Abstract
The purpose of our study was to investigate the role of hypoxia-inducible factor-1α (HIF-1α) in arsenic-induced carcinogenesis. We included 39 articles for meta-analysis. The results showed that low-dose exposure to arsenic (≤ 10 μmol/L) could promote the expression of phosphatidylinositol 3-kinase (PI3K) and phosphorylation-protein kinase B (p-AKT). High-dose arsenic exposure (> 10 μmol/L) promoted the expression of PI3K, HIF-1α, vascular endothelial growth factor (VEGF), and p38MAPK (P38). Acute arsenic exposure (< 24 h) promoted the expression of PI3K, HIF-1α, and VEGF. Chronic arsenic exposure (≥ 24 h) promoted the expression of PI3K, p-AKT, and P38. Moreover, for normal tissue-derived cells, arsenic could induce the increased expression of PI3K, p-AKT, HIF-1α, and VEGF. For tumor tissue-derived cells, arsenic could induce the expression of PI3K, p-AKT, and P38. We found that arsenic exposure could activate the PI3K/AKT pathway, further induce the high expression of HIF-1α, and then upregulate the levels of miRNA-21 and VEGF, promote the expression of proliferating cell nuclear antigen (PCNA), and ultimately lead to malignant cell proliferation. Our findings indicated that arsenic could increase the expression of HIF-1α by activating the PI3K/AKT pathway and eventually induce malignant cell proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic is a kind of non-metallic element, widely found in the atmosphere, soil, water, and food [1]. Arsenic in drinking water is one of the most important forms of exposure. Arsenic exposure to drinking water is very serious in developing countries, where groundwater is the main source of drinking water supply [2]. Epidemiological studies have shown that exposure to arsenic can induce a variety of cancerous diseases, including skin, bladder, lung, and liver cancers [3]. However, although arsenic exposure is associated with the development of various diseases, the mechanism of arsenic exposure-induced carcinogenesis is not yet fully elucidated. Recent studies have found that hypoxia-inducible factor-1α (HIF-1α) is involved in arsenic-induced cancer mechanisms. The purpose of this study is to investigate whether HIF-1α plays a role in the mechanism of arsenic-induced cancer.
Previous studies have found that hypoxia-inducible factor (HIF) family protein plays an important role in the development of inflammation and tumor growth. When HIF family protein binds to the target gene, it can induce a series of compensatory and pathological reactions in the body through transcription and post-transcriptional regulation, including the occurrence of inflammation and accelerated growth of the tumor. Some researchers found that HIFs are composed of two subunits, HIF-1α and HIF-1β, in which HIF-1β subunit can be expressed continuously and stably in cells, which mainly plays a structural role. HIF-1α can be expressed stably under hypoxia, and then, the combination of HIF-1α and HIF-1β can form active HIF protein, which leads to increase of HIF protein level, and then transfers to the cell to regulate the expression of various genes, and participate in the malignant transformation and proliferation of cells. Lei Wang [4] found that arsenic could activate HIF-1α through PI3K/AKT pathway, then upregulate the expression of vascular endothelial growth factor (VEGF), and finally promote the proliferation of colon cancer cells. In Lu Lu’s study [5], it was found that arsenic could affect the expression of HIF-1α through the AKT pathway in arsenic-exposed bronchial epithelial cells and then regulate the level of miRNA-21, ultimately promoting the proliferation of bronchial epithelial cells.
However, in different studies, the mechanism of arsenic on HIF-1α is different. Qing Zhou [6] found that when arsenic was exposed to human urothelial cells, it could induce the high expression of HIF-1α. Li Hongying [7] found that arsenic could inhibit the expression of HIF-1α in human synovial fibroblasts in a long time exposure. In addition, Yuan Xu [8] found that arsenic could not only inhibit the expression of HIF-1α but also increased the level of its subunit HIF-2α. Therefore, in the process of arsenic-promoting malignant proliferation, the mechanism of arsenic on HIF-1α is still controversial. We conducted this meta-analysis on the basis of previous studies on arsenic and HIF-1α, in order to reveal the role of HIF-1α in arsenic-promoting cell malignant proliferation.
Materials and Methods
Inclusion Criteria
Inclusion criteria were determined according to the PICO principles.
Study Design
Experimental studies published in Chinese and English.
Participants (P)
Cells, animals, or people.
Intervention (I)
All the experimental groups treated with arsenic or arsenic compounds. The arsenic model group related to cell proliferation, PI3K/AKT, HIF-1α, and VEGF in a dose or time relationship, and the highest dose or longest time was used in the analysis.
Comparison (C)
The control group was a blank control group without any intervention.
Outcome (O)
The indexes related to cell proliferation and HIF-1α. PI3K, AKT, HIF-1α, miRNA-21, VEGF, PCNA.
Exclusion Criteria
-
1.
Literature in languages other than Chinese and English.
