Abstract
Several studies indicate aluminum (Al) as a potent toxicant, mainly related to central nervous system disorders. However, investigations about the Al effects over salivary glands are still scarce. In this way, the present study aimed to investigate whether the Al chloride (AlCl3) is able of triggering oxidative stress in parotid and submandibular glands of mice and also, if any morphological impairment is observed. For this, twenty mice were divided into two groups: Exposed group (EG), which received 18.5 mg/kg of AlCl3 by intragastric gavage for 60 days and control group (CG), which received distilled water by intragastric gavage during the same period of time. After that, levels of reduced glutathione (GSH) and malonaldehyde (MDA) were analyzed and we performed morphological analyses by evaluating the area of parenchyma, stroma, acini, and ducts in both glands. Statistical analyses were performed by Student’s t test and two-way ANOVA, adopting p < 0.05. No abnormal body weight was observed and data indicates that although both major salivary glands are susceptible to Al-induced oxidative stress, by increasing MDA and reducing GSH, only submandibular glands decreased the parenchyma and increased stroma area. Moreover, the submandibular glands showed smaller total area of acini and higher total area of ducts, in comparison with the control group. Notably, AlCl3 induces oxidative stress in both glands, however, submandibular glands showed to be more susceptible to Al effects than parotid glands. Our study gives evidences about Al toxicity in parotid and submandibular glands and claims for new investigations to understand more mechanisms of Al-induced toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most abundant metallic element found in Earth’s crust is aluminum (Al) [1]. Exposure to Al is almost inevitable due to its widespread environmental presence. Acid rain can also result in a source of exposure due to solubilization of clay-derived Al particles. Thus, higher amounts of Al may be present in rivers and lakes, consequently related to drinking water acquisition [2].
Physical properties like electrical conductivity, corrosion resistance, and low melting point make Al highly embedded to many consumer products and industrial applications, such as water-treatment products [3, 4]. Moreover, some Al compounds present mechanisms not well characterized that act as adjuvant to vaccines by stimulating the immune response [5, 6].
The Al exposure has been associated to biological systems damage and it certainly can be considered a toxic agent. Some studies suggest that chronic exposure to Al can cause neurological and cognitive impairment and learning-memory dysfunction and is thought to be related to Alzheimer’s disease [2, 7,8,9,10].
Specifically, about oral cavity structures, morphological changes were also observed in salivary glands after Al chronic exposure in rats [11]. But in this study, the Al citrate that was used as a vehicle for the metal increased the absorption of this element from the gastrointestinal tract by a paracellular energy independent route [12, 13]. Thus, this experimental model was not representative of an environmental exposure and also the dose used was higher than recommended as safe by health agencies [14].
Salivary glands are important structures that produce and secrete an essential fluid to the stomatognathic system homeostasis, the saliva [15]. Saliva plays several roles as lubricant, immune, digestive, buffering, and other functions [16]. Saliva’s composition and flow rate are important factors to the effective activity of those structures and damage to the glandular morphology is associated with impairment of those factors [17]. Thus, changes in salivary glands can compromise oral cavity homeostasis.
In this perspective, we aimed to evaluate the effects of Al chloride exposure on the salivary glands of mice at doses proportional to those considered as a dietary consumption for humans and proportional to surface area. Dose values were about 6.16 mg/kg/day in accordance with the Agency for Toxic Substances and Disease Registry (ATSDR) (factor 3 for extrapolation from human to animal) [4, 18, 19]. Our hypothesis is that the experimental model of low-dose and long-term exposure of Al is able to promote oxidative imbalance and morphometric changes in salivary glands.
Material and Methods
Animals
Twenty male Swiss albino mice (Mus musculus, 21 days old) were provided by the Federal University of Pará Animal Facility. The animals were kept in cages (30 cm × 20 cm × 13 cm), 5 animals each. The animals received water and balanced food (Presence, Neovia, Brazil—pelleted diet ingredients on Supplementary Table 1) ad libitum and were housed under standard conditions (25 °C and 12-h dark/light cycle). All experiments were carried out in compliance with the guidelines provided by the Ethics Committee on Experimental Animals of Federal University of Pará that approved and authorized this project (CEUA-UFPA N 9037190417).
Experimental Groups and Exposure to Aluminum
The animals were divided into control group and intoxicated group using a simple randomization, each one with 10 animals. The intoxicated group received Al chloride (AlCl3) (18.5 mg/kg) with distilled water as vehicle by intragastric gavage for 60 days. The Al dose was calculated according to a formula used for dose conversion based on the body surface area [18, 19]. In the control group, only distilled water was administered following the same protocol. The animals were weighted weekly and dose adjustment was made if necessary.
