Abstract
The present study was devoted to investigate the PTE concentrations including cadmium (Cd), chromium (Cr), copper(Cu), iron (Fe), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb), arsenic(As), and zinc (Zn) among six types of edible wild, 23 cultivated mushroom samples collected from Iran’s market by the aid of an inductively coupled plasma-optical emission spectrometry (ICP-OES). Also, the related health risk assessment was established by the aid of the Monte Carlo simulation method (MSC). The limit of detection (LOD) and limit of quantification (LOQ) were ranged 0.001–0.048 and 0.003–0.160 ppm, respectively. The concentrations of As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn were determined in the ranges of 0.003–11. 5, 0.0008–5.89, 0.32–26.32, 9.15–110.08, 15.25–751.17, 0.16–2.24, 2.1–60.47, 1.21–24.22, 0.16–8.92, and 37.13–268.11 mg kg−1, respectively. According to findings, highest mean concentration of Cr in both types of mushrooms (cultivated and wild) was lower than recommended level by Codex Alimentarius/Food and Agriculture Organization/World Health Organization (CODEX/FAO/WHO) while the corresponding values for Hg (0.87 mg kg−1), As (1.39 mg kg−1), Ni (10.08 mg kg−1), Cu (36.65 mg kg−1), Cr (10.44 mg kg−1), Cd (0.589 mg kg−1), Fe (201.04 mg kg−1), Mn (10.30 mg kg−1), Zn (2266.43 mg kg−1), and Pb (3.81 mg kg−1) were higher than related standard levels. According to the health risk assessment, no concern regarding the non-carcinogenic risk due to the ingestion of PTEs via the consumption of the edible mushrooms, except Hg in wild mushrooms for children, was noted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mushroom consumption is growing all over the world, while this term refers to different species of fungi that belong to Basidomyecets or Ascomycetes. Basidomyecets or Ascomycetes can grow under moisture conditions and soils rich in organic matter [1,2,3]. According to literature, more than 70,000 species of fungi were identified in the world, while about 2000 species (31 genera) are primarily edible mushrooms [4]. However, about 10% of the 30 toxic mushroom species are considered lethal [5].
The edible mushrooms are appreciated, not only for their notable good flavor and texture but also as an essential source of the biologically active compound and nutritional properties [6]. In this regard, in many parts of the world, mushrooms are used as a part of the human diet due to their taste and aroma, as well as their high nutritional value such as dietary fiber, protein, fats, vitamins, and minerals [7,8,9]. In general, their fleshy body, on dry weight, contains almost 39.9% carbohydrate, 17.5% protein, and 2.9% fat, the residue constituting minerals [10]. Also, they have been reported as curative plants that are beneficial in preventing diseases such as high blood pressure [11], hypocholesteremia [12], and several types of cancer [13].
Human activities, such as the industry and the exploitation of raw materials, resulted in the formation of a huge amount of gas and sludge pollutants [14,15,16]. PTEs as the non-biodegradable, aggregate, and stable compounds are among the most important pollutants in the environment [17,18,19]. While recently, they are proposed as a global human health issue [18, 20,21,22,23] as a result of their adsorption via ingestion, inhalation, and dermal contact or even indirect way due to their accumulation in soil and water as well as other environmental factors [21, 24,25,26]. Generally, all PTEs have adverse effects on human health, such as disorders related to the nervous system, kidneys, and blood circulation [27,28,29]. Among PTEs, As, Cd, Cr, Hg, Ni, and Pb are the most dangerous metals [30,31,32,33,34]. The PTE concentrations in the mushroom are reported as considerably higher than those in agricultural crop plants, fruit, and vegetables [35], mainly due to effective mechanisms to absorb PTEs from the ecosystems by them [36]. This issue primarily depends on the type of fungus and the physiology of various mushrooms, especially their ecosystems [37]. In regard to the important relative segment of mushroom in the world food basket, the occurrence of high concentrations of PTEs in the mushroom is considered important because of a high toxicological hazard [20, 38, 39].
Therefore, it is necessary to evaluate the levels of toxic elements among the available mushrooms in Iran’s market. In this regard, for the first time, this study carried on to investigate the PTE content (Fe, Cu, Zn, Mg, Cd, Mn, Ni, Pb, Hg, As) in the wild edible and the cultivated mushroom samples collected from in Iran’s market by the aid of an ICP-OES. Also, the potential health risk assessment was carried out.
Materials and Methods
Chemicals and Reagents
All the reagents and chemicals such as HNO3, H2O2 in the analytical grade were obtained from Merck Company (Darmstadt, Germany).
Sample Collection
Twenty-nine mushroom samples, which contain six wild (Agaricus bisporus), were collected from South of Kermanshah in spring 2019, and 23 cultivated mushrooms (Agaricus bisporus) from different provinces (stations) of Iran in Spring (Table 1) were collected during 2019 year based on the recommended method and were analyzed in triplicate [15].
The mushroom samples were washed with distilled water and dried at 105 °C for 24 h. The dried samples were ground, then homogenized using an agate pestle, and sorted in polyethylene bottles until analysis [40]. All of the plastic and glassware were cleaned by means of soaking, overnight in a 10% nitric acid solution, and then rinsed with deionized water [41].
Sample Preparation
Concentrations of ten PTEs (Fe, Cu, Zn, Mg, Cd, Mn, Ni, Pb, Hg, and As) were analyzed. In this regard, a milestone Ethosup D closed vessel microwave (maximum power was 1400 W, and the maximum pressure in Teflon vessels—100 bar) (milestone Ethosup, Italia) besides the acid decomposition was used to minimize the effects of the organic matrix and also prevent the possibility of sample pollution and losses of analytes.