-
2.
The main contents of the literature were not related to arsenic or HIF-1α (arsenic or HIF-1α only appeared in the abstract or discussion, and there were no indicators related to arsenic or HIF-1α in the results).
-
3.
Repeated publications (published in both Chinese and English journals, repeatedly and included in different databases).
-
4.
Literature with incomplete data, data not available, or failure to provide valid data (failure to provide mean or standard deviation).
-
5.
Review literature, conference literature, degree literature, book materials (this study only included original studies, so the review literature was excluded; as the conference literature provided only the abstracts, and not the full text of the literature, so valid data could not be extracted; therefore, the conference literature was excluded; the author of the degree literature would publish the data obtained during the study period in the form of journal alone. If included, there would be the problem of repeated publication, so the degree literature would be excluded; some literature in books and works would be published separately in journals. If included, there would be problems with the repeated publication, so books would be excluded).
-
6.
No control group
Search Strategy
The databases from which studies were retrieved include the Cochrane library, PubMed, Web of Science, Excerpta Medica database (EMBASE), China National Knowledge Infrastructure (CNKI), Wan Fang Data databases, Wiper database, and China Biology Medicine disc (CBMdisc). The retrieval period was from the beginning of the publication to October 1, 2019.
We first determine the research indicators of this study according to the PICO principle, and then, we select the keywords of the corresponding indicators according to the Mesh thesaurus.
The search terms used included arsenic, arsenite, ATO, As2O3, hypoxia-inducible factor, HIF-1, HIF-1α, HIF-2α, Akt, PI3K, miRNA-21, microRNA-21, miR-21, MiR-21, P38, P65, caspase-3, VEGF, and PCNA. Take PubMed database as an example: ((((Arsenic) OR Arsenite) OR ATO) OR As2O3) and ((((Hypoxia-inducible factor) OR HIF-1) OR HIF-1α) OR HIF-2α) and (((((((((((Akt) OR PI3K) OR miRNA-21) OR microRNA-21) OR miR-21) OR MiR-21) OR P38) OR P65) OR caspase-3) OR VEGF) OR PCNA).
Search Results
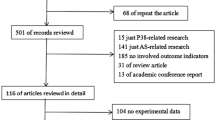
According to the search terms, we examined 8 databases and found 182 kinds of literature. According to the inclusion and exclusion criteria, we screened the retrieved literature and finally included 39. Our search strategy was to screen the retrieved literature independently by two researchers using the same search terms. At first, 182 kinds of literature with proper titles and abstracts were retrieved according to the PICO principle. Then, according to the inclusion and exclusion criteria, 31 articles repeatedly collected in different databases were excluded; 10-degree papers, 13 conference papers, and 3 books were excluded (the degree paper will be published repeatedly in the form of journal, the conference paper only contains the abstract of the paper without data to be extracted, and the literature in the books will be published separately in the form of journal). The remaining 125 articles were further screened, excluding 47 more articles that were not related to arsenic (arsenic or HIF-1α only appeared in the discussion section and no arsenic-related indicators were provided in the results). Furthermore, an additional 34 articles whose research content was not related to HIF-1α (arsenic or HIF-1α appeared only in the abstract or discussion sections, and no indicators related to HIF-1α were provided in the results) were excluded. With the remaining 44 articles, a full-text rescreening was made, and the literature that did not provide a mean or standard deviation among the five results was excluded. Finally, we included 39 articles for our study. The retrieval period taken was from the beginning of the publication to October 1, 2019. The search results are shown in Fig. 1.
Quality Evaluation
The Cochrane risk deviation quality assessment tool was used to evaluate the quality of the included 39 kinds of literature. The evaluation includes seven items: (1) random sequence generation (selection), (2) allocation concealment (selection), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias), and (7) other bias.
Statistical Analysis
Statistical analysis was performed using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2012, Portland, OR, USA) and Stata 12.0 (Stata Corp LP, College Station, TX, USA). The quality of the included studies was assessed using the scoring system in the Review Manager 5.3 software. The standard mean difference was used to compare and analyze the data. In this study, we analyzed the levels of many different indicators, including protein and mRNA levels. Since the units of these indicators were different, we used the standardized mean difference for statistical analysis so as to facilitate comparison among different articles, which avoided the problem that different indicators could not be compared due to different units. The formula for the standard mean difference was \( {\mathrm{d}}_i=\frac{\overline{x_{1i}}-\overline{x_{2i}}}{s_d} \), ⅈ=1, 2, 3…k. Heterogeneity was assessed according to I2 values, which were divided into four categories according to Cochrane Handbook: 0% to 40% meant low heterogeneity; 30% to 60% moderate heterogeneity; 50% to 90% denoted a significant heterogeneity; and finally, 75% to 100% suggested a very large heterogeneity. When P < 0.05 and I2 > 50%, the random effect model was used. When P > 0.05 and I2 ≤ 50%, the fixed effect model was used. The heterogeneous sources of 39 articles were detected according to subgroup analysis. Based on the study of the dose response relationship between Crippa and Orsini [9], we used the spline model and subgroup analysis to detect the bidirectional effect of arsenic on HIF-1α. Subgroup analysis was performed according to exposure time, exposure concentration, and tissue source of the cells. The results of the experimental and control groups were expressed by standard mean differences and 95% confidence intervals. We used funnel plots to detect whether there was a publication deviation. The Chi-square test was used for hypothesis testing. When P < 0.05, there was publication deviation. Sensitivity analysis was performed using Stat 12.0 software. Bilateral test was used in all statistical analysis; when P < 0.05, there was the statistical difference.