Oxidative Biochemistry Assays
To evaluate antioxidant system activity, reduced glutathione (GSH) levels were the chosen parameter. GSH is considered one of the most important agents in the cell’s antioxidant defense system because it protects it from injury resulting from exposure to offending agents.
One of the main mechanisms of injury is lipid peroxidation (LPO), which is the oxidation of the lipid layer of the cell membrane. Malondialdehyde (MDA) is one of the bioproducts of LPO. In this study, MDA was chosen as a pro-oxidant parameter.
After the exposure period, 5 animals of each group were euthanized and salivary glands (submandibular and parotid) were surgically removed, washed in physiological saline solution, frozen with liquid nitrogen, stored at − 80 °C, and then submitted to oxidative biochemistry assays. The samples were thawed and resuspended in 20 mM Tris-HCl2, pH 7.4, at 4 °C by sonic disaggregation (approximate concentration of 1 g/mL).
Determination of the GSH levels was based on the ability of GSH to reduce 5,5-dithiobis-2-nitrobenzoic acid (DTNB) to nitrobenzoic acid (TNB), which was quantified by spectrophotometry at 412 nm. The methodology described by Ellman [20] was adapted for this determination. Initially, an aliquot (20 μL) from supernatant obtained from the homogenate samples of glandular tissue was added in a tube containing distilled water (20 μL) and PBS solution pH 8.0 (3 mL) to carry out the first measurement. Afterwards, DTNB (100 μL) was added to the solution, and another measurement was carried out after 3 min. The GSH concentration was expressed as μg/mL.
The level of LPO was established by measurement of malonaldehyde (MDA) levels based on the method proposed by Esterbauer and Cheeseman [21]. Lysates were centrifuged at 3512g for 10 min at 4 °C. One hundred microliters of standard solutions of MDA or supernatant was added to 325 μL of 10.3 mM N-methyl-2-phenylindole diluted in methanol (1:3) and 75 μL of methanesulfonic acid. This mixture was heated at 45 °C for 40 min, with subsequent spectrophotometric reading (λ = 570 ηm). Also, we performed protein content determination in samples by Bradford’s method [22] in order to normalize the results of MDA levels. Then, the results were expressed as MDA nmol/mg of protein.
For the graphical representation, the results were expressed as percentages of the GSH and of the MDA concentrations of the control groups.
Morphometric Analyses
The other 10 animals were submitted to perfusion. Briefly, the animals were deeply anesthetized though intraperitoneal injection of ketamine hydrochloride (90 mg/kg) and xylazine hydrochloride (9 mg/kg). They were transcardially perfused with saline heparinized solution (NaCl 0.9%) followed by 4% paraformaldehyde. The salivary glands were removed and conducted to histological procedures. For this analysis, we evaluated the area of the tissues and their structures.
Submandibular and parotid glands were collected and post-fixed in 4% formaldehyde until processing. The glands were dehydrated in increasing solutions of ethanol (70%, 80%, 90%, absolute 1, absolute 2), diaphanized in xylol, and embedded in Paraplast. Sections of 5 μm thickness were obtained using a microtome. These sections were stained with hematoxylin and eosin.
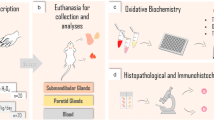
To perform morphometric analyses, images were taken from a color digital camera (Cyber Shot DSC W-230, × 4 optical zoom, Sony, Tokyo, Japan) coupled to a microscope (× 1.5, Eclipse E200, Nikon, Tokyo, Japan; × 40 magnification) of 5 random sagittal cuts of the glands, reducing 5 fields of each cut. The parameters of tissue morphology evaluated and expressed in μm2 were total acinar area, total duct area, total parenchyma area, and total stroma area [22]. The values were obtained using a digital image analyzer, ImageJ software (NIMH, NIH, Bethesda, MD, USA, http://rsbweb.nih.gov/ij/). For the graphical representation, the results were expressed as percentages of the control group. All experimental steps are summarized in Fig. 1.
