Abstract
Cancer incidence and mortality rates have been increasing rapidly worldwide. A growing body of evidence revealed that exposure to trace metals is the most important aetiology for development of the cancer. Therefore, present study was intended to evaluate the imbalances in the concentrations of selected metals (Na, K, Ca, Mg, Sr, Li, Fe, Zn, Cu, Co, Mn, Ag, Cd, Cr, Ni and Pb) in the blood of newly diagnosed thyroid cancer patients in comparison with counterpart healthy subjects/controls. Concentrations of the metals were quantified by flame atomic absorption spectrometry by employing nitric acid/perchloric acid–based wet digestion method. Average concentrations of Pb (774.6 μg/dL), Cr (757.9 μg/dL), Cd (472.5 μg/dL) and Ni (360.5 μg/dL) were found to be significantly higher in the blood of cancer patients than controls. Correlation study and multivariate analysis showed strong mutual relationships among Fe-Cd-Ca-Mg-Pb, Co-Sr-Zn, Li-Ag-Na-K and Cu-Ni in the blood of thyroid cancer patients while Na-K-Fe-Co-Pb, Zn-Sr-Cr, Ca-Mg and Li-Ag-Cu-Ni exhibited strong mutual associations in the blood of healthy donors. Significant variations in the trace metal levels were observed with the age, gender, habitat, food habits and smoking habits of both donor groups. Metal levels also exhibited considerable disparities with the stages and types of thyroid cancer. Multivariate analysis of the metal data revealed significantly divergent apportionment of the metals in the blood of cancer patients compared with the healthy group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Investigations related to the metals’ exposure are gaining considerable importance as diagnostic/prognostic tool in the assessment of different chronic diseases. Some metals play crucial role in various physiological processes in the human body, but an imbalance in metals exposure/intake may cause numerous disorders, such as cellular damage, inflammation, cardiovascular diseases and various types of cancer [1,2,3]. Generally, the carcinogenic progressions are associated with the excess or enrichment of toxic/carcinogenic metals while most of the essential elements exhibited depletion/deficiency in the cancer patients [4,4,6]. Thyroid cancer is one of the intimidating types of cancers which are associated with relatively higher rates of mortality throughout the world. Thyroid gland is located in the mid portion of the neck anterior to the trachea and larynx, just below the cricoid cartilage attached by a loose connective tissue capsule [7, 8]. Many factors are contributing towards the thyroid cancer; cigarette smoking, tobacco smoke, metal manufacturing, mining, asbestos and diet low in fruit/vegetable are the major reported risk factors [9,9,11].
The determination of metal contents in biological fluids and tissues is of vital importance due to their recognized roles in several biochemical processes [11, 12]. For this purpose, various biological specimens have been used in the past; some of the most commonly used specimens are blood, plasma, serum, hair, nails, urine, saliva and sweat [2, 7, 13, 14]. Monitoring the exposure of the essential and toxic metal has critical importance in human health [8]. The choice of biological specimen depends on various factors such as toxico-kinetics, the convenience or invasiveness of the specimen collection procedure and the potential for specimen contamination [1, 3, 15, 16]. Although hair analysis is an effective tool for evaluation of the metals because it reflects exposure from a long-term period, it has certain limitation including the occurrence of exogenous contamination, which can interfere with analysis making it less reliable [13, 17]. Another disadvantage of hair analysis is the lack of scientific knowledge about the kinetics of trace metal incorporation in it and there is insufficient epidemiological data that can support the predictions concerning health effect of a metal concentration [18]. Nail samples also require prewashing; therefore, nowadays blood is considered as an ideal matrix for trace metal research because of its significance and ease of sampling [19, 20]. It is in contact with all tissue and organs where metals are deposited. It provides the information about the materials, which are recently absorbed by the body [21]. As hair/nail samples require washing but blood does not need any washing prior to analysis, thus it is mostly free of external contamination. Moreover, blood is involved in transportation of the metabolic wastes and it helps in delivering the essential substances such as nutrient and oxygen [7, 22].
The current study is, therefore, intended to explore the relationships between enrichment and/or deficiency of the metals and the risk of thyroid cancer. Consequently, the main objectives of the present study are (i) to appraise the levels of selected essential/toxic metals (Na, K, Ca, Mg, Sr, Li, Fe, Zn, Cu, Co, Mn, Ag, Cd, Cr, Ni and Pb) in the blood of thyroid patients and matching controls; (ii) to find out any mutual variations between the metal concentrations by correlation study; (iii) to explore the multivariate methods for the apportionment of toxic/essential metals in the blood of the patients and controls; and (iv) to assess the levels of metals with respect to the cancer stages and types thereby investigating whether these metals had any presumptive link in the diagnosis and/or prognosis in the patients. The results of the present work may be useful for the treatment and prevention of thyroid cancer as well as can be used as a database for the researchers and oncologist.