Results
Basic Characteristics
The basic characteristics of the 39 articles included in this analysis are shown in Table 1. The experimental groups were treated with arsenic and arsenic compounds (NaAsO2, As2O3), the control groups were blank controls without arsenic exposure. According to the effect of different doses of arsenic on cell activity in the study, we conducted a dose-response analysis, and then according to the results of the dose-response relationship analysis of arsenic on cells, it was found that when the arsenic concentration was less than 10 μmol/L, the cell activity increased with the increase in arsenic concentration. When the concentration of arsenic is higher than 10 μmol/L, the cell activity decreases with the increase in arsenic concentration, so we use 10 μmol/L as the threshold of high or low dose. In the subgroup analysis, the arsenic exposure was divided into a low-dose group (≤ 10 μmol/L, n = 23) and a high-dose group (> 10 μmol/L, n = 16). According to the exposure time of arsenic, it was divided into a short time group (< 24 h, n = 15) and a long-time group (≥ 24 h, n = 24). The subjects were divided into normal tissue source group (n = 24) and tumor tissue source group (n = 15). Outcome variables were cell proliferation and apoptosis indicators (P38, P65, caspase-3, VEGF, and PCNA expression) and HIF-1α pathway-related (HIF-1α, AKT, PI3K, and miRNA-21 expression).
Quality Assessment
Quality assessment of the 39 articles included in the analysis and the low-risk rate was more than 75% (Fig. 2).
A Meta-analysis of Arsenic on Cell Viability, Proliferation, and Apoptosis of Cells
The cell viability in arsenic exposure groups was higher than those produced by the normal control groups (SMD = 2.02, 95% CI (0.02, 4.02)) (Fig. 3). PCNA levels in the arsenic-exposed group were 3.28 times higher than those in the control groups (95% CI (0.74, 5.81)), while there were no statistically significant differences in P38 (SMD = 1.22, 95% CI (− 0.19, 2.64)), P65 (SMD = 3.36, 95% CI (− 3.37, 10.09)), and caspase-3 (SMD = 5.01, 95% CI (− 0.09, 10.10)) (P > 0.05) expression in the arsenic exposure groups and normal control groups (Fig. 4). These results suggest that arsenic could increase the cell viability and upregulate the level of intracellular tumor proliferation index PCNA, leading to the malignant proliferation of cells.
A Meta-analysis of Arsenic on PI3K/AKT Pathway
The results showed that the level of PI3K in the arsenic-exposed groups was 2.01 times higher than in normal control group (95% CI (0.47, 3.56)) (Fig. 5). The level of p-AKT was 2.34 times higher than that of the normal control group (95% CI (0.57, 4.10)) (Fig. 6). In the study of Chi Yang,[10] AKT was found to be a downstream indicator of PI3K. In order to reveal the role of arsenic in the PI3K/AKT pathway, we observed the effect of arsenic on the PI3K/AKT pathway by taking the arsenic exposure group as the control group and the arsenic combined with PI3K inhibitor groups as experimental groups. The results showed that arsenic combined with PI3K inhibitor groups inhibited the level of intracellular p-AKT (SMD = − 22.11, 95% CI (− 43.51, − 0.70)) (Fig. 7). These results suggested that arsenic can activate the PI3K/AKT pathway and affect the expression of p-AKT by regulating PI3K.
A Meta-analysis of the Effects of Arsenic and PI3K/AKT Pathways on HIF-1α
The results showed that the level of HIF-1α in arsenic-exposed groups was higher than in the normal control groups (SMD = 6.32, 95% CI (0.01, 12.62)) (Fig. 8). We took an arsenic exposure group as a control group and arsenic combined with PI3K inhibitor groups as an experimental group to observe the effect of the PI3K/AKT pathway on HIF-1α. The results showed that the level of HIF-1α in arsenic combined with PI3K inhibitor groups was inhibited compared with arsenic exposure groups (SMD = − 3.27, 95% CI (− 5.55, − 0.99)) (Fig. 9). These results indicated that arsenic could activate the expression of HIF-1α and regulate the level of HIF-1α through PI3K/AKT pathway.