Statistical Analyses
The values obtained from the biochemistry assays and histomorphometry were plotted on GraphPad Prism 5.0 software (San Diego, CA, USA). Although the structures analyzed are two salivary glands, they are considered as different organs, therefore, the results were evaluated individually, for each gland, being compared only the groups exposed to AlCl3 and control. The normality of each group was evaluated by the Shapiro-Wilk method and then morphometric variables and oxidative stress parameters were analyzed by Student’s t test with significance level of p < 0.05. The results were expressed in percentage of control, mean, and standard error. For statistical comparison of body weight gains between control and intoxicated groups, two-way analysis of variance (ANOVA) with repeated measures was used, considering p < 0.05. The test power was calculated using the difference between the groups’ averages with the OpenEpi software (Version 2.3.1), considering the type I error of 5% and a power of 0.8000 (for all values, see supplementary material 2).
Results
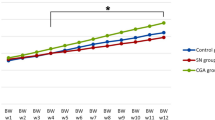
The Long-term Exposure to AlCl3 Does Not Affect the Body Weight of Mice
Systemic administration of AlCl3 for 60 days did not affect the gain or loss of body weight of mice. Furthermore, there was a natural gain in body mass due to animal growth (p > 0.05), as observed in Fig. 2.
AlCl3 Chronic Exposure Impairs the Oxidative Biochemistry
Our data showed that AlCl3 promotes changes in oxidative parameters related to GSH. We observed a decrease in GSH levels in submandibular about 26.09% (± 7.402, p = 0.0077) and in parotid about 14.85% (± 4.376, p = 0.0134), when compared with the control group (Fig. 3).
Animals exposed to AlCl3 increased lipid peroxidation by higher levels of MDA in their salivary glands (Fig. 4). When compared with the control group, both glands presented higher levels of MDA: submandibular increased about 38.20% (± 10.21, p = 0.0158) and parotid, 51.20% (± 11.68, p = 0.0031).
AlCl3 May Impair Salivary Glands Structures, Especially the Submandibular Glands
The results of the morphometric evaluation showed that the exposure to AlCl3 was able to modify the submandibular tissue with decreased parenchyma (64.76 ± 6.816, p = 0.0011) and increased stroma (303.5 ± 51.81, p = 0.0046) when compared with the control group, as shown in Fig. 5a–c. However, no statistically differences in the parenchyma and in the stroma were observed in the parotid glands compared with the control group (Fig. 5d–f).
Effects of AlCl3 exposure (18.5 mg/kg/day) during 60 days on morphometric parameters of submandibular and parotid glands. Photomicrographs showing the submandibular glands of the control group (a) and the exposed group (b) and the parotid glands of the control group (d) and the exposed group (e) stained with hematoxylin and eosin. Area (μm2) of parenchyma and stroma in the submandibular (c) and parotid glands (f). Results are expressed as mean ± standard error of mean. Student’s t test, p < 0.05. Scale bar 40 μm
We also observed that the AlCl3 exposure changed the area of the submandibular gland structures. When compared with the control group, the salivary glands exposed to AlCl3 showed a decrease in the total area of acini (61.1 ± 8.522, p = 0.0339) and an increase in the total area of ducts (325.5 ± 29.83, p < 0.0001) (Fig. 6a). In the parotid glands, these differences were not observed (Fig. 6b).
The results were submitted to evaluation of power test regarding comparison between the control and aluminum group (t test, two independent means). The values of the comparisons with statistical differences were between 0.8625 and 1.000.
Discussion
In this study, we showed for the first time that low-dose and long-term systemic exposure to AlCl3 is capable of promoting oxidative stress in mice salivary glands, in which the submandibular gland is more susceptible to this biochemical imbalance, resulting in damage to the epithelial structure.
Saliva is a product of three pairs of major salivary glands (parotid, submandibular, and sublingual) and numerous minor salivary glands distribute in the submucosa under most soft tissues in the mouth [23]. For the present investigation, we have chosen to study submandibular and parotid gland because they are the two largest glands in mice and together they produce more than a half of the saliva present in the mouth cavity [24]. The sublingual gland was excluded from this investigation, because in mice its dimensions are very small and fused in submandibular gland [25], making it difficult to remove it exclusively, without including other tissue, which could interfere with the biochemical analysis. Besides that, its contribution to saliva’s production is smaller [24].
The dose used in this investigation was a human dose adjusted to rodents following the methods proposed by Reagan-Shaw [19], using an allometric scale, which considers the differences between the species. This adjustment allows a representative adaptation of the human’s dose to a rodent equivalent considering their different metabolisms, excretion mechanisms, weights, and body surface areas [26].
Al in humans is absorbed and accumulated through the gastrointestinal system by inhalation of particles. Its absorption happens via the gastrointestinal tract and through connections between the blood components, it can circulate the plasma in different organs. From that, Al can trigger oxidative stress and inflammatory events resulting in tissue damages [27].