Duplicate mushroom samples (0.25 g) were digested with 9 mL of HNO3 (65%) and 1 mL of H2O2 (70%) in the microwave digestion system for 30 min under pressure 100 bar and maximum temperature 300 °C. The residue was then diluted to 10 mL with deionized water in a 10-mL volumetric flask. A blank sample was digested with the same procedure [41].
Inductively Coupled Plasma Atomic Emission Spectrometry Conditions
All prepared samples (duplicate) were analyzed by the aid of an ICP-OES (Spectoro, Arcos, Germany) with Torch type of flared end EOP Torch 2.5 mm. The optimum functioning parameters were RF generator (1400 W); argon gas grade 6 was used for plasma, nebulizer, and auxiliary gas. The gas flows of plasma, auxiliary, and nebulizer were 14.50, 0.90, and 0.85 (L min−1), respectively. Afterward, initial stabilization time, rinse time, and sample uptake time were 240 s total and 45 s for preflush. Also, the time between replicate analysis and delay time was zero. The analysis was a three-time replicate, and the frequency (resonance frequency) of the generator of RF was 27.12 MHz. The types of solid state, detector, and spray chamber were cyclonic, CCD, and modified Lichte, respectively. The type of pump of sample delivery was four-channel, software-controlled; peristaltic pump enables exact sample flows. The speed of prewash pump was 60 rpm (for 15 s), 30 rpm (for 30 s), and the time of prewash was 45 s, and finally, the speed of the pump of sample injection was 30 rpm.
Validation of the Analytical Method
The validation of the analytical procedure for quantitative analysis of elements in mushroom and its aqueous extracts was performed by evaluating selectivity, working and linear ranges, detection limit (LOD), quantification limit (LOQ), repeatability, and reproducibility(precision). Matrix effects were studied using standard addition method, by adding 200 μL of mixed standard solutions to the original samples (Mix standard CRM: 92091 Supelco LOT BCCB9855, TraceCERT®, 33 elements, 10 mg L−1 in nitric acid, Hg standard CRM:28941 Supelco, LOT BCCB8927, 1000 mg L−1, Hg in nitric acid). The recoveries were within 92% and 106% for all the studied elements.
The wavelengths used for determination of the concentration of elements, based on baseline signals and their interferences at selected lines observed experimentally during the measurements, are presented in Table 2.
Health Risk Assessment
In this study, estimated weekly intake (EWI, mg kg−1 body weight week−1) for PTEs through consuming edible mushroom was determined according to Eq. 1:
where W is the weight of mushroom consumed (25 g week−1); C, the PTE concentration of mushroom (mg kg−1); BW, the average body weight (70 kg) [42]. The dietary exposure levels were also estimated and compared with the provisional tolerable weekly intake (PTWI) Joint FAO/WHO Expert Committee on Food Additives [43].
For estimation of a non-carcinogenic hazard of PTEs due to edible mushrooms, the chronic daily intake (CDI) was calculated by the following equation:
In this equation, C is the concentration of PTEs in individual edible mushrooms (mg kg−1); IRi is the daily intake of edible mushrooms set as 3.56 g day−1 [44]; ED is the time period of PTE exposure for adults (70 years); EF is the frequency exposure of PTEs (350 days years−1) [26, 45,46,47,48]; BW, the average body weight of Iranian adult consumers (for children 15 and adults 70 kg) [49] and AT is the average time (365 × 70) which for adults is 25,550 days [17, 50, 51].
The non-carcinogenic hazard was determined according to Eq. 3:
where THQ is the target hazard quotient, CDI chronic daily intake (mg kg−1 day), RfD, the oral reference dose (mg kg−1 day) [52]. RfD for Cd, Pb, Hg, Ni, Cr, Mn, and As is 0.0005, 0.0035, 0.0001, 0.011, 0.003, 0.140, and 0.0001 mg kg−1 day, respectively [51].
If THQ > 1 value, the exposed population is at considerable health risk, but if THQ ≤ 1, the health risk is not likely [26, 53, 54].
For considering the effect of PTEs in non-carcinogenic risk, total target hazard quotient (TTHQ) was calculated by the equation below:
Besides, the carcinogenic health risk (CR) of As in edible mushrooms was calculated according to the following equation [55, 56]:
where CDI is the chronic daily intake (mg kg−1 day over a lifetime) and SF is the cancer slope factor for As that is 1.5 mg−1 kg day [57]. The Monte Carlo simulation with Oracle Crystal Ball software (Release 11.1.2.4) was applied to calculate the probabilistic distribution of health risk (THQ and CR).
Statistical Analysis
The data are expressed as median, mean rank, lowest, and highest. In this study, Mann-Whitney tests were applied to determine the statistical significance for PTEs between the subjects as classified by type of mushroom (cultivated versus wild). Statistical analyses were performed using the statistical package SPSS for Windows, PC software, version 20 (SPSS Inc., Chicago, IL, USA). The statistical significance was defined as P value < 0.05.
Results and Discussion
Evaluation of the Analytical Method
The graphs of calibration for the ten PTEs were prepared from the standard solutions at five points, ranged from 0.50–10 mg L−1. All the PTEs displayed linear relationships of the instrumental response and the solutions containing the metals with insignificant intercepts and correlation coefficients of 0.994 or higher. Perceived limits of detection (LOD) and limits of quantification (LOQ) are ranged 0.001–0.048 and 0.03–0.16 ppm, respectively. The recoveries gained were found to vary from 92 to 106% that is a suitable range for all PTEs (Table 2).