A Meta-analysis of Arsenic and HIF-1α on miRNA-21 and VEGF
The results showed that the levels of miRNA-21 (SMD = 5.20, 95% CI (0.71, 9.69)) (Fig. 10) and VEGF (SMD = 2.20, 95% CI (0.05, 4.35)) (Fig. 11) in the arsenic exposure groups were higher than those in the normal control group. We used the arsenic exposure group as a control group, arsenic combined with HIF-1α inhibitor groups and arsenic combined with HIF-1α activity groups as an experimental group to observe the effect of HIF-1α on the expression of miRNA-21. The results showed that arsenic combined with HIF-1α activity could upregulate the level of miRNA-21 (SMD = 7.64, 95% CI (0.09, 15.19)), arsenic plus HIF-1α inhibitor could down-regulate the level of miRNA-21 (SMD = − 9.42, 95% CI (− 18.66, − 0.18)). We took the arsenic exposure group as the control group, arsenic combined with HIF-1α inhibitor groups, and arsenic combined with miRNA-21 inhibitor groups as the experimental group to observe the effects of HIF-1α and miRNA-21 on VEGF. The results showed that arsenic combined with HIF-1α inhibitor groups could down-regulate the expression of VEGF (SMD = − 5.42, 95% CI (− 10.90, 0.05)), arsenic combined with miRNA-21 inhibitor groups could also down-regulate the expression of VEGF (SMD = − 7.89, 95% CI (− 17.74, 1.96)) (Fig. 12). These results suggested that arsenic could upregulate the levels of miRNA-21 and VEGF by HIF-1α.
The Role of HIF-1α in the Process of Arsenic-Promoting Malignant Cell Proliferation
We synthesized the results of the study on the effect of HIF-1α inhibitor combined with arsenic treatment on cell viability and further revealed the effect of HIF-1α on cell viability in the process of arsenic-promoting cell malignant proliferation. The results showed that the effect of HIF-1α inhibitor combined with arsenic treatment on cell viability was lower than that of arsenic treatment alone (SMD = − 1.74, 95% (− 3.78, 0.29)). The results showed that HIF-1α inhibitor could inhibit the promoting effect of arsenic on cell activity, that is, HIF-1 α inhibitor inhibited cell malignant proliferation.
Dose-Response of Arsenic to HIF-1α
We used the spline model to quantitatively analyze the included literature to demonstrate the bidirectional effect of arsenic on HIF-1α. We examined some of the data included in the study, and the basic information is shown in Table 2. The results showed that the mean difference at diverse doses was 5 μmol/L (SMD = 6.109, 95% CI (3.405, 8.918)), 10 μmol/L (SMD = 10.092, 95% CI (5.486, 14.921)), and 15 μmol/L (SMD = 7.725, 95% CI (3.36, 12.019)). The results showed that when the dose of arsenic exposure was less than 10 μmol/L, the dose-response relationship between arsenic and HIF-1α was positive, and when the dose of arsenic exposure was more than 10 μmol/L, the dose-response relationship between arsenic and HIF-1α was negative (Fig. 13). These results suggested that arsenic has a bidirectional effect on HIF-1α regulation.
Subgroup Analysis Based on Exposure Dose of Arsenic
The results showed that the levels of PI3K (SMD = 1.70, 95% CI (0.05, 3.35)), p-AKT (SMD = 2.31, 95% CI (0.37, 4.26)), and cell viability (SMD = 2.33, 95% CI (0.32, 4.98)) were increased when the arsenic exposure dose was less than 10 μmol/L. When the dose of arsenic exposure was more than 10 μmol/L, the levels of intracellular PI3K (SMD = 2.31, 95% CI (0.43, 5.06)), HIF-1α (SMD = 4.16, 95% CI (0.63, 7.70)), VEGF (SMD = 3.87, 95% CI (0.05, 7.74)), and P38 (SMD = 8.04, 95% CI (0.11, 15.96)) were increased, but there was no significant difference in cell viability (SMD = 3.21, 95% CI (− 3.18, 9.59)) (Fig. 14). These results suggested that a low arsenic dose could promote the expression of PI3K, p-AKT and increase cell viability and promote cell proliferation. High-dose arsenic could promote the expression of PI3K, HIF-1α and VEGF, P38, but had no significant difference in cell activity.