The exact mechanism of Al toxicity is not fully understood, but recent studies [27, 28] suggested that Al exhibits pro-oxidant activity because it binds to pro-oxidants metals such as iron (Fe), modulating the ability to generate oxidative events. According to this hypothesis, Al participates in the Fenton reaction, which is a catalytic process triggered in the peroxide hydrogen presence forming reactive oxygen species [28].
In the present study, we show that oral exposure to AlCl3 was able to trigger biochemical imbalance in both salivary glands studied. We showed that the dose and the exposure time were able to reduce the endogen antioxidant response, by the reduced GSH levels, as well as trigger LPO by increasing MDA concentration.
GSH acts as a cysteine carrier and reservoir and participates in the detoxification of chemical agents and the elimination of lipoperoxidation products. Also, it is required for the synthesis of DNA, proteins, and some prostaglandins. GSH has a reducing capacity determined by the SH group present in cysteine [29]. The MDA is one of the secondary products of lipid peroxidation (LPO) and is considered a potential candidate as a general biomarker of tissue oxidative stress [30].
In a previous study from our group [11], we showed that oral exposure to Al citrate was able to increase the levels of Al in the glandular parenchyma. Although in our current investigation we did not measure Al levels in glandular tissue, we believe that the diagnosed oxidative alterations could be an important validation of this metal’s absorption by the exposed animals.
Moreover, in the same study mentioned above [11], we showed that Al was able to trigger damages to intracellular components of acinar and myoepithelial cells; however, we could not show whether this would impact the histological and morphometric structure of these glands. In the present investigation, we showed that exposure to AlCl3 changed the proportion between parenchyma and stroma in submandibular glands, suggesting a loss of the epithelial component and the formation of a reparative conjunctive tissue.
This probable explanation is based in fact that, in addition to the finding of a reduced submandibular parenchyma in exposed animals, we also found a decrease in the acinar component, which is the cellular component responsible for salivary production and a considerable increase in tubular component. Among duct-type structures, granular and intercalated ducts have been reported to overpass possible injuries through stem-cells activity within these structures [31, 32]. Thus, acinar damages related to AlCl3 toxicity may be reduced through intercalated and granular repair mechanisms.
We did not detect morphometric changes in parotid glands. Importantly, although both are salivary glands, structures predominantly composed of acinar cells, and a complex ductal system associated with contractile myoepithelial cells, which helps the salivary secretion into the ducts [33], the salivary glands are independent organs, with different cellular organization [34], biochemical and tissue structure [35, 36], and proteomic profile [37], which could explain the different tissue response.
Submandibular glands are composed of mixed acini with mucous and serous components and it is mainly responsible for secreting a viscous saliva composed of glycoproteins and epidermal growth factors, promoting lubrication and protection of the oral mucosa. The parotid glands contain mostly serous acini and make mostly serous fluid rich of water and ions [16, 36, 38].
Other previous studies that used different experimental models, but also included the evaluation of oxidative stress and histological parameters, demonstrate this lower susceptibility or probable cytoprotection of the parotid glands in rats exposed to methylmercury [39, 40] and inorganic mercury [37, 41], in which the exposed animals although presented higher levels of total mercury in the parotid glands, did not present changes in parenchyma and stroma proportions, neither in the acinar nor ductal organization [40, 41].
Under the experimental conditions of this study, the data demonstrate that chronic exposure to AlCl3, even at low doses, impairs the oxidative biochemistry as well as promotes morphological changes in salivary glands of mice. Further investigations including other tissue parameters are necessary in order to elucidate whether these changes could affect important functions of these salivary glands, especially about flow and quality of saliva, repercussion on the maintenance of oral homeostasis, and digestive-immune response.
