) settling the tubes in a rack for 15–18 h before measuring the concentrations.
The reagents (nitric acid, alkali, and ammonia solution) of chemically pure grade were used for sample preparation. All solutions were prepared using bidistilled water with electrical conductivity not more than 1 μS/cm. To prevent the interaction of reagents with glass, polypropylene chemical vessels were used. Calibration solutions were prepared from certified reference samples.
Devices, Methods, and Analytical Conditions
The AAS with electrothermal atomization (KAS 120.1, “SELMI,” Ukraine) was employed to determine the content of microelements in bone samples. The analytical complex consisted of a single-beam spectrophotometer S-115M equipped with a deuterium corrector of background absorption and an A-5 electrothermal atomizer with a closed tubular graphite furnace (analog of Perkin Elmer HGA-500). The lamps with a hollow cathode served as line radiation sources. The furnace temperature was programmed and controlled by a tungsten-rhenium thermocouple. The error of measurement of the furnace surface temperature during atomization did not exceed 5%. The samples were dosed with an MD-10 manual sampler (20 ± 0.4 μl). Spectral measurement conditions (wavelength, width of the spectral slit, lamp current) were standard [19]. The temperature program of the furnace was selected and adjusted for each element individually to achieve the optimal analysis conditions. For the purity of the experiment and the prevention of possible chemical contamination, no additional spectral buffers and modifiers were used.
For each measured element, five repetitions were performed, and the results were averaged with the determination of the standard deviation. The concentrations were recalculated to the weight of the dried initial sample. The averaged data are represented in mg/kg of dry weight.
The structural characteristics of the samples were studied by X-ray diffraction (XRD) using the diffractometer DRON4-07 (“Burevestnik,” Russia. The Ni-filtered CuKα radiation (wavelength 0.154 nm) with a conventional Bragg-Brentano θ-2θ geometry was used (2θ is the Bragg’s angle). The Ca/P molar ratios in the bones were determined using XRD phase analysis of annealed bone tissue [20].
Results and Discussion
Before AAS examination, the bone samples (in the powdered form) were characterized by XRD (Fig. 1). The XRD patterns of the initial bones have shown the presence of poorly crystalline apatite phase. In the annealed samples (at 1000 °C for 3 h), the phase analysis has revealed the formation of two phase system: hydroxyapatite (Са10(РО4)6(OH)2, JCPDS 9-0432) and β-tricalcium phosphate (Са3(РО4)2, JCPDS 9-169), which indicates the Ca deficiency in the initial bioapatite. The results of XRD quantitative phase analysis and following evaluation of Ca/P ratio (according to the procedure described in [20, 21]) have shown that the Ca deficiency is more pronounced in the osteoporosis spongy bone than in the healthy cortical bone. For the first one, the Ca/P molar ratio was found to be 1.59 while for the second 1.63 (compared with 1.67 for stoichiometric hydroxyapatite). These results fairly well correlate with the data available from literature about Ca/P ratio for cortical and trabecular (spongy) bones obtained by energy dispersive X-ray spectroscopy [17, 18, 22].
The bulk analysis of chemical composition of bone samples shows (Fig. 2) that the AAS technique allows reliable measurement of both the concentrations of macroelements (Mg) and microelements, including those present in the bone tissue at the level of ppm (Cu and Mn). The determined element concentrations are well consistent with literature data [1, 7, 16, 17]. The increased concentration of Sr in the cortical bone of cow compared with the osteoporosis spongy bone illustrates the high susceptibility of bone tissue to adverse environmental factors, as the first sample originated from heavily industrialized region. Also, Fig. 2 shows that the total concentrations of Fe and Cu are noticeably higher in the osteoporosis spongy bone compared with the cortical cow bone. The total concentrations of Mg, Al, Zn, and Mn are nearly identical in both analyzed specimens of bone. The work [17] as well indicates an increased content of Fe and Cu in osteoporotic bone compared with control, although the reasons for this are not clarified. They also note that the concentrations of Fe and A1 may vary widely from one subject to another. The relatively high content of Al in the samples we studied may be the consequence of Al accumulation in bones with age.
The labile fraction of trace elements was determined after the special preparation procedure. The chemical separation of bone components is based on the fact that calcium apatites of any origin are practically insoluble in water and alkaline solutions but soluble in acids [7, 23].
A method of the isolation of the original bioapatite crystals from bone tissue by combination of chemical and ultrasonic treatment has been suggested in a series of papers [24,25,26]. Using transmission electron microscopy [24, 25] and atomic force microscopy [26], it has been shown that ultrasonic treatment can separate bioapatite crystals and non-apatite components.