PTE Concentrations in the Mushrooms
Tables 1 and 3 show the concentration of the PTEs among different species of mushroom (cultivated and wild) and the location of the samples. The concentration of Zn, Fe, Mn, Cd, Cr, Cu, Ni, As, Hg, and Pb in mushroom species was found to be 928.29–6702.86 mg kg−1, 15.25–1662.19 mg kg−1, 2.10–60.48 mg kg−1, 0.001–5.89 mg kg−1, 0.32–26.32 mg kg−1, 9.15–110.08 mg kg−1, 1.21–24.23 mg kg−1, 0.003–11.50 mg kg−1, 0.16–2.24 mg kg−1, and 0.160–8.93 mg kg−1, respectively.
According to Table 1, the order of the mean levels of PTEs in the cultivated mushroom samples was found to be as Zn > Fe > Cu > Cr > Ni > Mn > Pb > Hg > As > Cd and the wild mushroom samples were found to be as Zn > Fe > Cu > Cr > Mn > Ni > Pb > As > Cd > Hg. The statistical analysis confirmed the existence of significant differences between the concentrations of the PTEs in cultivated and wild mushroom (p < 0.05).
Based on research conducted in Iran and other countries, the concentration of these metals in the mushroom is mainly affected by factors of compost or soil, heavily polluted areas (such as highways with heavy traffic), and species of mushroom; increasing the concentration of PTE in mushroom is in many respects different from other plants. Some studies have shown that Agaricus bisporus is more susceptible to increasing content of Hg and to a lesser extent Cd in the substrate, whereas this is not the case in the present study. Some studies have done about the soil and compost contamination by PTE in many cities of Iran like Hamadan, Khozestan, Kurdestan, Isfahan, Sabzevar, babol [58,59,60,61,62,63,64,65,66,67,68]. On the other hand, various studies have found that the mushroom has a good texture for absorbing PTEs [69,70,71,72,73]. Inorganic arsenic is intensely toxic and intake of high quantities leads to inorganic As that is intensely toxic, and intake of high quantities leads to gastrointestinal symptoms, serious disturbances of the cardiovascular and central nervous systems, and finally death [29]. In the present study, it was found that the highest As level was 11.50 mg kg−1 in sample WM25, whereas the lowest As level was 0.003 mg kg−1 in samples WM26, WM27, WM29, CM2, CM3, CM4, CM5, CM6, CM7, CM8, CM9, CM10, CM12, CM15, according to the FAO/WHO/CODEX standard which has declared a standard As level of 0.5 mg kg−1 [43]. The amount of As in samples CM1, CM11, CM13, CM14, CM16, CM17 (the cultivated mushroom sample of Kerman city), CM18, CM19 (the cultivated mushroom sample of Ahvaz city), CM20, CM21 (the cultivated mushroom sample of Zahedan city), CM22 (the cultivated mushroom sample of Hamadan city), CM23 (the cultivated mushroom sample of Rafsanjan city), and WM24, WM25, WM28 is higher than the maximum limit. However, in other samples, it is less than the allowed limit; this difference can be due to various factors such as genetics and the type of primary material (a type of compost) that the mushroom on it has grown and the type of soil in the area is different. In previous investigations, As levels in edible mushrooms were 73.91 to 104.96 mg kg−1 [66], 0.50–5.00 mg kg−1 [74], 0.04 mg kg−1, and 212.30 mg kg−1 [75]. In the present study, the highest concentration of As, like previous studies, is higher than the allowed limit.
Cd is one of the most dangerous rare elements because the amount of its entry through the diet is very high, and its accumulation in organs of the body for a long time causes renal impairment [76]. Cd is often found in soil and enters the food chain through plants. Therefore, it is necessary to determine the rate of absorption, distribution, and bioaccumulation in order to prevent or reduce the contamination of the food chain [77].
In the present study, the highest Cd content was found as 5.89 mg kg−1 in sample WM24. The lowest Cd level was 0.00 mg kg−1 in sample CM12. According to the FAO/WHO/CODEX standard, it has declared the maximum limit of the Cd of 0.5 mg kg−1 [43]. Cd is obtained in sample CM4, CM17, WM24, WM25, WM26, WM28, and WM29 that is higher than the maximum levels, but in other samples, it is less than the allowed limit. This difference can be due to the early composition of the environment where the mushrooms grew. Cd reported in mushroom samples in other studies has been reported as follows.
Reported Cd levels in wild mushrooms in previous studies were 0.08–3.37 mg kg−1 [78], 0.52–5.27 mg kg−1 [79], and 0.06–0.58 mg kg−1 [35]. The highest concentration of previous studies, such as our study, was more than the maximum levels of the FAO/WHO/CODEX standard.
Cr is a mineral that plays a very effective role in maintaining health. Cr 3 is a rare PTE that is required for the natural metabolism of cholesterol, fat, and sugar [35]. The highest Cr level was determined as 26.32 mg kg−1 in sample CM18, and the lowest Cr level was 0.32 mg kg−1 in sample CM5, according to the FAO/WHO/CODEX standard that has declared the maximum limit of the Cr 120 mg kg−1 [43]. In all examined mushrooms, the Cr obtained was less than the FAO/WHO/CODEX allowed limit mushrooms that have less ability to absorb this metal. Previous studies reported 0.87–2.66 mg kg−1 [80], 10.70–42.70 mg kg−1 [35], and 0.54–73.80 mg kg−1 [81]. The Cr concentrations in this study were similar to those from previous studies, and all reports were less than the allowed limit specified by the FAO/WHO/CODEX standard.