Materials and Method
Study Subjects
Subjects included in the present study were thyroid cancer patients and healthy donors. All the subjects were initially briefed about the objectives of the study and a consent form was signed by each participant before the sample collection. Thyroid cancer patients of different ages, gender, food habits, smoking habits and habitat were included in the present study. Blood samples were collected from the patients admitted in Nuclear Oncology and Radiotherapy Institute (NORI), Islamabad. Before the sample collection protocol of the study was approved by ethical review committee of the institute (Ref. No. QAUC-2017-A77). The selected donors were newly diagnosed thyroid cancer patients without any treatment, such as radiotherapy or chemotherapy. They were not taking any kind of mineral supplements as well. Blood samples were collected from a total of 110 thyroid cancer patients on volunteer basis. Clinical diagnosis was confirmed in the institute by histopathological examinations of all the patients. The controls were not suffering from any type of cancer. They were not taking any kind of mineral supplements on regular basis. Mostly, the controls had close relationship with the patients; thus, they had similar socioeconomic status and environmental exposure. The controls were matched with the patients regarding age, habitat, gender and food habits. A total of 108 blood samples were collected from the controls on volunteer basis. A proforma was filled out to record the information regarding the donor’s name, age, gender, social/general health status, nutrition habits, job description, socioeconomic status past diseases etc. at the time of sample collection.
Sample Collection and Processing
For the collection of blood sample, specially designed evacuated tubes were used. Skin of subjects was properly cleaned with 70% isopropyl alcohol. Blood sample was collected from the antecubital vein by venipuncture method. Approximately 3 to 5 mL of the blood sample was collected from each subject using BD syringes (5 mL). The samples were stored at − 15 °C until further analysis. Proper precautions were taken in order to avoid any kind of external contamination. For the digestion of the blood sample, it was transferred from the storage tube to a digestion flask. Sample was correctly measured and weighed on an analytical balance, and then digested in nitric acid and perchloric acid (10:1, v/v) mixture. Samples were heated on hot plate at 80 °C to a soft boil until white dense fumes evolved, which marked the completion of the digestion. Samples were then cooled to room temperature and diluted to appropriate volume with 0.1 N HNO3. Blanks containing all the reagents in the same sequence (without blood sample) were also processed with each batch of five samples.
Quantification of Selected Metals
Atomic absorption spectrometry (AAS) is an analytical technique frequently used to quantify the metals at trace and ultra-trace level; therefore, it has many applications including clinical analysis, environmental analysis and biological analysis. Moreover, it is used in pharmaceuticals, industry and mining. In the present study, selected essential and toxic metals including Na, K, Ca, Mg, Sr, Li, Fe, Zn, Cu, Co, Mn, Ag, Cd, Cr, Ni and Pb were quantified using a flame atomic absorption spectrophotometer (Shimadzu AA-670, Japan). Various analytical conditions (such as detection wavelength, hollow cathode lamp current, slit width, flame type and fuel/oxidant flow rates) were optimized for the analysis of each element independently. The instrument was operated with automatic background compensation under optimum analytical conditions which are given in Table S1 (supplementary material). Average level of every metal in each sample was computed based on the measurement of three sub-samples of each sample that were run separately onto the spectrophotometer. Standard Reference Material (Animal Serum, NIST SRM 1598a) was used for quality assurance and accuracy. These results showed very good agreement/recoveries (96–103%). For comparison of the data, samples were also analysed at an independent laboratory and the two results showed almost comparable levels (± 2% difference). All the reagents used were obtained from E-Merck or BDH and they were of ultrahigh purity (certified > 99.99%). Working standards were prepared by serial dilution of the stock standard solutions (1000 mg/L) just before the analysis of the metal on the instrument [6].
Statistical Analysis
Statistical analyses were applied on the results of metal data using MS Excel and STATISTICA software. Descriptive analysis was conducted to obtain the basic statistical parameters including range, mean, median, relative standard deviation (RSD) and skewness in the blood samples. In addition, Anderson-Darling test was employed to investigate the normality in the distribution of selected metal levels in the blood of the patients and controls. The data were further analysed by Wilcoxon rank-sum test and t test (assuming unequal variances) to compare the measured levels of the metals between various sub-groups in each category. The level of p < 0.05 was noted as statistically significant. Pearson correlation analysis was used to investigate the mutual associations among the metals. Apportionment of the metals in the blood of the patients and controls was done by multivariate methods including cluster analysis (CA) and principal component analysis (PCA). PCA has been proven to be an efficient chemometric approach frequently employed for pattern recognition in a large dataset. It is an excellent tool used to acquire the hidden information that is not apparent from conventional data analyses. CA involves grouping of the variables with similar characteristics into clusters which result in internal homogeneity and external heterogeneity. It is informative to examine the CA in conjunction with PCA as they provide similar information in different forms [6, 7].
Results and Discussion
Demographic Characteristics of the Donors
The summary of demographic data related to the thyroid cancer patients and healthy donors/controls is presented in Table 1. Blood samples were collected from 110 patients and 108 healthy subjects. Among the patients, 57 were female and 53 were male donors, while 45% cases in the patient group and 48% in the control group were drawn from rural areas. About 54% of the patients were addicted of tobacco on continuous basis, while only 28% of healthy subjects were smokers. Among the selected subjects, 59% of the patients and 57% of the controls were vegetarian. Among the patients, 45% were diagnosed at stage I, 18% at stage II and 16% at stage III and 20% at stage IV during the present study (Table 1). Based on different types of thyroid cancer, 30% of the patients were suffering from anaplastic thyroid cancer while 21% were diagnosed with medullary thyroid cancer; however, an almost comparable number of the patients were suffering from follicular thyroid cancer (25%) and papillary thyroid cancer (24%).