Subgroup Analysis Based on the Exposure Time of Arsenic
The results showed that when the arsenic exposure time was less than 24 h, the levels of intracellular PI3K (SMD = 2.31, 95% CI (0.43, 5.06)), HIF-1α (SMD = 4.53, 95% CI (0.04, 9.10)), and VEGF (SMD = 5.29, 95% CI (2.85, 7.73)) were increased, but the cell viability (SMD = 0.03, 95% CI (− 1.57, 1.64)) had no significant difference. When the arsenic exposure duration was more than 24 h, the levels of intracellular PI3K (SMD = 2.12, 95% CI (0.40, 4.64)), p-AKT (SMD = 3.67, 95% CI (1.07, 6.26)), and P38 (SMD = 8.04, 95% CI (0.11, 15.96)) were upregulated, and the cell viability (SMD = 2.77, 95% CI (0.29, 5.25)) was significantly increased (Fig. 15). These results suggested that acute arsenic exposure could promote PI3K, HIF-1α, and VEGF expression, but could not induce malignant proliferation of cells. Chronic arsenic exposure could promote PI3K, p-AKT, P38 expression and induced malignant proliferation of cells.
Subgroup analysis of arsenic exposure dose. SMD, standardized mean difference. Low-dose exposure to arsenic (≤ 10 μmol/L) can promote the expression of PI3K and p-AKT, increase cell activity, and promote cell proliferation. High-dose exposure to arsenic (> 10 μmol/L) promoted the expression of PI3K, HIF-1α, VEGF, and P38, but had no significant effect on cell activity
Subgroup Analysis of Different Tissue Sources
According to different tissue sources, the subjects were divided into normal tissue and tumor tissue sources for subgroup analysis. The results showed that the levels of PI3K (SMD = 4.25, 95% CI (0.15, 8.66)), p-AKT (SMD = 2.80, 95% CI (0.33, 5.92)), HIF-1α (SMD = 3.79, 95% CI (0.74, 6.84)), and VEGF (SMD = 3.42, 95% CI (0.16, 7.00)) were upregulated and the cell viability (SMD = 2.64, 95% CI (0.19, 5.09)) was significantly increased in normal tissues. In the tumor tissue source, the levels of intracellular PI3K (SMD = 1.70, 95% CI (0.05, 3.35)), p-AKT (SMD = 2.37, 95% CI (0.11, 4.85)), and P38 (SMD = 8.04, 95% CI (0.11, 15.96)) were increased, but the cell viability (SMD = 1.29, 95% CI (− 6.10, 8.68)) was not significantly changed (Fig. 16). These results suggested that for cells from normal tissues, arsenic exposure could upregulate the expression of PI3K, p-AKT, HIF-1α, and VEGF and promote the malignant proliferation of cells. While, for cells from tumor tissue, arsenic exposure could increase the expression of PI3K, p-AKT, P38, but had no significant effect on cell viability.
Subgroup analysis of arsenic exposure time. SMD, standardized mean difference. Acute arsenic exposure (< 24 h) promoted the expression of PI3K, HIF-1α, and VEGF, but did not induce malignant cell proliferation. Chronic arsenic exposure (≥ 24 h) can promote the expression of PI3K, p-AKT, and P38, significantly increase cell activity, and promote malignant cell proliferation
Publication Bias Analysis
The results of the funnel plot showed that all the studies were evenly distributed on both sides of the ineffective line, indicating that the literature included in this study was stable and there was no publication bias (Fig. 17).
Subgroup analysis of different tissue sources. SMD, standardized mean difference. For normal tissue-derived cells, arsenic exposure can induce the increased expression of PI3K, p-AKT, HIF-1α, and VEGF and promote the malignant proliferation of cells. For tumor tissue-derived cells, arsenic exposure can induce the increased expression of PI3K, p-AKT, and P38 expression, but has no significant effect on cell activity
Sensitivity Analysis
Taking the sensitivity analysis of arsenic to HIF-1α as an example, we found that all the studies were evenly distributed on both sides of the midline, with no obvious deviation. It showed that the data included in this study were more robust, and there were no individual studies affecting the overall results (Figs. 18 and 19).
Discussion
Arsenic exposure can induce a variety of cancers, such as liver, lung, and bladder cancers. It has been found that HIF-1α played an important role in carcinomatous diseases induced by arsenic. Especially in malignant cell proliferation, HIF-1α can promote malignant transformation and malignant proliferation by regulating its upstream and downstream proteins. In the study of Lili Song et al. [17], it was found that arsenic could upregulate the level of HIF-1α by activating the AKT signal pathway and further promoting the malignant proliferation of cells. However, in the study of WANG Song Mei et al. [30], it was found that arsenic could down-regulate the level of HIF-1α through the AKT signaling pathway, but had no significant effect on malignant proliferation of cells. To sum up, the regulatory effects of arsenic on HIF-1α are different, so the regulatory effect of arsenic on HIF-1α is not yet fully elucidated [43]. Therefore, this study systematically evaluated the relationship between arsenic and HIF-1α. The results showed that exposure to arsenic could activate the PI3K/AKT pathway, induce the high expression of HIF-1α, and then upregulate the levels of miRNA-21 and VEGF through HIF-1α. When miRNA-21 and VEGF were upregulated, the expression of cell proliferation protein PCNA was increased, and eventually leading to the malignant proliferation of cells. The results provide a theoretical basis for the molecular mechanism of arsenic carcinogenesis.