References
Kumar V, Gill KD (2009) Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol 83:965–978. https://doi.org/10.1007/s00204-009-0455-6
Nie J (2018) Exposure to aluminum in daily life and Alzheimer’s disease. Neurotoxicity of Aluminum:99–111. https://doi.org/10.1007/978-981-13-1370-7_6
Mu Z, Zhou F, Zhang S, Liang Y, Liu W (2005) Effect of the functional groups in ionic liquid molecules on the friction and wear behavior of aluminum alloy in lubricated aluminum-on-steel contact. Tribol Int 38:725–731
Agency for Toxic Substances and Disease Registry (ATSDR) (2006) Toxicological profile for aluminum (draft for public comment). U.S. Department of Health and Human Services, Public Health Service, Atlanta
Ghimire TR (2015) The mechanisms of action of vaccines containing aluminum adjuvants: an in vitro vs in vivo paradigm. SpringerPlus 4(1):1–18. https://doi.org/10.1186/s40064-015-0972-0
McFarland G, La Joie E, Thomas P, Lyons-Weiler J (2019) Acute exposure and chronic retention of aluminum in three vaccine schedules and effects of genetic and environmental variation. J Trace Elem Med Biol 126444. https://doi.org/10.1016/j.jtemb.2019.126444
Varner JA, Jensen KF, Horvath W, Isaacson RL (1998) Chronic administration of aluminum–fluoride or sodium–fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Res 784(1–2):284–298. https://doi.org/10.1016/s0006-8993(97)01336-x
Bondy SC (2016) Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. NeuroToxicology 52:222–229. https://doi.org/10.1016/j.neuro.2015.12.002
Niu Q (2018) Overview of the relationship between aluminum exposure and health of human being. Neurotoxicity of Aluminum, pp 1–31. https://doi.org/10.1007/978-981-13-1370-7_1
Zhang X, Long Y, Huang J, Xia J (2019) Molecular mechanisms for coping with Al toxicity in plants. Int J Mol Sci 20(7):1551. https://doi.org/10.3390/ijms20071551
Costa N, Correa R, Júnior I, Figueiredo A, Vilhena K, Farias-Junior PMA, Teixeira FB, Ferreira NMM, Pereira-Junior JB, Dantas KGF, Silva MCF, Silva-Junior AF, Alves-Junior SM, Pinheiro JJV, Lima RR (2014) Physical, chemical, and Immunohistochemical investigation of the damage to salivary glands in a model of intoxication with aluminium citrate. Int J Environ Res Public Health 11(12):12429–12440. https://doi.org/10.3390/ijerph111212429
Cunat L, Lanhers MC, Joyeux M, Burnel D (2000) Bioavailability and intestinal absorption of aluminum in rats : effects of aluminum compounds and some dietary constituents. Biol Trace Elem Res 76(1):31–56. https://doi.org/10.1385/bter:76:1:31
Moore PB, Day JP, Taylor GA, Ferrier IN, Fifield LK, Edwardson JA (2000) Absorption of aluminium-26 in Alzheimer’s disease, measured using accelerator mass spectrometry. Dement Geriatr Cogn Disord 11(2):66–69. https://doi.org/10.1159/000017216
World Health Organization (1997) Aluminium. World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 194), Geneva
Holmberg, KV, Hoffman, MP (2014) Anatomy, biogenesis and regeneration of salivary glands. Saliva: Secretion and Functions, 1–13. https://doi.org/10.1159/000358776
Amano O, Mizobe K, Bando Y, Sakiyama K (2012) Anatomy and histology of rodent and human major salivary glands. Overview of the Japan Salivary Gland Society-Sponsored Workshop. Acta Histochem Cytochem 45(5):241–250. https://doi.org/10.1267/ahc.12013
Lamy E, Neves S, Ferreira J, Rodrigues L, da Costa G, Cordeiro C, Fialho L, Lima M, Costa AR, Antunes CM, Lopes O, Amado F, Capela e Silva F (2018) Effects of hyperleptinemia in rat saliva composition, histology and ultrastructure of the major salivary glands. Arch Oral Biol 96:1–12. https://doi.org/10.1016/j.archoralbio.2018.08.005
Martinez CS, Alterman CDC, Peçanha FM, Vassallo DV, Mello-Carpes PB, Miguel M, Wiggers GA (2016) Aluminum exposure at human dietary levels for 60 days reaches a threshold sufficient to promote memory impairment in rats. Neurotox Res 31(1):20–30. https://doi.org/10.1007/s12640-016-9656-y
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3):659–661. https://doi.org/10.1096/fj.07-9574lsf
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Proctor GB, Carpenter GH (2007) Regulation of salivary gland function by autonomic nerves. Auton Neurosci 133:3–18. https://doi.org/10.1016/j.autneu.2006.10.006
