Abstract
A method for the preconcentration of Cu(II), Co(II), and Ni(II) based on their complex formation with the potassium salt of 2.6-dimethyl-morpholinedithiocarbamate (DMMDTC) and the Amberlite XAD-4 resin as a solid support in a column was suggested. Cu(II), Co(II), and Ni(II) were detected by using the suggested spectrophotometric method in Triton X-100 media. The analytes were adsorbed as DMMDTC complexes on Amberlite XAD-4 column at the pH range of 4–6 and eluted with 0.5 M HNO3 in acetone. The best possible enrichment factors for trace metal ions were achieved by optimizing the experimental conditions including reagent amount, eluent type, sample and eluent flow rates, sample volume, and the effects of matrix ions. The detection limits of Cu(II), Co(II), and Ni(II) were found to be 11.2, 26.1, and 1.37 μg L−1, respectively. The accuracy of the proposed method was confirmed by determining the analytes in two Certified Reference Materials (TMDA-70.2 Ontario Lake Water and BCR-715 Waste Water) with the recoveries of more than 90%. The proposed method was successfully applied to the environmental and pharmaceutical samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of non-biodegradable heavy metals at trace amounts is one of the targets for the researchers around the world, due to their toxicity and accumulation tendency in human vital organs [1,2,3].
The concentration of heavy metal ions in environmental samples such as waters, biological fluids, and soils is often lower than the detection limit of modern instrumental equipment such as EDXRF, FAAS, inductively coupled plasma optical emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), and NAA. Besides, the main other restriction comes from the matrix effects of the IA (alkaline) and IIA (alkaline earth) metal ions such as Na+, Ca2+, K+, Mg2+, Sr2+, and Ba2+.
To determine the low levels of trace metal ions more accurately, sensitively, and selectively using the available techniques, a preconcentration procedure must generally be used [4]. Among the commonly used preconcentration procedures, the solid-phase extraction (SPE) based on solid supports [5,6,7], functionalized [8, 9] with chelating reagent can easily be applied using a column filled with a solid support without any chemical treatment. The adsorption of trace metal ion complexes with chelating reagent on solid support is auspicious respecting the adsorption capacity because the free chelating reagent is not retained. Especially, complexes of the metal ions can be selectively adsorbed on solid support [10].
Various sorbents have been used as solid supports such as hydrophobic resins (Amberlite XAD derivatives), multiwalled carbon nanotubes (MWCNTs), silica gel, activated carbon [11,12,13,14,15].
Amberlite XAD 4 is known to have high-surface area, uniform pore size distribution, and a promising neutral matrix for designing chelating resins, hence making it an efficient solid support [16, 17].
In recent years, dithiocarbamate (DTC) reagent has been given more attention due to forming stable complexes with heavy metal ions with a wide pH range for the spectrophotometric determination of metal ions [18,19,20]. DTCs have also been used as a chelating reagent for the separation and preconcentration of trace metal ions from various water samples [21,22,23,24]. The choice of complexation reagent is based on its fast and effective reaction with metal ions, strength in a wide pH range, and solubility in water. Dithiocarbamates (DTCs) have the advantage of offering remarkable analytical properties, particularly for the separation of the heavy metals. DTCs can form colored chelates with heavy metal ions such as Cu(II), Co(II), Ni(II), and Mn(II), and these chelates are stable during SPE separation procedures. Furthermore, DTCs do not form complexes with the alkali and alkaline earth metals [25]. Therefore, it is a suitable and selective method for heavy metal determinations in environmental water samples including alkali and alkaline earth metals.
The aim of the present work is to investigate the optimal analytical conditions of Amberlite XAD-4 (AXAD-4) with DMMDTC including sample pH and volume and eluent type for the separation and preconcentration of Cu(II), Co(II), and Ni(II) with SPE method before ultraviolet–visible (UV–VIS) spectrophotometric determination.
Experimental
Reagents and Samples
Single standard solutions of Cu(II), Co(II), and Ni(II) (Merck, Darmstadt, Germany) at 1000 μg mL−1 in 0.5 mol L−1 HNO3 were used. Working solutions of metal ions were prepared by serial dilutions of the stock standard solution with ultra-pure water (Merck, Darmstadt, Germany). AXAD-4 resin (polystyrene divinylbenzene type, 20–60 mesh, surface area of 725 m2/g) was obtained from (Sigma, St Louis, USA). It was washed with methanol, water, 1 mol L−1 HNO3 in acetone, water, 1 mol L−1 NaOH, and water, respectively, for removing organic and inorganic contaminants.
Dilute solutions (0.1 mol L−1) of either nitric acid or ammonium hydroxide solutions were used to examine the pH effect on sorption efficiency. Na2HPO4/KH2PO4 (Merck, Darmstadt, Germany) Sorenson’s buffer solution (0.133 M, pH 4.0–6.0) was used for keeping the pH constant. Triton X-100 (TX-100, 1%, v/v) was prepared in deionized water.