In this study, we focused on the role of HIF-1α in the malignant proliferation induced by arsenic. In the study of Zukaa al Taleb [14], it was found that exposure to arsenic could increase the expression of HIF-1α in cells, and a variety of cell signaling pathways involved in regulating the expression of HIF-1α, the PI3K/AKT pathway is one of the known pathways involved in cell proliferation, differentiation, and apoptosis and related to cancer. In the study of Dong Zhang [21], it was found that high-dose and short time arsenic exposure could inhibit PI3K/AKT pathway and cell viability; Fei Wang [26] found that low-dose and long-term arsenic exposure could activate PI3K/AKT pathway and increase cell viability. Based on previous studies, this study found that exposure to arsenic can increase the expression of PI3K and p-AKT. In the subgroup analysis, we found that low-dose, long-term arsenic exposure had a stronger activation effect on the PI3K/AKT pathway, in which cells from normal tissue sources were more obvious. Then, we analyzed the group of PI3K inhibitor combined with arsenic, further analyzed the interaction in the PI3K/AKT pathway; the result showed that PI3K inhibitor could inhibit the activation of p-AKT induced by arsenic. These results suggest that exposure to arsenic upregulated the expression of PI3K and activated the AKT pathway to promote the expression of p-AKT. In addition, the promoting effect of high dose and short time arsenic exposure on HIF-1α was more obvious in normal tissues cells; the PI3K inhibitor could inhibit the expression of HIF-1α. These results suggested that arsenic could increase the expression of HIF-1α and induce malignant proliferation of cells by activating the PI3K/AKT pathway.
In addition to the PI3K/AKT pathway, miRNA-21 and VEGF can also interact with HIF-1α in arsenic-induced malignant cell proliferation and play an important role in the process of malignant cell proliferation (Fig. 20). miRNA-21 is one of the most important oncogenes in the miRNA family. In Xiao Yang’s study [20], the results showed that miRNA-21 could be regulated by HIF-1α as a downstream indicator. When the level of miRNA-21 was upregulated, it could increase cell activity and promote cell proliferation. In this study, it was also found that arsenic could increase the expression of miRNA-21. We also compared the effect of HIF-1α activity groups and HIF-1α inhibitor groups to observe the effect of HIF-1α on miRNA-21. The results showed that HIF-1α activity groups could increase the expression of miRNA-21, and HIF-1α inhibitor groups could inhibit the expression of miRNA-21. The results suggested that arsenic could regulate the expression of miRNA-21 through HIF-1α and affect the proliferation of cells. VEGF mainly exists on the surface of vascular endothelial cells. VEGF plays an important role in promoting the growth of endothelial cells, inflammatory response, growth, and metastasis of tumor cells. In the study of LingZhi Liu [32] and Minyoung Lee et al. [35], the results showed that HIF-1α could regulate the expression of VEGF in both humans immortalized lung epithelial cells and human prostate cancer cells. These results indicated that VEGF was likely to be the downstream target protein of HIF-1α. We found that arsenic could increase the expression of VEGF. In normal tissues, high dose and short time arsenic exposure had a more obvious effect on the activation of VEGF. Then, we analyzed the effect of HIF-1α inhibitor group to observe the effect of HIF-1α on VEGF. The results showed that HIF-1α inhibitor could inhibit the expression of VEGF, which indicated that arsenic could regulate the expression of VEGF through HIF-1α. In order to demonstrate the interaction between miRNA-21 and VEGF, we found that miRNA-21 inhibitor could inhibit the expression of VEGF. The above results revealed that arsenic could promote the expression of miRNA-21 through HIF-1α. Furthermore, the level of VEGF was increased, and finally caused the malignant proliferation of cells.
Mechanism of arsenic on HIF-1α. Arsenic exposure could activate the PI3K/AKT pathway, further induce the high expression of HIF-1α, and then upregulate the levels of miRNA-21 and VEGF through HIF-1α, promote the expression of cell proliferation protein PCNA, and ultimately lead to malignant cell proliferation
There were still high levels of heterogeneity in this study; we analyzed the source of heterogeneity by subgroup analysis. The results showed that arsenic exposure dose and exposure time had great heterogeneity in the expression of HIF-1α and VEGF. It showed that dose and time were the main sources of heterogeneity. In the published migration, we found that the literature was not completely evenly distributed on both sides of the ineffective line, and in the sensitivity analysis, we still found that the effect values of some literature deviated from the overall results. Therefore, the results of this study still need to be further confirmed by the experimental results. Moreover, a large number of studies have found that the effect of arsenic on HIF-1α is not only related to PI3K/AKT pathway but also affected by NF-κB, Nrf2, mTOR, and other signal pathways [44,45,46]. We also need to further explore the relationship between other signaling pathways and HIF-1α and further clarify the regulation mechanism of arsenic on HIF-1α.