The possibility to determine the preferential localization of Mg, Na, and K in bone apatite has been studied earlier [27, 28] by using stage heating (from 560 to 720°С) and ultrasonication of the samples before AAS measurements.
There is also a work in which chemical removal of organic material with hydrazine was used to determine the preferential localization of Na and K in bones [29]. Determination of the ionic composition of the samples prepared that way was done by secondary ion mass spectrometry (SIMS). As a result, it was found that it is the organic part that contains the majority (predominant part) of sodium and potassium of the bone.
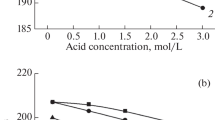
Thus, the elements weakly bound to the mineral phase of the bone readily dissolve in water if pH is in the range of 7–8. Increasing pH to 10–12 causes the dissolution of the organic bone tissue portion. At the other hand, at high pH, undesirable precipitation of phases not present in the original bone mineral can take place. This impediment was bypassed by analyzing the solutions with different pH values (6.5; 10 and 12). In addition, the ultrasonication was applied to the powdered samples during chemical treatment as an improvement of the sample preparation procedure described in [30]. Figure 3 shows the concentrations of each of the analyzed elements in total and in water-soluble and alkali-soluble fractions. The reliability of the results can be estimated from the Table 1.
The actual detection limits for each element determined using blank solutions 3–5 times exceeded the data in Table 1; nevertheless, it does not influence the conclusive results.
As it can be seen (Fig. 3a), the fraction of soluble Mg is about 3% of the total content. In alkaline medium Mg dissolved significantly less than in water, probably due to the formation of Mg(OH)2. This complicates the estimation of the location of labile Mg. There are no principal differences between osteoporotic spongy bone and healthy cortical bone except that the proportion of water-soluble Mg in the first one is slightly higher (~ 2.7%) than in the second one (~ 2.0%).
The total strontium content in the bone sample examined (Fig. 3b) lies within the concentration range known from the literature [7] (an order of magnitude less than magnesium). However, unlike magnesium, almost all strontium is bound to the crystalline phase, and only very little is in a water-soluble state. In the osteoporosis spongy bone, the proportion of water-soluble Sr is significantly higher than in the cortical bone.
Iron in both bone samples (Fig. 3c) to a much greater extent than strontium, but less than magnesium, can be in the mobile (water-soluble and alkali-soluble) state (approximately 1% of the total content). In the osteoporotic spongy bone, the concentration of total Fe and the fraction of mobile Fe are noticeably higher in comparison with the cortical bone. It seems from the diagrams that for both samples, the mobile Fe is localized more in organic components of tissue than in water.
In aluminum content (Fig. 3d), there are no fundamental differences between osteoporotic spongy bone and healthy cortical bone. The mobile fraction of aluminum is quite appreciable in both samples. In spongy osteoporotic bone, the portion of water-soluble Al is slightly higher than in the cortical bone (approximately 1.2% vs. 0.7% of the total content).
In zinc content (Fig. 3e), there are no noticeable differences in osteoporotic spongy bone and healthy cortical bone. In the solutions with high pH values, the measured concentrations of zinc are noticeably higher, this may be due to the formation of insoluble Zn(OH)2 in low pH and neutral solutions. In the ammonia solutions with pH 12, zinc hydroxide dissolves:
Zn(OH)2↓ + 6NH4+ → [Zn(NH3)6]2+ + 2H2O + 4H+. The obtained results show that a significant portion of Zn is associated with mineral component of the bone and indicate the localization of labile Zn predominantly in alkali-soluble organic components of the bone tissue.
The concentration of Cu for almost all solutions (Fig. 3f) exceeds a little the detection limit of the AAS method. In the spongy osteoporotic bone, compared with cortical, the fraction of soluble Cu is much larger, and the concentration of the total copper is 2.5 times higher. This is quite similar to the observed distribution of Fe in the investigated samples. Generally, the obtained data indicate that the predominant Cu fraction is localized in the mineral component of the bone tissue.
There are no significant differences in the content of Mn (Fig. 3g) in spongy osteoporotic bone and cortical bone. The concentration of Mn in all solutions barely exceeds the limit of detection for AAS method. This convincingly indicates that practically all manganese found in the bone tissue is deposited in the crystalline phase of both bone samples.
The obtained numerical values do not display the complete picture of the localization of microelements in bone tissue, providing, however, information about their relative migration ability in biological tissue, which can be represented by the following descending series: Mg ≥ Zn > Al > Fe > Mn > Cu > Sr. Besides, some common and individual features for the both type of bones can be pointed.