Cu is one of the rare elements in the human body, which has the third rank. Its effect is on living systems like vitamins. A small amount of it is in the human body that plays an important role in biochemical processes [35]. Cu amounts in mushrooms are higher than other vegetables, which can be considered as a nutritional source for consumers. However, the bioavailability of the mushroom in humans is limited due to low absorption from the intestine [82]. In this study, the highest concentration of Cu 110.08 mg kg−1 was found in sample CM20, while the lowest Cu level was 9.15 mg kg−1 in sample CM8. According to the FAO/WHO/CODEX standard, it has declared a maximum limit of the Cu of 40 mg kg−1 [43]. Cu concentration in samples CM16, CM17, CM18, CM19, CM20, CM23, WM24, and WM29 is higher than the maximum levels, but in other samples, it is less than the allowed limit. The similar studies conducted, Cu content ranged from 18.00 to 54.00 mg kg−1, 17.50–122.00 mg kg−1, 15.50–63.40 mg kg, respectively [78, 82, 83]. In the present study, the Cu concentrations detected were similar previously reported in the literature.
Fe is the third element from the point of view abundant after Zn and Mn in mushroom, which is almost vital for all living organisms, and it has a broad role in all metabolic activities, including oxygen transfer, DNA synthesis, and electron transfer. It is also specified that enough Fe is too important in the diet to reduce the incidence of anemia. The high concentrations of Fe may be to tissue damage due to the formation of free radicals [35]. In the present study, it was found that the highest concentration of Fe 751.17 mg kg−1 in sample WM28, whereas the lowest Fe level was 15.25 mg kg−1 in sample CM6. According to the FAO/WHO/CODEX standard that has specified the maximum limit of the Fe of 15 mg kg−1 [43]. In all tested mushrooms, the Fe obtained was higher than the FAO/WHO/CODEX allowed limit, which can be due to the ability of the mushroom to absorb Fe. In the previous studies, the concentration of Fe in edible mushrooms ranged from 834 to 870 mg kg−1, 1714–1172 mg kg−1, 834–870 mg kg−1, and 97.2–3919 mg kg−1, respectively [78, 79, 84, 85].
Hg is a naturally occurring metal found in air, water, and soil. It exists in various forms: elemental (or metallic), inorganic, and organic. Acute Hg exposure may give rise to lung damage. Chronic poisoning is specified by neurological and psychological symptoms, such as changes in personality, restlessness, anxiety, sleep disorder, and depression. The symptoms are reversible after the termination of exposure [86]. In this study, the highest concentration of Hg 2.44 mg kg−1 was found in sample CM20, while the lowest Hg level was 0.16 mg kg−1 in sample CM8. According to the China standard, it has specified the maximum limit of the Hg of 1 mg kg−1 [87]. Hg concentration in samples CM11, CM16, CM17, CM18, CM19, CM20, CM21, CM22, and CM23 is higher than the maximum levels, but in other samples, it is less than the allowed limit. The amount of Fe in edible mushrooms in the study of Xin-Hua Chen et al. was 0.28–3.92 mg kg−1 [86], while other values, 1.10–8.40 mg kg−1 and 0.82–3.80 mg kg−1, were reported previously, respectively [88, 89]. The highest concentration of previous studies, such as our study, was more than the maximum levels of the China standard.
Mn is one of the vital elements and is available in metalloproteins (metal-containing proteins) such as carboxylase pyruvate and the glial cytoplasmic enzyme, glutamine synthase. The most important effect of Mn is on the respiratory system and the brain; high doses of Mn cause side effects on the lungs and brain [35]. In the present study, it was found that the highest amount of Mn was 60.47 mg kg−1 in sample WM27, while the lowest Mn level was 2.10 mg kg−1 in sample CM8. Previous studies have shown the amount of Mn in the mushroom as follows.
In the studies of Kalac and Svoboda, Mn level ranged from 12.90 to 93.30 mg kg−1 [90]. In previous investigations, Mn content ranged from 5.50 to 135.00 mg kg−1, 4.61–102.00 mg kg−1, and 5.00–60.00 mg kg−1, respectively. In this study, measured Mn values are consistent with some previous studies. Accordingly, according to Table 2, the amount of studied Mn in our study was lower than the reported Mn concentration in previous studies.
Trace amounts of Ni are useful, because they activate some enzyme systems, while their higher levels can Pb to serious toxicity [91]. In this study, the highest (24.22 mg kg−1) and lowest (1.21 mg kg−1) levels of Ni were found in samples WM25 and CM3. It reported that Ni uptake increased commensurable with the metal increase in the substrate [91]. In the previous studies, amount of Ni content in edible mushrooms ranged from 12.70 to 24.20, 1.72 to 24.10, and 8.2 to 26.7 mg kg−1, respectively [69, 78, 92]. The range of Ni obtained in this study and previous studies was higher than 0.05–5 mg kg−1 reported for plant foods by National Academy of Sciences [86].