References
Barajas-Olmosa FM, Ortiz-Sánchezb E, Imaz-Rosshandlerc I (2019) Analysis of the dynamic aberrant landscape of DNA methylation and gene expression during arsenic-induced cell transformation. Gene 711:143941
Yu C, Wu F, Liu X (2019) Early life and adolescent arsenic exposure from drinking water and blood pressure in adolescence. Environ Res 178:108681
Sun Z, Li M, Bai L (2019) Arsenic trioxide inhibits angiogenesis in vitro and in vivo by upregulating FoxO3a. Toxicol Lett 315:1–8
Wang L, Son Y-O, Ding S (2012) Ethanol enhances tumor angiogenesis in vitro induced by low-dose arsenic in colon cancer cells through hypoxia-inducible factor 1 alpha pathway. Toxicol Sci 130(2):1–32
Lu L, Xu H, Yang P (2018) Involvement of HIF-1α-regulated miR-21, acting via the Akt/NF-κB pathway, in malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol Lett 289:1–25
Zhou Q, Jin P, Liu J (2018) HER2 and Src co-regulate proliferation, migration and transformation by downstream signaling pathways in arsenite-treated human uroepithelial cells. Metallomics 10(8):1–76
Hong-ying LI, Xue-min WEI, Yu-yan HAN (2016) Role of arsenic trioxide in expression of hypoxia-inducible factor and correlated factors in rheumatoid synovial fibroblasts. J Harbin Med Univ 50(5):383–387
Xu Y, Li Y, Pang Y (2012) EMT and stem cell-like properties associated with HIF-2a are involved in arsenite-induced transformation of human bronchial epithelial cells. PLoS One 7(5):e37765
Crippa A, Orsini N (2016) Dose-response meta-analysis of differences in means. BMC Med Res Methodol 16:91
Yang C, Liu X, Zhao K (2019) miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther 10(1):65
Banerjee N, Bandyopadhyay AK, Dutta S (2017) Increased microRNA 21 expression contributes to arsenic induced skin lesions, skin cancers and respiratory distress in chronically exposed individuals. Toxicology 3:378
Jiao X, Xu X, Teng J (2017) miR-21 contributes to renal protection by targeting prolyl hydroxylase domain protein 2 in delayed ischaemic preconditioning. Nephrology (Carlton) 22(5):1–21
Luo F, Liu X, Ling M (2016) The lncRNA MALAT1, acting through HIF-1α stabilization, enhances arsenite-induced glycolysis in human hepatic L-02 cells. Biochim Biophys Acta 1862(9):1685–1695
al Taleb Z, Petry A, Chi TF (2016) Differential transcriptional regulation of hypoxia-inducible factor-1α by arsenite under normoxia and hypoxia: involvement of Nrf2. J Mol Med 94(10):1–14
Lu X, Yang L, Luo F (2016) MicroRNA-21 activation of Akt PTEN is involved in the epithelial-mesenchymal transition and malignant transformation of human keratinocytes induced by arsenite. Toxicol Res 5(4):1140–1147
Liu X, Luo F, Ling M (2016) MicroRNA-21 activation of ERK signaling via PTEN is involved in arsenite-induced autophagy in human hepatic L-02 cells. Toxicol Lett 252:1–10
Song L, Liu S, Liang Z (2016) MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1α feedback loop and the Akt-mTOR signaling pathway. Tumour Biol 37(9):1–8
Rachel J. Person, Ntube N. Olive Ngalame, Ngome L. Makia (2015) Chronic inorganic arsenic exposure in vitro induces a cancer cell phenotype in human peripheral lung epithelial cells. Toxicol Appl Pharmacol 286(1): 36–43
Luo F, Ji J, Liu Y (2015) MicroRNA-21, up-regulated by arsenite, directs the epithelial-mesenchymal transition and enhances the invasive potential of transformed human bronchial epithelial cells by targeting PDCD4. Toxicol Lett 232(1):301–309
Yang X, Cheng Y, Li P (2015) A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol 36(1):383–391
Zhang D, Liu J, Mi X (2014) The N-terminal region of p27 inhibits HIF-1α protein translation in ribosomal protein S6-dependent manner by regulating PHLPP-Ras-ERK-p90RSK axis. Cell Death Dis 5:e1535
Ganapathy S, Xiao S, Yang M (2014) A low-dose arsenic-induced p53 protein-mediated metabolic mechanism of radiotherapy protection. J Biol Chem 289(8):5340–5347
Watcharasit P, Suntararuks S, Visitnonthachai D (2014) β-Catenin involvement in arsenite-induced VEGF expression in neuroblastoma SH-SY5Y cells. Environ Toxicol 29(6):672–681
Li Y-N, Xi M-M, Yu G (2014) NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylases activity. Toxicol Lett 224(2):165–174
Yang L, Nie H, Zhang K (2014) A feedback regulatory loop between HIF-1a and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett 588:3137–3146