Distribution of the Metals
Basic statistical parameters related to the distribution of selected essential and toxic metal levels (μg/dL) in the blood samples of thyroid cancer patients are shown in Table 2. A wide range of concentrations as shown by the minimum and maximum levels were exhibited by most of the metals. Noticeably higher mean concentrations were found for Na (124,380 μg/dL), K (90,828 μg/dL) and Fe (26,661 μg/dL), followed by moderately higher levels of Ca (3682 μg/dL), Mg (2903 μg/dL), Zn (1008 μg/dL) and Co (977.3 μg/dL). Among the rest of the metals, relatively higher contributions were noted for Pb (774.6 μg/dL), Cr (757.9 μg/dL), Sr (685.5 μg/dL), Cd (472.5 μg/dL), Ni (360.5 μg/dL), Mn (337.9 μg/dL) and Cu (281.4 μg/dL), while the least concentrations were noted for Ag (78.60 μg/dL) and Li (52.14 μg/dL) in the blood of thyroid cancer patients. Overall, average concentrations of the metals showed the following decreasing order: Na > K > Fe > Ca > Mg > Zn > Co > Pb > Cr > Sr > Cd > Ni > Mn > Cu > Ag > Li. Relatively narrow dispersion was exhibited by most of the metals as demonstrated by their RSD values on one hand and almost equivalent mean and median levels on the other hand. Large skewness values for Fe, Cu, Ca, Cr, Na, Mg, Sr, Zn, Li and Mn indicated their significant asymmetric distribution while modest skewness values for rest of the metals showed moderately symmetrical distribution of these metals in the blood of thyroid cancer patients. Anderson-Darling normality plots (as shown in Fig. S1, supplementary material) revealed considerably non-normal distribution for all the metal levels in the blood of thyroid cancer patients; however, relatively lower randomness and somewhat normal distribution (p > 0.01) was observed for Pb and Ni levels in the blood of the patients.
Basic statistical parameters pertaining to the distribution of selected essential and toxic metal levels (μg/dL) in the blood of the controls/healthy subjects are also shown in the Table 2. On the average basis, predominantly higher concentrations were found for Na (121,158 μg/dL), K (80,678 μg/dL) and Fe (23,850 μg/dL), followed by moderately higher levels of Ca (3800 μg/dL), Mg (2647 μg/dL), Co (2073 μg/dL) and Zn (1488 μg/dL). Among the remaining metals, some significant contributions were noted for Sr (856.1 μg/dL), Cr (588.8 μg/dL), Pb (416.2 μg/dL), Mn (360.5 μg/dL), Cd (344.6 μg/dL), Cu (261.1 μg/dL) and Ni (200.0 μg/dL), however, Ag (67.35 μg/dL) and Li (54.73 μg/dL) were found at the lowest levels. The selected metals in the blood of controls exhibited following decreasing order in their average concentrations: Na > K > Fe > Ca > Mg > Co > Zn > Sr > Cr > Pb > Mn > Cd > Cu > Ni > Ag > Li. Relatively normal distribution pattern and lower dispersion was displayed by most of the metals as reflected by their RSD values as well as almost similar mean and median levels in the blood of controls. Large skewness values for Cu, Ca, Cr, Fe, K, Ni, Mg and Sr indicated their mostly asymmetric distribution in the blood of healthy subjects. Overall, on comparative basis, the extent of asymmetry and randomness in the elemental distributions was found to be reasonably less in case of the controls than those of the patients which showed reasonably higher randomness in their concentrations. Anderson-Darling probability plot (Fig. S1, supplementary material) revealed that the distribution of the metal levels in the blood of controls was not normal (except Na) and the metal levels displayed noticeably higher randomness in their distribution. Only Na levels in the blood of controls exhibited relatively normal distribution (p > 0.05) while Li levels showed relatively lower randomness (p > 0.01) than other metals in the blood of controls.
As most of the metals showed non-normal distribution in both donor groups included in the present study, therefore, the measured metal levels in the blood of the patients and controls were compared by Wilcoxon rank-sum test (Table 2). The comparative study showed that the concentrations of Cd, Cr, Ni and Pb were found to be significantly higher (p < 0.05) while those of Zn, Sr and Co were significantly lower (p < 0.05) in the blood of the patients compared with the counterpart controls. The comparative evaluation therefore revealed a buildup of toxic metals (Cd, Cr, Ni and Pb) and deficiency of the essential metals (Zn, Sr and Co) in case of the cancer patients than the healthy subjects. Most of the elevated metals found in the patients are proven carcinogen and their higher contents can further promote the carcinogenesis. Moreover, the deficiency of essential metals (particularly Zn) is associated with poor immunity and excessive accumulation of the free radicals which further deteriorate the health of the patients.
Correlation Study
The data on metal-to-metal correlations in the blood of thyroid cancer patients are shown in Table 3 (below the diagonal), wherein the italic r-values are significant at p < 0.05. Among the selected metals, strong and significant positive correlations were observed between Li-Ag (r = 0.53), Ni-Cu (r = 0.42), Ni-Mn (r = 0.37), Pb-Ni (r = 0.37), Sr-Co (r = 0.37), Sr-Ni (r = 0.36), Cu-Ag (r = 0.34), Li-Cr (r = 0.34), Zn-Sr (r = 0.33), K-Ag (r = 0.28), Ni-Co (r = 0.27), Fe-Cd (r = 0.27), Na-Co (r = 0.27), Li-Fe (r = 0.26), Na-Li (r = 0.25), Zn-Co (r = 0.25), Mn-Cr (r = 0.24) and Ni-Ag (r = 0.23). Some of the metal pairs such as Ag-Mg (r = − 0.30), Cd-Ni (r = − 0.27), K-Cu (r = − 0.26) and K-Mg (r = − 0.31) exhibited inverse relationships and opposing distributions in the blood of thyroid cancer patients. The correlation study showed mutual association among Li, Ag, Ni, Cu, Mn and Pb in the blood of the thyroid cancer patients while one of the major electrolytes (K) revealed contrasting variations with most of the metals thus demonstrating the disproportional distribution of the metals in the blood of cancer patients.