Pb ions are known to cause neurotoxic effects, such as mental retardation and loss of intellectual performance in children, as well as increased blood pressure and the risk of developing cardiovascular disease for adults [93]. In this study, the highest concentration of Pb 8.92 mg kg−1 was found in sample WM27, while the lowest Pb level was 0.16 mg kg−1 in sample CM1. According to the FAO/WHO/CODEX standard, it has declared a maximum limit of the Pb of 2 mg kg−1 [43]. Pb is obtained in the samples CM4, CM7, CM11, CM13, CM15, CM16, CM17, CM18, CM19, CM20, CM21, CM22, CM23, WM25, WM26, WM27, WM28, and WM29 that is higher than the maximum levels, but in other samples, it is less than the allowed limit. The amount of Pb in edible mushrooms was ranged from 0.64 to 12.50, 0.48 to 10.18, and 1.68 to 2.56 mg kg−1, respectively [20, 58, 79]. In the present study, the highest concentration of Pb, like previous studies, is more than the maximum limit.
Zn is one of the integral parts of a wide range of enzymes that is responsible for catalytic, structural, and regulative roles. Zn deficiency can be due to inadequate food that can cause impairment in absorption and deficiency in metabolism. Mushrooms are known as Zn storage plants [35]. Zn content ranged from 37.13 to 268.11 mg kg−1 in the present study. The highest Zn content was seen in the sample CM20 and the lowest in the sample CM5. According to the FAO/WHO/CODEX standard, it has specified the maximum limit of the Zn of 60 mg kg−1 [43]. Zn is obtained in the samples CM6, CM11, CM16, CM17, CM118, CM19, CM20, CM21, CM22, CM23, WM24, WM27, and WM28 that is higher than the maximum levels, but in other samples, it is less than the allowed limit. In previous investigations, the amount of Zn in edible mushrooms ranged from 43.50 to 205.00, 1714 to 1172, and 34.4 to 225 mg kg−1, respectively [58, 78, 83]. In the present study, the highest concentration of Zn in our findings, like previous studies, is more than the highest limit.
Human Risk Assessment and Exposure to PTEs
The EWIs of Pb, Hg, Cd, Ni, As, Mn, Cr, Cu, Fe, and Zn for wild mushrooms were 10.25, 11.25, 11.25, 125.025, 0.075, 137.50, 138.75, 407.75, 1522.25, and 1160.50 μg kg−1 bw, and for cultivated mushrooms, 4.00, 4.00, 0.02, 30.25, 0.09, 52.5, 8.06, 228.75, 381.25, and 928.25 μg kg−1 bw, respectively. EWIs of selected metals for consuming edible mushrooms included in this study are well below the PTWI values, except Ni in wild mushrooms, which was higher than PTWI recommended for this element (Table 4).
Non-carcinogenic Risk
The non-carcinogenic risk due to the ingestion of PTEs through edible mushroom consumption for the adults and children in Iran is listed in Table 5.
For adults, the rank order of PTEs based on P95 THQ through edible cultivated mushroom consumption was Hg (0.1182) > Ni (0.0083) > Cr (0.0082) > Pb (0.0035) > As (0.0027) > Mn (0.0012) > Cd (0.0001). The THQ of PTEs for children is similar to that for adults.
For edible wild mushrooms, the rank order of PTEs based on P95 THQ for adults was Hg (0.3183) > Cr (0.1379) > Cd (0.0664) > Ni (0.0349) > Pb (0.0089) > Mn (0.0031) > As (0.0023). Also for children, it was Hg (1.1622) > Cr (0.4952) > Cd (0.2387) > Ni (0.1210) > Pb (0.0307) > Mn (0.0105) > As (0.0080).
The THQs of all PTEs in edible mushrooms in the cultivated and wild for both adults and children were lower than 1 value, except Hg in edible wild mushrooms; for children, it was considerably higher than 1 value.
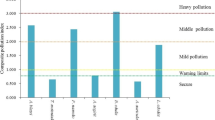
The results of edible mushrooms examined suggest that Hg exposures in humans have the highest potential health risk of adverse effects for children and Cd and As exposures have the minimum risk for cultivated and wild mushrooms, respectively (Fig. 1).
A comparison between HQ of PTEs in edible mushrooms shows that non-carcinogenic risk due to the ingestion of wild mushrooms was higher than cultivated mushrooms.
The TTHQ95 of PTEs in edible mushrooms (cultivated and wild) for adults were 0.14 and 0.57, and for children, 0.51 and 2.07, respectively. The total target hazard quotient (TTHQ) in the edible wild mushrooms due to PTEs residues was ~ 4 times higher than the edible cultivated mushrooms.
Also, when TTHQ value is higher than 10, adverse health effects are considered high for the exposed population [94]. These findings were reflected that, based on the TTHQ value, edible mushrooms are not adverse health effects for the exposed population.
Carcinogenic Risk
The results of carcinogenic risk (CR) due to the ingestion of edible mushrooms are presented in Fig. 2. Percentile 95% CR indexes of PTE ingestion due to consumption of edible mushrooms (cultivated and wild) for adults and children were 3.99E-07 and 3.33E-07, and for children, 1.36E-06 and 1.16E-06, respectively. USEPA recommended that when CR < 10–6, cancer risk is negligible, but if CR > 10–4, cancer risk is unacceptable, and when CR is between 10–6 and 10–4, cancer risk is tolerable for consumers [95, 96]. Therefore, no carcinogenic risk was found based on the results of the health risk assessment.