Common Features
Among the analyzed elements, Mg and Zn show the highest ability to form mobile/labile (non-apatite) fractions in bone tissue. Non-crystalline Zn is located more in the alkali-soluble organic components of bone than in the biological liquids. Al and to the less extent Fe may have the appreciable mobile fractions in bone tissue. Sr, Cu, and Mn are practically not detected in solutions, i.e., they all are predominantly localized in the crystalline phase. The total concentrations of Mg, Al, Zn, and Mn are nearly identical in the both analyzed specimens of bone.
Individual Features
The total concentrations of Fe and Cu are noticeably higher in the osteoporosis spongy bone comparing with the cortical cow bone, while for Sr the situation is reverse. In the osteoporosis spongy bone, the proportion of water-soluble (labile) Sr and Mg is higher than in the cortical bone.
We realize that these features cannot represent statistically significant fractionation of microelements in two kinds of bone species due to limited number of samples. Nevertheless, the proposed approach allows comparing the ratio of labile and mineral-bound microelements in bone tissue of different samples. In summary, our results show that there is a potential for the development of inexpensive and simple approach to study the localization and migration ability of microelements in bone tissue. Such approach could be employed to study biological effects of mobile ionic species in normal and pathological bone.
Conclusions
An approach including the chemical separation of bone tissue components based on their selective solubility and the subsequent determination of microelements by AAS is proposed to compare the ratio of labile and mineral-bound microelements in bone tissue of different samples. The total concentrations of Mg, Zn, Fe, Sr, Al, Cu, and Mn and the concentrations of these elements in solutions with pH 6.5, 10, and 12 after their ultrasonically activated interaction with the powder of dried bone were determined.
To verify the suggested approach, the two quite diverse bone samples were analyzed: cortical fragment of femur of a mature healthy cow and spongy part of human hip joint affected by osteoporosis. Some common and individual features for the both type of bone are revealed; the ability of elements to form labile fractions in the bone tissue could be arranged in the following descending series: Mg ≥ Zn > Al > Fe > Mn > Cu > Sr.
References
Combes C, Cazalbou S, Rey C (2016) Apatite biominerals. Minerals. 6. https://doi.org/10.3390/min6020034
Pasteris JD (2016) A mineralogical view of apatitic biomaterials. Am Mineral 101:2594–2610. https://doi.org/10.2138/am-2016-5732
Drouet C, Aufray M, Rollin-Martinet S, Vandecandelaère N, Grossin D, Rossignol F, Rey C (2018) Nanocrystalline apatites: the fundamental role of water. Am Mineral 103:550–564. https://doi.org/10.2138/am-2018-6415
Neuman WF, Neuman MW (1953) The nature of the mineral phase of bone. Chem Rev 53:1–45
Legeros RZ (1981) Apatites in biological systems. Progr Cryst Growth Charact 4:1–45
Betts F, Blumenthal NC, Posner AS (1981) Bone mineralization. J Cryst Growth 53:63–73
Elliott JC (2002) Calcium phosphate biominerals. In: Kohn MJ, Rakovan J, Hughes JM (eds) Phosphates: geochemical, geobiological, and materials importance, vol 48. Reviews in mineralogy and geochemistry. Mineralogical Society of America, Washington, DC, pp 427–453
Cazalbou S, Combes C, Eichert D, Rey C (2004) Adaptative physico-chemistry of bio-related calcium phosphates. J Mater Chem. https://doi.org/10.1039/b401318b
Frankær CG, Raffal AC, Stahl K (2014) Strontium localization in bone tissue studied by X-ray absorption spectroscopy. Calcif Tissue Int 94:248–257
Porcaro F, Roudeau S, Carmona A, Ortega R (2018) Advances in element speciation analysis of biomedical samples using synchrotron-based techniques. Trends Anal Chem 104:22–41
Bazin D, Dessombz A, Nguyen C, Ea HK, Lioté F, Reh J, Daudon M (2014) The status of strontium in biological apatites: an XANES/EXAFS investigation. J Synchrotron Radiat 21:136–142
Pemmer B, Roschger A, Wastl A, Hofstaetter JG, Wobrauschek P, Simon R, Streli C (2013) Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone 57:184–193