The correlation coefficient matrix for trace metals pertaining to the blood of healthy subjects is shown in Table 3 (above the diagonal), wherein the significant r-values are shown in italic at p < 0.05. Significantly strong positive relationships were observed between Cu-Ni (r = 0.47), Fe-Ni (r = 0.45), K-Na (r = 0.41), Cr-Zn (r = 0.41), Sr-Zn (r = 0.40), Ag-K (r = 0.38), Li-Mg (r = 0.34), Mg-Zn (r = 0.34), Co-Cr (r = 0.33), Li-Zn (r = 0.33), Ca-Mg (r = 0.31), Cr-Sr (r = 0.31), Co-Ni (r = 0.30), Ag-Cu (r = 0.27), K-Pb (r = 0.27), Cr-Na (r = 0.27), Cr-Mn (r = 0.25), Ag-Li (r = 0.24) and Ni-Sr (r = 0.22). Nevertheless, Mg-Ag (r = − 0.27) and Mn-Ag (r = − 0.40) exhibited inverse relationship and opposing distributions in the blood of healthy donors. In comparison to the patients, correlation study demonstrated mutual associations among most of the essential metals while toxic/trace metals showed separate grouping in the blood of controls. Consequently, the correlation study pointed out considerably diverse associations among the metals in the cancerous patients and healthy subjects.
Comparison of the Metal Levels Based on Demographic Characteristics
Average metal levels found in the blood samples of various demographic groups of both patients and controls were compared by t test (two-tailed, assuming unequal variance) in the present study. Gender-based comparison in the average concentrations of selected metals in the blood of thyroid cancer patients and controls is shown in the Fig. 1a. The comparative evaluation revealed significantly elevated average concentration of Pb, Ni, Cu, Sr and Zn in the male patients than the female patients, while average levels of Cd, Ca, Na, Co, Mg and K were more or less comparable in both male and female patients. Nevertheless, mean levels of Ag, Cr, Fe, Li and Mn were found to be considerably higher in the female patients than the male subjects. In the case of controls, mean levels of Ag, Cd, Li and Ni were found to be significantly higher in the male donors, while average concentrations of Co, Cr, Mn and Zn were found to be higher in the blood of female controls. Nonetheless, average concentrations of Ca, Cu, Fe, K, Mg, Na, Pb and Sr were almost comparable in both male and female controls. The comparative study revealed considerable gender-based disparities in the distribution of the metals among the patients and controls. It has been reported that the gender factor may had a major effect on thyroid hormone/TSH levels [19].
Comparative assessment of average metal levels in the blood based on the habitat of the subjects is shown in Fig. 1b. It revealed comparatively high concentrations of Cd, Co, Cr, K and Mn in the blood of urban patients than the rural counterparts which exhibited relatively elevated levels of Ag, Cu, Li, Mg, Ni and Pb in their blood samples. Nevertheless, average levels of Ca, Sr, Fe, Zn and Na were found to be nearly equivalent in both patient groups. In the case of controls, mean contents of Ag, Cu, Fe and Pb were found to be considerably higher in the blood of rural subjects than urban subjects, whereas mean levels of Ca, Co, Cr, K, Mg, Mn and Na were almost comparable in the blood of both control groups. Nonetheless, average blood levels of Cd, Li, Ni, Sr and Zn were found to be comparatively higher in the urban than rural controls. It has been reported that habitat may has a major influence on the human physiology; people living in the industrial areas are more exposed to toxic metals which in turn are threat for thyroid cancer [8,8,10, 23, 24].
Comparison of average concentrations of selected metals in the blood of the patients and controls based on their food habits is depicted in the Fig. 1c. Comparative assessment of the metals showed approximately equivalent contributions of Ca, Co, Cr, Sr, Cu, Fe, K and Na in the blood of vegetarian and non-vegetarian patients. However, mean contents of Ni and Mn showed significantly higher contribution in the blood of vegetarian patients, while mean levels of Ag, Li, Cd, Mg, Pb and Zn were found to be comparatively higher in the blood of non-vegetarian patients than the vegetarian patients. On the other hand, in case of controls, relatively higher mean concentrations of Ca, Li, Sr and Zn were recorded in the blood of non-vegetarian subjects compared with the vegetarian donors. However, mean levels of Ag, Cd, Cr, Cu and Ni were relatively higher in the blood of vegetarian controls than the non-vegetarian donors. Average concentration of Co, K, Na, Fe, Mg, Mn and Pb was found to be almost comparable in the blood of both control groups.
Smoking-based comparison of the selected metals in the blood of the patients and controls is shown in Fig. 1d, which showed that significantly higher average concentrations of Co, Ni, Pb and Zn were found in the blood of smoking patients than of the non-smoking patients. Average contents of Ca, Cr, Cu, K, Mg, Na, Cd and Sr were almost comparable in both smoking and non-smoking patients, while average levels of Ag, Fe, Mn and Li were found to be relatively higher in the blood of non-smoking patients than the smoking counterparts. In the case of controls, mean levels of Ag, Cd, Fe, Ni, Sr and Zn showed relatively higher contributions in the blood of smoking subjects while mean level of Ca, Li, K, Mn, Na, Cu and Pb showed comparable levels in both donor groups. Besides, average concentrations of Cr and Mg were found to be considerably higher in the blood of non-smoking controls than the smoking controls. It has been reported that smoking is the main factor for hyperthyroidism, while alcohol consumption may have a deteriorating influence on the thyroid function [19].