Conclusion
In this study, the measured values of As, Cd, Cu, Fe, Hg, Mn, Ni, Pb, and zn were higher than standard level as well as Cr was obtained lower than the set limit. The results of this study showed that in analyzed wild and cultivated mushroom samples for first time in different parts of Iran. The health risk assessment (Monte Carlo simulation method) indicated that there is no concern regarding the non-carcinogenic risk due to the ingestion of PTEs via the consumption of the edible mushrooms, except Hg in edible wild mushrooms, for children. However, no carcinogenic risk (CR) was found since all the CR index for As did not exceed the acceptable value. Finally, due to the high concentration of toxic PTEs in some samples of wild mushrooms studied and because of the high mortality rate of people due to consumption of these mushrooms in the past, we recommend that people, especially villagers, refrain from eating these mushrooms.
References
Zied DC, Pardo-Giménez A (2017) Edible and medicinal mushrooms: technology and applications. Wiley
Wang Y, Xu B (2014) Distribution of antioxidant activities and total phenolic contents in acetone, ethanol, water and hot water extracts from 20 edible mushrooms via sequential extraction. Austin J Nutr Food Sci 2(1):5
Zied DC et al (2011) Soybean the main nitrogen source in cultivation substrates of edible and medicinal mushrooms. Soybean Nutr 22:433–452
Waktola G, Temesgen T (2018) Application of mushroom as food and medicine. Adv Biotechnol Microbiol 113:1–4
Muleta D et al (2013) Mushroom consumption habits of Wacha Kebele resident, southwestern Ethiopia. Glob Res J Agric Biol Sci 4(1):6–16
Melgar MJ, Alonso J, García MA (2016) Cadmium in edible mushrooms from NW Spain: bioconcentration factors and consumer health implications. Food Chem Toxicol 88:13–20
Zavastin DE et al (2018) Metal content and crude polysaccharide characterization of selected mushrooms growing in Romania. J Food Compos Anal 67:149–158
Cheung P (2010) The nutritional and health benefits of mushrooms. Nutr Bull 35(4):292–299
Kalač P (2013) A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J Sci Food Agric 93(2):209–218
Latiff LA et al (1996) Relative distribution of minerals in the pileus and stalk of some selected edible mushrooms. Food Chem 56(2):115–121
Talpur NA, Echard BW, Fan AY, Jaffari O, Bagchi D, Preuss HG (2002) Antihypertensive and metabolic effects of whole Maitake mushroom powder and its fractions in two rat strains. Mol Cell Biochem 237(1–2):129–136
Jeong SC et al (2010) White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr Res 30(1):49–56
Lavi I et al (2006) An aqueous polysaccharide extract from the edible mushroom Pleurotus ostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett 244(1):61–70
Krupa P, Kozdroj J (2004) Accumulation of heavy metals by ectomycorrhizal fungi colonizing birch trees growing in an industrial desert soil. World J Microbiol Biotechnol 20(4):427–430
Peykarestan B et al (2020) The concentration and non-carcinogenic risk assessment of aluminium in fruits, soil, and water collected from Iran. Int J Environ Anal Chem:1–16
Rezaei H et al (2019) Health-risk assessment related to the fluoride, nitrate, and nitrite in the drinking water in the Sanandaj, Kurdistan County, Iran. Human Ecol Risk Assess 25(5):1242–1250
Heshmati A, Ghadimi S, Mousavi Khaneghah A, Barba FJ, Lorenzo JM, Nazemi F, Fakhri Y (2018) Risk assessment of benzene in food samples of Iran's market. Food Chem Toxicol 114:278–284
Khaneghah AM et al (2018) Impact of unit operations during processing of cereal-based products on the levels of deoxynivalenol, total aflatoxin, ochratoxin A, and zearalenone: a systematic review and meta-analysis. Food Chem 268:611–624
Shariatifar N et al (2017) Assessment of heavy metal content in refined and unrefined salts obtained from Urmia, Iran. Toxin Rev 36(2):89–93
Liu B et al (2015) Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem 188:294–300
Fathabad AE, Shariatifar N, Moazzen M, Nazmara S, Fakhri Y, Alimohammadi M, Azari A, Mousavi Khaneghah A (2018) Determination of heavy metal content of processed fruit products from Tehran's market using ICP-OES: a risk assessment study. Food Chem Toxicol 115:436–446
Mateo-Sagasta, J., et al., (2018) More people, more food, worse water?: a global review of water pollution from agriculture. Rome, Italy: FAO Colombo, Sri Lanka: International Water Management …
Razzaghi N, Ziarati P, Rastegar H, Shoeibi S, Amirahmadi M, Conti GO, Ferrante M, Fakhri Y, Mousavi Khaneghah A (2018) The concentration and probabilistic health risk assessment of pesticide residues in commercially available olive oils in Iran. Food Chem Toxicol 120:32–40
Stihi C et al (2011) Studies on accumulation of heavy metals from substrate to edible wild. Roman J Physics 56(1–2):257–264
Welch RM (2005) Biotechnology, biofortification, and global health. Food Nutr Bull 26(4_suppl3):S304–S306
Yousefi M, Shemshadi G, Khorshidian N, Ghasemzadeh-Mohammadi V, Fakhri Y, Hosseini H, Mousavi Khaneghah A (2018) Polycyclic aromatic hydrocarbons (PAHs) content of edible vegetable oils in Iran: a risk assessment study. Food Chem Toxicol 118:480–489
Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on modified jute fibres. Bioresour Technol 96(13):1430–1438