Dessombz A, Nguyen C, Ea HK, Rouzière S, Foy E, Hannouche D, Réguer S, Picca FE, Thiaudière D, Lioté F, Daudon M, Bazin D (2013) Combining μX-ray fluorescence, μXANES and μXRD to shed light on Zn2+ cations in cartilage and meniscus calcifications. J Trace Elem Med Biol 27:326–333
Bazin D, Carpentier X, Brocheriou I, Dorfmuller P, Aubert S, Chappard C, Thiaudière D, Reguer S, Waychunas G, Jungers P (2009) Revisiting the localisation of Zn2 cations sorbed on pathological apatite calcifications made through X-ray absorption spectroscopy, Biochimie. https://doi.org/10.1016/j.biochi.2009.05.009
Bigi A, Cojazzi G, Panzavolta S, Ripamonti A, Roveri N, Romanello M, Moro L (1997) Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J Inorg Biochem 68:45–51
Lanocha N, Kalisinska E, Kosik-Bogacka DI, Budis H, Sokolowski S, Bohatyrewicz A (2012) Concentrations of trace elements in bones of the hip joint from patients after hip replacement surgery. J Trace Elem Med Biol 26:20–25
Baslé MF, Rebel A, Mauras Y, Allain P, Audran M, Clochon P (1990) Concentration of bone elements in osteoporosis. J Bone Miner Res 5:41–47. https://doi.org/10.1002/jbmr.5650050108
Kuhn LT, Grynpas MD, Rey CC, Wu Y, Ackerman JL, Glimcher MJ (2008) A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif Tissue Int 83:146–154
Schlemmer G, Radziuk B (1999) Analytical graphite furnace atomic absorption spectrometry. A laboratory guide. Birkhäuser Basel, Basel-Boston-Berlin
Balmain N, Legros R, Bonel G (1982) X-ray diffraction of calcined bone tissue: a reliable method for the determination of bone Ca/P molar ratio. Calcif Tissue Int 34:S93–S98
Raynaud S, Champion E, Bernache-Assollant D, Laval JP (2001) Determination of calcium/phosphorus atomic ratio of calcium phosphate apatites using X-ray diffractometry. J Am Ceram Soc 84:359–366
Kourkoumelis N, Balatsoukas I, Tzaphlidou M (2012) Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J Biol Phys 38:279–291
Wang L, Nancollas GH (2008) Calcium orthophosphates: crystallization and dissolution. Chem Rev 108:4628–4669
Kim HM, Rey C, Glimcher MJ (1995) Isolation of calcium-phosphate crystals of bone by non-aqueous methods at low temperature. J Bone Miner Res 10:1589–1601
Kim HM, Rey C, Glimcher MJ (1996) X-ray diffraction, electron microscopy, and Fourier transform infrared spectroscopy of apatite crystals isolated from chicken and bovine calcified cartilage. Calcif Tissue Int 59:58–63
Eppell SJ, Tong W, Katz JL, Kuhn L, Glimcher MJ (2001) Shape and size of isolated bone mineralites using atomic force microscopy. J Orthop Res 19:1027–1034
Danil’chenko SN, Kulik AN, Pavlenko PA, Kalinichenko TG, Bugai AN, Chemeris II, Sukhodub LF (2006) Thermally activated diffusion of magnesium from bioapatite crystals. J Appl Spectrosc 73:437–443
Danilchenko SN (2013) The approach for determination of concentration and location of major impurities (Mg, Na, K) in biological apatite of mineralized tissues. J Nano Electron Phys 5:03043 (5pp)
Bushinsky DA, Gavrilov KL, Chabala LM, Levi-Setti R (2000) Contribution of organic material to the ion composition of bone. J Bone Miner Res 15:2026–2032
Danilchenko SN, Rogulsky YV, Kulik AN, Kalinkevich AN (2019) Determination of labile and structurally bound trace elements of bone tissue by atomic absorption spectrometry. J Appl Spectrosc 86:264–269
Acknowledgments
The authors are grateful to Dr. R.A. Moskalenko and E.V. Husak from the Medical Institute of Sumy State University (Ukraine) for supplying the human hip joint affected by osteoporosis, to M. Zhovner (Institute of Applied Physics, NAS Ukraine) for the pretreatment and delivery of the cortical fragment of the mature healthy cow femur from Lanzhou (China), and to A.V. Kochenko (Institute of Applied Physics, NAS Ukraine) for XRD analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danilchenko, S., Rogulsky, Y., Kulik, A. et al. A Simple Method to Determine the Fractions of Labile and Mineral-Bound Microelements in Bone Tissue by Atomic Absorption Spectrometry. Biol Trace Elem Res 199, 935–943 (2021). https://doi.org/10.1007/s12011-020-02234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02234-4