The metal levels in the blood of both donor groups were also compared to explore the age-based variations and for this purpose the donors were classified into four age groups: < 30 years, 31–40 years, 41–50 years and > 50 years. Age-based comparison among the patients is shown in Fig. 2a, which revealed considerably higher concentrations of Ca, Cr, Cu and Mg in the blood of the patients of <30 years; while 31–40 years age group showed higher contents of K and Zn. Patients of age group 41–50 years showed relatively higher mean levels of Pb in their blood. Nonetheless, relatively higher levels of Ag, Cd, Li, Mn and Ni were observed in the blood of the patients of > 51 years age. Age-based comparison for the controls is shown in Fig. 2b. Comparatively higher contributions of Na, Cd and Sr were observed in the blood of < 30 years of controls, while those of 41–50 years showed significantly higher concentrations of Ag and Li. However, relatively higher levels of Cr and Pb were shown by > 51 years controls. The comparative study revealed large buildup and higher accumulation of toxic metals with age of the subjects in case of the patients compared with the controls. Age is one of the pivotal factors towards the functioning of thyroid gland as reported earlier [19].
Comparative evaluation of mean metal levels in the blood of various stages (I, II, III and IV) of thyroid cancer patients is displayed in Fig. 3. Based on the stage, Na, K and Ca showed insignificant differences among their mean levels at all four cancer stages. However, Cr, Cu and Ni exhibited significant increase in the mean levels at stage IV compared with the initial stages, while Fe, Ag, Li, Sr and Zn showed considerable decline in the mean levels moving from stage I/II to stage III/IV. In the case of Cd, and Mn mean levels found at stage III were found to be significantly higher than the remaining stages while the mean levels of Co, Li and Mg at stage II were higher than other stages. Among the selected metals, Cr, Cu, Mn and Ni showed gradual buildup in their concentrations with cancer stages; thereby indicating more accumulation of these metals with the progression of disease (Fig. 3).
Comparison of the average metal levels measured in the blood of various types of thyroid cancer (anaplastic, follicular, medullary and papillary) patients is shown in Fig. 4. Significantly higher contributions of Ag, Cr, Cu, Li, Ni and Sr were found in the blood of anaplastic thyroid cancer patients, while mean concentrations of Ca, Ca, Mg, Mn and Pb were noted at elevated levels in the blood of follicular thyroid cancer patients. Mean contents of Ag, Cd, Cu, K, Mn and Ni were found at the lowest levels in the blood of medullary thyroid cancer patients than other types of the patients. However, relatively higher and comparable concentrations of Cd and Fe were recorded in the blood of papillary thyroid cancer patients and follicular thyroid cancer patients (Fig. 4).
Multivariate Analysis
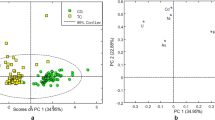
Another important aspect of the present study was the multivariate apportionment of the metal levels in the blood of thyroid cancer patients and controls using PCA and CA. The PC loadings extracted by varimax-normalized rotation on the metals data for the patients and healthy donors are shown in Table 4. In case of the patients, PCA yielded seven significant PCs with eigenvalue greater than 1, commutatively explaining approximately 66% of the total variance of data. The CA of metal data pertaining to the cancer patients is shown in Fig. 5a. PC 1 showed higher loading for Sr, Zn and Co with a similar cluster of these metals in CA. PC 2 showed maximum loadings for Cd and Fe with a parallel cluster of the metals in CA. This PC showed the interference of toxic metals (Cd) with the essential metal (Fe) in the patients and they were believed to be mainly contributed by anthropogenic sources. PC 3 indicated higher loading for Li and Ag along with a similar cluster in CA. PC 4 showed higher loadings of K and Cr along with significant contributions of Mg and Ca, while PC 5 showed higher loadings of Cu and Ni which also exhibited common cluster in CA. PC 6 revealed higher loadings of Ca and Pb with significant contribution of Fe; this PC also indicated the interference of toxic metal (Pb) with essential metals (Ca and Fe). PC 7 showed higher loadings for Na and Mn with significant contributions of Co and Cr. These metals are mostly derived from the nutritional habits of the subjects. The PCA and CA results were in very good agreement with each other.
In the case of controls, PCA of the metals data yielded seven PCs with eigenvalue > 1, commutatively explaining approximately 65% of the total variance of data (Table 4). The CA of metals data pertaining to the blood of controls is shown as dendrogram in Fig. 5b. PC 1 showed higher loadings for Cr, Sr and Zn whereas PC 2 showed higher loadings for Mg and Li. These metals were mostly associated with the food habits and significantly affected by the anthropogenic contamination. PC 3 showed higher loadings for Pb, Fe and Mn while PC 4 showed elevated loadings for Cd and Li. PC 5 indicated higher loadings for Ni, while PC 6 showed higher loading for K and Na which also showed a strong cluster in CA. PC 7 showed higher loadings for Ag, Ca and Cu. These metals were mostly regulated by internal body metabolism in healthy donors. It is important to note that in the case of controls, the toxic metals were not primarily associated with the essential metals as was the case in the cancer patients thus indicating a disproportion among the metals in thyroid cancer patients. Overall, PCA and CA showed significantly diverse apportionment of the essential and toxic metals in the blood of the cancer patients and healthy subjects which might be ascribed to the imbalances of trace metals in the cancer patients. Consequently, the multivariate methods can be employed for diagnostic and prognostic purpose in clinical studies, but they required further validation by considering more variables on larger population groups from different geographical areas around the world.