Mahurpawar M (2015) Effects of heavy metals on human health. Int J Res Granthaalayah 530:1–7
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182
Cheraghi M et al (2013) Evaluation of heavy metal concentration in compost, soil cover and button mushroom in Kurdistan greenhouses. J Food Hygiene 2(4)
Atamaleki A et al (2020) The concentration of potentially toxic elements (PTEs) in eggs: a global systematic review, meta-analysis and probabilistic health risk assessment. Trends Food Sci Technol 95:1–9
Atamaleki A et al (2019) The concentration of potentially toxic elements in eggs: a systematic review-meta-analysis and probabilistic health risk assessment. Trends Food Sci Technol
Fakhri Y et al (2019) Bioaccumulation of potentially toxic elements (PTEs) in muscle Tilapia spp fish: a systematic review, meta-analysis, and non-carcinogenic risk assessment. Toxin Rev:1–11
Khaneghah AM et al (2019) Potentially toxic elements (PTEs) in cereal-based foods: a systematic review and meta-analysis. Trends Food Sci Technol
Zhu F et al (2011) Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. 179(1–4):191–199
Raj DD et al (2011) Mushrooms in the remediation of heavy metals from soil. Int J Environ Pollut Control Manag 3(1):89–101
Demirbaş A (2001) Heavy metal bioaccumulation by mushrooms from artificially fortified soils. Food Chem 74(3):293–301
Fakhri Y et al (2019) The concentration of potentially toxic elements (PTEs) in honey: a global systematic review and meta-analysis and risk assessment. Trends Food Sci Technol
Rezaei M, Ghasemidehkordi B, Peykarestan B, Shariatifar N, Jafari M, Fakhri Y, Jabbari M, Khaneghah AM (2019) Potentially toxic element concentration in fruits collected from Markazi Province (Iran): a probabilistic health risk assessment. Biomed Environ Sci 32(11):839–853
Udochukwu U et al (2014) Bioaccumulation of heavy metals and pollutants by edible mushroom collected from Iselu market Benin-city. Int J Curr Microbiol App Sci 3(10):52–57
Türkmen, M. and N. Dura (2016) Assessment of heavy metal concentrations in fish from south western black sea
Joint F et al (2004) Evaluation of certain veterinary drug residues in food: sixty-second report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization
Jamuna, P.J.J.o.f.s. and technology (2010) Evaluation of certain food additives. Sixty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Technical Report Series No. 952. 2009. World Health Organization. Geneva, pages 208 47(4): 465–467
Abdollahi, M., et al. (2014) Socio-economic differences in dietary intakes: the comprehensive study on household food consumption patterns and nutritional status of IR Iran
Nazaroff W, Alvarez-Cohen L (2001) Environmental engineering science. Wiley, New York
Jahanbakhsh M et al (2019) Probabilistic health risk assessment (Monte Carlo simulation method) and prevalence of aflatoxin B1 in wheat flours of Iran. Int J Environ Anal Chem:1–12
Madani-Tonekaboni M et al (2019) Monitoring and risk assessment of lead and cadmium in milks from east of Iran using Monte Carlo simulation method. Nutr Food Sci Res 6(2):29–36
Ihugba UA et al (2018) Heavy metal determination and health risk assessment of oyster mushroom Pleurotus tuberregium (Fr.) Singer, collected from selected markets in Imo State. Nigeria. Am J Environ Protect 6(1):22–27
Sharafi K, Yunesian M, Nodehi RN, Mahvi AH, Pirsaheb M (2019) A systematic literature review for some toxic metals in widely consumed rice types (domestic and imported) in Iran: human health risk assessment, uncertainty and sensitivity analysis. Ecotoxicol Environ Saf 176:64–75
EPA, U.,(2002) A review of the reference dose and reference concentration processes. EPA/630/P-02
EPA, U.,(2015) United States Environmental Protection Agency, in Quantitative Risk Assessment Calculations 7–9. 2015
Moradi-Khatoonabadi Z et al (2015) Synthetic food colours in saffron solutions, saffron rice and saffron chicken from restaurants in Tehran, Iran. Food Additives Contaminants: Part B 8(1):12–17
Dadar M et al (2017) Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev 36(3):222–227
Ghasemidehkordi, B., et al. (2018) Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: Non-carcinogenic risk assessment. Food Chem Toxicol
Sultana MS et al (2017) Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ Sci 3(1):1291107
Antoine JM et al (2017) Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol Rep 4:181–187
Siddique, F and N. Vigasini (n.d.) Assessment of levels of specific synthetic food colours and preservatives in selected commonly consumed food products in Chennai
Anbari, M., et al. (2011) Investigation of lead and cadmium contents of cultivated edible mushrooms consumed in Tehran
Sobhan Ardakani S, Jahangard A (2017) Toxicological assessment of inorganic arsenic and zinc content in button mushrooms. J Adv Environ Health Res 5(4):246–251
Murray H et al (2011) Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. J Soils Sediments 11(5):815–829
Kalač P, Svoboda Lr (2000) A review of trace element concentrations in edible mushrooms. Food Chem 69(3):273–281
Owaid MN (2015) Mineral elements content in two sources of Agaricus bisporus in Iraqi market. J Adv Appl Sci 3(2):46–50
Asgharzadeh, F., et al. (2014) Evaluation of cadmium, lead and zinc content of compost produced in Babol Composting Plant