Sas R, Dawes C (1997) The intra-oral distribution of unstimulated and chewing-gum-stimulated parotid saliva. Arch Oral Biol 42(7):469–474. https://doi.org/10.1016/s0003-9969(97)00045-9
Maruyama CL, Monroe MM, Hunt JP, Buchmann L, Baker OJ (2019) Comparing human and mouse salivary glands: a practice guide for salivary researchers. Oral Dis 25(2):403–415. https://doi.org/10.1111/odi.12840
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm
Nagaoka MH, Maitani T (2005) Binding affinity of aluminium to human serum transferrin and effects of carbohydrate chain modification as studied by HPLC/high-resolution ICP-MS -speciation of aluminium in human sérum. J Inorg Biochem
Ruiperez F, Mujika JI, Ugalde JM, Exley C, Lopez X (2012) Pro-oxidant activity of aluminum: promoting the Fenton reaction by reducing Fe (III) to Fe (II). J Inorg Biochem
Gaucher C, Boudier A, Bonetti J, Clarot I, Leroy P, Parent M (2018) Glutathione: antioxidant properties dedicated to nanotechnologies. Antioxidants 7(5):62. https://doi.org/10.3390/antiox7050062
Grotto D, Barcelos GRM, Valentini J, Antunes LMG, Angeli JPF, Garcia SC, Barbosa F (2008) Low levels of methylmercury induce DNA damage in rats: protective effects of selenium. Arch Toxicol 83(3):249–254. https://doi.org/10.1007/s00204-008-0353-3
Kwak M, Alston N, Ghazizadeh S (2016) Identification of stem cells in the secretory complex of salivary glands. J Dent Res 95(7):776–783. https://doi.org/10.1177/0022034516634664
Porcheri C, Mitsiadis TA (2019) Physiology, pathology and regeneration of salivary glands. Cells 8(9):976. https://doi.org/10.3390/cells8090976
Dodds MW, Johnson DA, Yeh CK (2005) Health benefits of saliva: a review. J Dent Mar 33:223–233
Aure MH, Konieczny SF, Ovitt CE (2015) Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 33(2):231–237. https://doi.org/10.1016/j.devcel.2015.02.013
Martinez-Madrigal F, Micheau C (1989) Histology of the major salivary glands. Am J Surg Pathol 13(10):879–899. https://doi.org/10.1097/00000478-198910000-00008
Triantafyllou A, Fletcher D (2017) Comparative histochemistry of posterior lingual salivary glands of mouse. Acta Histochem 119(1):57–63. https://doi.org/10.1016/j.acthis.2016.11.007
Bittencourt LO, Puty B, Charone S, Aragão WAB, Farias-Junior PM, Silva MCF, Crespo-Lopez ME, Leite AM, Buzalaf MAR, Lima RR (2017) Oxidative biochemistry disbalance and changes on proteomic profile in salivary glands of rats induced by chronic exposure to methylmercury. Oxidative Med Cell Longev 2017:1–15. https://doi.org/10.1155/2017/5653291
Ferraris MEG, Munõz AC (2006) Histologia e Embriologia Bucodental: Bases Estruturais da Patologia, diagnóstico, tratamento e prevenção odontológica, 2°
Farias-Junior PMA, Teixeira FB, Fagundes NCF, Miranda GHN, Bittencourt LO, Silva MCF, Sagica F, de Oliveira Paraense RS, Crespo-Lopez ME, Lima RR (2017) Chronic intoxication by methylmercury leads to oxidative damage and cell death in salivary glands of rats. Metallomics 9(12):1778–1785. https://doi.org/10.1039/c7mt00168a
Lima LAO, Bittencourt LO, Puty B, Fernandes RM, Nascimento PC, Silva MCF, Alves-Junior SM, Pinheiro JJV, Lima RR (2018) Methylmercury intoxication promotes metallothionein response and cell damage in salivary glands of rats. Biol Trace Elem Res 185(1):135–142. https://doi.org/10.1007/s12011-017-1230-9
Aragão WAB, Costa NMM, Fagundes NCF, Silva MCF, Alves-Junior SM, Pinheiro JJV, Amado L, Crespo-Lopez ME, Maia CSF, Lima RR (2017) Metallomics. https://doi.org/10.1039/C7MT00123A
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) from Brazilian Ministry of Science, Technology, Innovation and Communications.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were carried out in compliance with the guidelines provided by the Ethics Committee on Experimental Animals of Federal University of Pará that approved and authorized this project (CEUA-UFPA N 9037190417).
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza-Monteiro, D., de Oliveira Nunes, P.B., de Oliveira Ferreira, R. et al. Aluminum-Induced Toxicity in Salivary Glands of Mice After Long-term Exposure: Insights into the Redox State and Morphological Analyses. Biol Trace Elem Res 198, 575–582 (2020). https://doi.org/10.1007/s12011-020-02091-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02091-1