In the present study, concentration of some essential metals such as Zn was found to be significantly higher in healthy subjects than the patients. It is considered as vital component of cells and it can prevent the formation of free radicals that are capable of mutating with antioxidant effects. It also participates in cell immunity of T-lymphocytes, thus preventing the tumour development. It is vital for the proper functioning of the thyroid hormone metabolism; its deficiency can have detrimental effect on the thyroid activity which may be involved in carcinogenic activity [1, 3, 13, 15, 25]. Zinc is the constituent of superoxide dismutase, an enzyme that eliminate free radicals [26]. It is a cofactor of many proteins which regulate critical cellular functions including response to oxidative stress, DNA replication, cell cycle progression and immunity [27]. Zinc plays a key role in antiangiogenic activity of endostatin cell proliferations and intracellular signalling pathways [28]. It has been reported that higher Zn intake was linked with reduced cancer risk [29]. A study revealed that decreased serum Zn level may be associated with an increased risk of cancer [26]. Co and Sr were also found to be higher in the controls than the patients; they are part of many important biological processes/structural components and acting as cofactor for enzymes. Their imbalances can lead to serious problems [18].
The cancer patients exhibited considerably higher concentrations of toxic metals (such as, Cd, Ni, Cr and Pb) compared to healthy donors in the present study. Elevated doses of these elements may impair normal functioning of body [30]. Activities of many enzymes and division of cells as well as synthesis of DNA and RNA can be affected by their elevated concentrations [31]. Higher concentration of Cd in the patients may affect the thyroid gland at cellular level, disturbing the DNA synthesis and cell proliferation and ultimately act as a carcinogen ([1, 5, 20, 31,32,33]. Cadmium is one of the major culprits towards carcinogenicity which may act by interfering with essential metals (such as Fe and Ca). It can affect multiple cellular processes including cell cycle progression, proliferation, differentiation, DNA replication and apoptosis [34, 35]. It can interfere via substitution with essential metals in various cytoplasmic and membrane proteins and enhancing the cellular amount of free redox active metals could induce oxidative stress via Fenton reaction [34]. In addition, it can induce oxidative stress via ROS which might be involved in genotoxicity [36]. Chronic exposure to Cd could inhibit the activity of superoxide dismutase as one of the strongest antioxidant enzymes [35]. It can accelerate cancer development by activating protooncogenes and genes involved in cell proliferation and by inhibiting DNA methylation, which increases clonal expansion of damaged and mutated cells, as well as mitigates p53 function thus accelerating cancer development [37].
Cluster of Ni with Cu (Ni being carcinogenic) is another example of interference of toxic metal with essential metal [31]. Significantly higher level of Pb was noted in the patients; it is a carcinogenic metal and associated with decreased concentration of thyroid binding protein, thus major culprit of cardiovascular diseases, stroke and cancer [19]. Lead is involved in the components of cellular damage by inducing oxidative stress via formation of reactive oxidants [38]. It can inhibit DNA repair and acts synergistically with other mutagens [39]. A number of epidemiologic studies suggested possible relationship of Pb exposure and cancers [40, 41]. An increased risk of cancer was reported among the workers with higher exposure to Pb [42]. The mechanisms involved can be DNA repair inhibition, oxidative DNA damage, gene amplification, genomic instability, aneuploidy and epigenetic effects [43]. The present study exhibited significantly elevated level of Pb in the blood of cancer patients than healthy donors.
Cellular metabolism of Cr can cause both oxidative and non-oxidative forms of DNA damage and it also revealed genotoxic effects [44]. Chromium-induced mutations can be generated through different types of DNA damage such as DNA protein cross-links, DNA-DNA cross-links, Cr-DNA adducts and oxidative damage [45]. In China, high incidence in cancer mortality was noted in an ecologic study of villagers exposed to Cr in drinking water [46]. In the present study, Cr level was found to be significantly higher in the patients compared to the controls clearly showing the adverse effect of Cr overload in the patients. Most of the carcinogenic metals produce ROS via the Fenton reaction of superoxide anion radical and hydroxyl radical eventually resulting in damage to the cellular macromolecules including DNA, RNA, proteins, lipids and alteration of cell homeostasis [47, 36]. Although the mechanism of the action of metals is not yet clear, the mechanisms concerned in metal-mediated carcinogenesis resulting from oxidative stress may cause genetic and epigenetic changes, uncontrolled cell growth and abnormal cellular signalling [38]. Similarly, the patients originating from urban/industrial areas were more exposed to the anthropogenic chemicals and toxic pollutants; it is high time to implement the environmental preventive measures, as well as the disposal of chemicals, and to initiate the remediation of the areas declared at risk [22]. Many studies have shown that diet is more important; greater consumption of fruits/vegetables can significantly reduce the risk of cancer [48]. Thus, fruits/vegetables should be the most important and desirable diet ingredient for the cancer patients.