HOODAJI M et al (2015) The effect of compost on release and transport of heavy metals (Zn, Cu) in soil. Cumhuriyet Üniversitesi Fen-Edebiyat Fakültesi Fen Bilimleri Dergisi 36(3):685–692
Jafari J et al (2014) Survey of the effects of soil type on the leaching and adsorption of heavy metals (chromium, lead and cadmium) after compost application on the soils. Iran J Health Environ 6(4):523–534
CHERAGHI, M., et al. (2013) Evaluation of heavy metal concentration in compost, SOIL COVER AND BUTTON MUSHROOM IN KURDISTAN GREENHOUSES
Sobhanardakani S et al (2016) Assessment of heavy metal contamination in surface soils of Ahvaz IV industrial estate, Khuzestan province, Iran. Iran J Health Sci 4(1):53–61
Khanlari ZV, Jalali M (2008) Concentrations and chemical speciation of five heavy metals (Zn, Cd, Ni, Cu, and Pb) in selected agricultural calcareous soils of Hamadan Province, western Iran. Arch Agron Soil Sci 54(1):19–32
Mendil D et al (2004) Determination of trace elements on some wild edible mushroom samples from Kastamonu, Turkey. 88(2):281–285
Isildak Ö et al (2004) Analysis of heavy metals in some wild-grown edible mushrooms from the middle black sea region, Turkey. 86(4):547–552
Khodabakhshi A et al (2016) Investigation of heavy metals in edible mushrooms consumed in Shahrekord. J Shahrekord Univ Med Sci 18(1):54–62
Das, N. (2005) Heavy metals biosorption by mushrooms
Sinha, S.K., et al. (n.d.) Heavy metals detection in white button mushroom (Agaricus bisporus) cultivated in state of Maharashtra, INDIA
Kalač PJFC (2010) Trace element contents in European species of wild growing edible mushrooms: a review for the period 2000–2009. 122(1):2–15
Cheng J, Zhang X, Tang Z, Yang Y, Nie Z, Huang Q (2017) Concentrations and human health implications of heavy metals in market foods from a Chinese coal-mining city. Environ Toxicol Pharmacol 50:37–44
Yang H et al (2013) Cross-species extrapolation of prediction models for cadmium transfer from soil to corn grain. 8(12):e80855
FAO/WHO, C.A.C.J.G (1995) Position paper on cadmium
Türkmen M, Budur DJFC (2018) Heavy metal contaminations in edible wild mushroom species from Turkey’s Black Sea region. 254:256–259
Radulescu C et al (2010) Studies concerning heavy metals bioaccumulation of wild edible mushrooms from industrial area by using spectrometric techniques. 84(5):641–646
Tüzen MJMJ (2003) Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. 74(3):289–297
Yamaç M et al (2007) Heavy metals in some edible mushrooms from the Central Anatolia, Turkey. 103(2):263–267
Turkekul I et al (2004) Determination of iron, copper, manganese, zinc, lead, and cadmium in mushroom samples from Tokat, Turkey. 84(3):389–392
Sesli E, Tuzen M, Soylak M (2008) Evaluation of trace metal contents of some wild edible mushrooms from Black sea region, Turkey. J Hazard Mater 160(2–3):462–467
Sarikurkcu C et al (2011) Metal concentration of wild edible mushrooms in Soguksu National Park in Turkey. Food Chem 128(3):731–734
Zhu F, Qu L, Fan W, Qiao M, Hao H, Wang X (2011) Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ Monit Assess 179(1–4):191–199
Chen X-H et al (2009) Analysis of several heavy metals in wild edible mushrooms from regions of China. 83(2):280
Maskan M (2001) Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J Food Eng 48(2):177–182
Falandysz J, Bielawski LJFC (2007) Mercury and its bioconcentration factors in Brown Birch Scaber Stalk (Leccinum scabrum) from various sites in Poland. 105(2):635–640
Falandysz J, Gucia M (2008) Bioconcentration factors of mercury by parasol mushroom (Macrolepiota procera). Environ Geochem Health 30(2):121–125
Kalac, P and L. Svoboda (2004) Contents of detrimental metals mercury, cadmium and lead in wild growing edible mushrooms: a review, Energy Education Science and Technology
Širić I et al (2016) Heavy metal bioaccumulation by wild edible saprophytic and ectomycorrhizal mushrooms. 23(18):18239–18252
Sarikurkcu C, Tepe B, Kocak MS, Uren MC (2015) Metal concentration and antioxidant activity of edible mushrooms from Turkey. Food Chem 175:549–555
Khodabakhshi, A., et al. (2016) Investigation of heavy metals in edible mushrooms consumed in Shahrekord 18
Hashish SM et al (2012) Mineral and heavy metals content in eggs of local hens at different geographic areas in Egypt. Global Vet 8(3):298–304
USEPA (2016) U.S. Environmental Protection Agency, Supplemental guidance for assessing susceptibility from early-life exposure to carcinogens. http://www3.epa.gov/ airtoxics/childrens supplement final.pdf (Accessed on Jan 25, 2016)
Huang C-L, Bao LJ, Luo P, Wang ZY, Li SM, Zeng EY (2016) Potential health risk for residents around a typical e-waste recycling zone via inhalation of size-fractionated particle-bound heavy metals. J Hazard Mater 317:449–456
Funding
The Tehran University of Medical Sciences supported this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karami, H., Shariatifar, N., Nazmara, S. et al. The Concentration and Probabilistic Health Risk of Potentially Toxic Elements (PTEs) in Edible Mushrooms (Wild and Cultivated) Samples Collected from Different Cities of Iran. Biol Trace Elem Res 199, 389–400 (2021). https://doi.org/10.1007/s12011-020-02130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02130-x