Wang F, Liu S, Xi S (2013) Arsenic induces the expressions of angiogenesis-related factors through PI3K and MAPK pathways in SV-HUC-1 human uroepithelial cells. Toxicol Lett 222(3):303–311
Zhao Y, Xu Y, Luo F (2013) Angiogenesis, mediated by miR-21, is involved arsenite-induced carcinogenesis. Toxicol Lett 223(1):35–41
Lu S, Ling M, Li Y (2013) Feedback regulations of miR-21 and MAPKs via Pdcd4 and Spry1 are involved in arsenite-induced cell malignant transformation. PLoS One 8(3):e57652
Luo F, Xu Y, Ling M (2013) Arsenite evokes IL-6 secretion, autocrine regulation of STAT3 signaling, and miR-21 expression, processes involved in the EMT and malignant transformation of human bronchial epithelial cells. Toxicol Appl Pharmacol 273(1):27–34
Song-Mei WANG, Hong-Ying WU, Jun-Qian HUANG (2012) Effects of hyperbaric oxygenation combined with As2O3 on proliferation of K562 cells and associated mechanism. J Exp Hematol 20(4):863–866
Bao B, Ali S, Ahmad A (2012) Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One 7(12):e50165
Liu L-Z, Jiang Y, Carpenter RL (2011) Role and mechanism of arsenic in regulating angiogenesis. PLoS One 6(6):e20858
Guo W, Yang Z, Xia Q (2011) Arsenite stabilizes HIF-1a protein through p85a-mediated up-regulation of inducible Hsp70 protein expression. Cell Mol Life Sci 68:475–488
Yin-hua TANG, You-lin YANG, Guang SONG (2009) Study of growth inhibition by arsenic trioxide in human colon carcinoma cell. Prog Mod Biomed 13(9):2549–2552
Lee M, Hwang J-T, Yun H (2006) Critical roles of AMP-activated protein kinase in the carcinogenic metal-induced expression of VEGF and HIF-1 proteins in DU145 prostate carcinoma. Biochem Pharmacol 72:91–103
Kamat CD, Green DE, Curilla S (2005) Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol Sci 86(2):248–257
Nathaniel Roybal C, Hunsaker LA, Barbash O (2005) The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem 280(21):20331–20339
Soucy NV, Klei LR, Mayka DD (2004) Signaling pathways for arsenic-stimulated vascular endothelial growth factor-A expression in primary vascular smooth muscle cells. Chem Res Toxicol 17:555–563
Gao N, Shen L, Zhang Z (2004) Arsenite induces HIF-1α and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem 255:33–45
Skinner HD, Zhong X-s, Gao N (2004) Arsenite induces p70S6K1 activation and HIF-1αexpression in prostate cancer cells. Mol Cell Biochem 255:19–23
Duyndam MCA, Hulscher STM, van der Wall E (2003) Evidence for a role of p38 kinase in hypoxia-inducible factor 1-independent induction of vascular endothelial growth factor expression by sodium arsenite. J Biol Chem 278(9):6885–6895
Duyndam MCA, Hulscher TM, Fontijn D (2001) Induction of vascular endothelial growth factor expression and hypoxia-inducible factor 1α protein by the oxidative stressor arsenite. J Biol Chem 276(51):48066–48076
Koutsourakia E, Pellsa S, De Sousaa PA (2019) Sufficiency of hypoxia-inducible 2-oxoglutarate dioxygenases to block chemical oxidative stress-induced differentiation of human embryonic stem cells. Stem Cell Res 34:101358
Lin Q, Geng Y, Zhao M (2017) MiR-21 regulates TNF-α-induced CD40 expression via the SIRT1-NF-κB pathway in renal inner medullary collecting duct cells. Cell Physiol Biochem 41:124–136
Chen QY, Costa M (2018) PI3K/Akt/mTOR signaling pathway and the biphasic effect of arsenic in carcinogenesis. Mol Pharmacol 94(1):784–792
ZHENG S-B, ZHENG Y, JIN L-W (2018) Microvesicles containing microRNA-21 secreted by proximal tubular epithelial cells are involved in renal interstitial fibrosis by activating AKT pathway. Eur Rev Med Pharmacol Sci 22:707–714
Funding
This work was supported by the National Natural Science Foundation of China (No. 81560517, 81760584), the Key Areas of Science and Technology Research Project of Xinjiang Production and Construction Corps (No. 2014BA039, No. 2015AG014), and the International Cooperative Project of Shihezi University (No. GJHZ201602).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Niu, Q., Hu, Y. et al. The Mechanism of Trivalent Inorganic Arsenic on HIF-1α: a Systematic Review and Meta-analysis. Biol Trace Elem Res 198, 449–463 (2020). https://doi.org/10.1007/s12011-020-02087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02087-x