Conclusion
The current study is based on the measurement of selected essential and toxic metals (Ag, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Na, Ni, Pb, Sr and Zn) in the blood of thyroid cancer patients in comparison with healthy donor. The average levels of Cd, Cr, Ni and Pb were significantly higher (p < 0.05) in the blood of thyroid cancer patients, while mean levels of Zn, Sr and Co were significantly elevated in the blood of healthy donors. The correlation study revealed divergent relationships among the toxic/essential metals in the patients and controls; it was further clarified by PCA and CA which revealed divergent sources/grouping of the metals in the patients and controls. Most of the metals exhibited considerable disparities in their average concentrations based on age, habitat, food habits, smoking habits and gender of the subjects. Among the stages of cancer, mean levels of Cr, Cu, Mn and Ni in the blood exhibited higher accumulation and buildup from stage I to stage IV of the cancer patients. Majority of the metals showed elevated levels in the blood of anaplastic thyroid cancer patients while least contents were noted in the blood of medullary thyroid cancer patients. Consequently, the disruption in the balance of essential and toxic metals in the blood may possibly indicate the development and progression of thyroid malignancy.
References
Przybylik-Mazurek E, Zagrodzki P, Kuźniarz-Rymarz S, Hubalewska-Dydejczyk A (2014) Thyroid disorders—assessments of trace elements, clinical, and laboratory parameters. Biol Trace Elem Res 141(1–3):65–75
Qayyum MA, Shah MH (2014) Comparative assessment of selected metals in the scalp hair and nails of lung cancer patients and controls. Biol Trace Elem Res 158(3):305–322
Zhang F, Liu N, Wang X, Zhu L, Chai Z (2004) Study of trace elements in blood of thyroid disorder subjects before and after 131I therapy. Biol Trace Elem Res 97(2):125–133
Binkowski ŁJ, Rogoziński P, Roychoudhury S, Bruliński K, Kucharzewski M, Łaciak T, Massanyi P, Stawarz R (2015) Accumulation of metals in cancerous and healthy tissues of patients with lung cancer in southern Poland. J Environ Sci Health A Tox Hazard Subst Environ Eng 50(1):9–15
Kim HS, Kim YJ, Seo YR (2015) An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev 20(4):232–240
Pasha Q, Malik SA, Shaheen N, Shah MH (2010) Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clin Chim Acta 411(7–8):531–539
Hanif S, Ilyas A, Shah MH (2017) Statistical evaluation of trace metals, TSH and T4 in blood serum of thyroid disease patients in comparison with controls. Biol Trace Elem Res 183(1):58–70
Vigneri R, Malandrino P, Giani F, Russo M, Vigneri P (2017) Heavy metals in the volcanic environment and thyroid cancer. Mol Cell Endocrinol 457:73–80
Fei X, Lou Z, Christakos G, Liu Q, Ren Y, Wu J (2018) Contribution of industrial density and socioeconomic status to the spatial distribution of thyroid cancer risk in Hangzhou, China. Sci Total Environ 613:679–686
Malandrino P, Russo M, Ronchi A, Minoia C, Cataldo D, Regalbuto C, Giordano C, Attard M, Squatrito S, Trimarchi F, Vigneri R (2016) Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 53(2):471–479
Vigneri R, Malandrino P, Vigneri P (2015) The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol 27(1):1–7
Qayyum MA, Shah MH (2017) Study of trace metal imbalances in the blood, scalp hair and nails of oral cancer patients from Pakistan. Sci Total Environ 593–594:191–201
Baltaci AK, Dundar TK, Aksoy F, Mogulkoc R (2017) Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol Trace Elem Res 175(1):57–64
Rosati MV, Montuori L, Caciari T, Sacco C, Marrocco M, Tomei G, Scala B, Sancini A, Anzelmo V, Bonomi S, Tomei F (2016) Correlation between urinary cadmium and thyroid hormones in outdoor workers exposed to urban stressors. Toxicol Ind Health 32:1978–1986
Jain RB (2014) Thyroid function and serum copper, selenium, and zinc in general US population. Biol Trace Elem Res 159(1–3):87–98
Kudabayeva KI, Koshmaganbetova GK, Mickuviene N, Skalnaya MG, Tinkov AA, Skalny AV (2016) Hair trace elements are associated with increased thyroid volume in schoolchildren with goiter. Biol Trace Elem Res 174(2):261–266
El-Fadeli S, Bouhouch S, Skalny AV, Barkouch Y, Pineau A, Cherkaoui M, Sedki A (2016) Effects of imbalance in trace element on thyroid gland from Moroccan children. Biol Trace Elem Res 170(2):288–293
Jain RB, Choi YS (2016) Interacting effects of selected trace and toxic metals on thyroid function. Int J Environ Health Res 26(1):75–91
Mendy A, Gasana J, Vieira ER (2013) Low blood lead concentrations and thyroid function of American adults. Int J Environ Health Res 23(6):461–473
Park S, Lee BK (2013) Strong positive association of traditional Asian-style diets with blood cadmium and lead levels in the Korean adult population. Int J Environ Health Res 23:531–543
Ilyas A, Shah MH (2015) Abnormalities of selected trace elements in patients with coronary artery disease. Acta Cardiol Sin 31(6):518–527
Petrosino V, Motta G, Tenore G, Coletta M, Guariglia A, Testa D (2018) The role of heavy metals and polychlorinated biphenyls (PCBs) in the oncogenesis of head and neck tumors and thyroid diseases: a pilot study. Biometals 31(2):285–295
Arrebola JP, Fernández MF, Martín-Olmedo P, Molina-Molina JM, Sánchez-Pérez MJ, Sánchez-Cantalejo E, Molina-Portillo E, Expósito J, Bonde JP, Olea N (2014) Adipose tissue concentrations of persistent organic pollutants and total cancer risk in an adult cohort from southern Spain: preliminary data from year 9 of the follow-up. Sci Total Environ 500:243–249
Marcello MA, Malandrino P, Almeida JF, Martins MB, Cunha LL, Bufalo NE, Pellegriti G, Ward LS (2014) The influence of the environment on the development of thyroid tumors: a new appraisal. Endocr Relat Cancer 21(5):T235–T254
Hashimoto A, Kambe T (2015) Mg, Zn and Cu transport proteins: a brief overview from physiological and molecular perspectives. J Nutr Sci Vitaminol 61(Supplement):S116–S118
Zhang WH, Wu XJ, Niu JX, Yan H, Wang XZ, Yin XD, Pang Y (2012) Serum zinc status and Helicobacter pylori infection in gastric disease patients. Asian Pac J Cancer Prev 13:5043–5046
Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, Wu Y (2014) Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin Nutr 33:415–420
Gumulec J, Masarik M, Adam V, Eckschlager T, Provaznik I (2014) Serum and tissue zinc in epithelial malignancies, a meta-analysis. PLoS One 9(6):e99790
Pakseresht M, Forman D, Malekzadeh R, Yazdanbod A, West RM, Greenwood DC (2011) Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control 22(5):725–736
Hordyjewska A, Popiołek Ł, Kocot J (2014) The many “faces” of copper in medicine and treatment. Biometals 27(4):611–621
Chung HK, Nam JS, Ahn CW, Lee YS, Kim KR (2016) Some elements in thyroid tissue are associated with more advanced stage of thyroid cancer in Korean women. Biol Trace Elem Res 171:54–62
Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri E, Tsatsakis A, Schweitzer A, Wallace D (2018) Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci 19(5):1501
Kim JH, Lee JY, Seo JE, Jeong JY, Jung KK, Yoon HJ, Park KS (2012) Lead, cadmium and mercury levels in the 2010 Korean diet. Food Addit Contam Part B Surveill 5(4):260–264
Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A (2015) Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac J Cancer Prev 16(1):9–21
Ostadrahimi A, Payahoo L, Somi MH, Hashemzade SH, Esfahani A, Asgharijafarabadi M, Mobasseri M, Samadi N, Faraji S, KhajeBishak Y (2017) The association between blood cadmium levels and the risk of gastrointestinal cancer in Tabriz, northwest of Iran. Pol Ann Med 24(2):133–137
Valko M, Jomova K, Rhodes CJ, Kuca K, Musielk K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90(1):1–37
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88(11):1549–1559
Lee JC, Son YO, Kumar P, Shi X (2012) Oxidative stress and metal carcinogenesis. Free Radic Biol Med 53:742–757
Zhao Q, Wang Y, Cao Y, Chen A, Ren M, Ge Y, Yu Z, Wan S (2014) Potential health risks of heavy metals in cultivated topsoil and grain, including correlations with human primary liver, lung and gastric cancer, in Anhui province, eastern China. Sci Total Environ 470-471:340–347
Lam TV, Agovino P, Niu X, Roche L (2007) Linkage study of cancer risk among lead-exposed workers in New Jersey. Sci Total Environ 372:455–462
Rousseau MC, Parent ME, Nadon L, Latreille B, Siemiatycki J (2007) Occupational exposure to lead compounds and risk of cancer among men: a population-based case-control study. Am J Epidemiol 166(9):1005–1014
Lopes ACBA, Peixe TS, Mesas AE, Paoliello MMB (2015) Lead exposure and oxidative stress: a systematic review. Rev Environ Contam Toxicol 236:193–238
Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F (2009) A review of human carcinogens-part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10:453–454
Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: a double-edged sword. Chem Biol Interact 188(2):276–288
Gatto NM, Kelsh AM, Mai DH, Suh M, Proctor DM (2010) Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract, a meta-analysis. Cancer Epidemiol 34:388–399
Beaumont JJ, Sedman RM, Reynolds SD, Sherman CD, Li LH, Howd RA (2008) Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology 19(1):12–23
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogensis of gastrointestinal mucosal diseases. Physiol Rev 94(2):329–354
Li Q, Chuang SC, Eluf-Neto J, Menezes A, Matos E, Koifman S, Wünsch-Filho V, Fernandez L, Daudt AW, Curado MP, Winn DM (2012) Vitamin or mineral supplement intake and the risk of head and neck cancer: pooled analysis in the INHANCE consortium. Int J Cancer 131:1686–1699
Acknowledgements
We are grateful to the administrations of the Nuclear Oncology and Radiotherapy Institute (NORI), Islamabad, Pakistan, for their invaluable help during the sample collection. Technical and financial help by the Quaid-i-Azam University, Islamabad, Pakistan, to execute this project is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethical Review Committee, NORI, Islamabad REF. NO. QAUC-2017-A77) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 3195 kb)
Rights and permissions
About this article
Cite this article
Bibi, K., Shah, M.H. Appraisal of Metal Imbalances in the Blood of Thyroid Cancer Patients in Comparison with Healthy Subjects. Biol Trace Elem Res 198, 410–422 (2020). https://doi.org/10.1007/s12011-020-02088-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02088-w