TMDA-70.2 Ontario Lake (Environment Canada) and BCR-715 Waste Water samples (European Commission-Joint Research Centre-Institute of Reference Materials) Certified Reference Materials were employed to test the accuracy of the proposed method. Before use, all the environmental water samples were filtered through 0.45-μm pore cellulose acetate membrane filters (Merck Millipore, Germany). Potassium salt of 2.6-dimethyl-morpholinedithiocarbamate (KDMMDTC) was synthesized and purified as described in our previous work [26]. All other reagents used were of analytical reagent-grade (Merck, Darmstadt, Germany).
Preparation of Water, Fertilizer, and Pharmaceutical Samples
A tap water sample was collected from the research laboratory, a lake water sample was collected from Gölköy in Bolu, and a dam water sample was collected from Yumrukaya Dam in Bolu, Turkey. Water samples were acidified immediately after collection using 1.0 mL of concentrated nitric acid per liter of sample and subsequently filtered through 0.45-μm pore cellulose membrane filter in order to remove the particulate matter.
Fertilizer material was bought from NPK companies. Digestion of the fertilizer was carried out using the method of the Association of Official Agricultural Chemists [26].
Supradyn and Decavit vitamin samples, to be used for the determination of Cu(II) ions, were purchased from a local pharmacy. A known weight of sample was crushed and heated in a furnace at 400 °C for 2 h in nickel crucible. Five-milliliter concentrated HCl was used to dissolve the residue, and the contents were transferred into a 100-mL volumetric flask by filtering through Whatman No. 1 filters, and the residues were washed into a calibrated 100-mL flask [27].
Preparation of the Column
After soaking for 24 h in water, 200 mg of Amberlite XAD-4 resin was transferred into a 10 × 100-mm glass column on glass wool as a plug at one end of the column. Then, another small glass wool plug was placed onto the tap of the adsorbent column. Before each use, 1 mol L−1 HNO3, deionized water, and 1 mol L−1 of HCl and deionized water, respectively, were used to clean the column. Subsequently, for obtaining the desired pH, the column was conditioned with proper buffer solutions. In order to prevent the drying of resin, it was kept in ultra-pure water after each use.
Proposed Method
Preconcentrations of Cu (II), Co(II), and Ni(II) ions in real samples were carried out as follows: pH of a known volume of an original sample solution (200 to 1000 mL) was adjusted to pH range of 4–6 with Sorenson’s buffer solution. One milliliter of 0.1% DMMDTC ligand solution was added and then the sample solution containing metal-DMMDTC chelate was passed through the preconditioned AXAD-4 column with a flow rate of 4.5 mL min−1. The chelate was sorbed on AXAD-4 column. The sorbed metal-DMMDTC chelates were then extracted from the column by the addition of 8.0 mL of 0.5 mol L−1 HNO3 (in acetone) eluent. One milliliter of 1.0% TX-100 and 0.1% DMMDTC solutions was added on to the eluate which made the final volume 10.0 mL. The eluate was analyzed for Cu(II), Co(II), and Ni(II) ions by using UV–VIS spectrometry according to the proposed method in “Determination of Metal Ions.”
Determination of Metal Ions
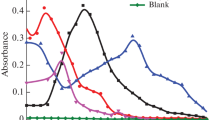
Cu(II), Co(II), and Ni(II) ions were simultaneously measured by UV–VIS spectrophotometer after elution with 0.5 M HNO3 (in acetone) from the AXAD-4 column. The principal details of UV–VIS method are listed in Table 1. The absorption spectra of DMMDTC and its Cu(II), Co(II), and Ni(II) chelates in 1% TX-100 medium are shown in Fig. 1.
Results and Discussion
Effects of Sample pH and DMMDTC Concentration
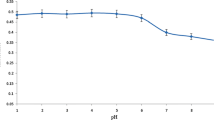
Complexation reaction of DMMDTC with heavy metal ions strongly depends on the pH of the solution. Because DMMDTC forms complexes in the wide pH range (2–10) and the fact that at pH values lower than 2.0 the DMMDTC ligand decomposes and at the pH values higher than 8.0 precipitation of ions as hydroxides develop, optimal pH for extraction procedure needs to be balanced. Thus, the effect of sample pH on the chelate formation of DMMDTC with Cu(II), Co(II), and Ni(II) ions and their following adsorption on AXAD-4 was inspected by changing the pH within the 2–10 range. The pH values were adjusted either by ammonium hydroxide or nitric acid solutions. Figure 2a shows the results of the sample pH influence on the recoveries of Cu(II), Co(II), and Ni(II) metal ions. As shown in Fig. 2a, as the pH increases up to 3, the recovery of the analytes also increases and at this pH, the highest recovery (≥ 95%) was observed. This can be explained by the stabilization of DMMDTC reagent, which is beneficial to the formation of DMMDTC chelates. Further increase of the sample pH causes slight reduction in the recovery due to the precipitation of the ions as hydroxide forms. Thus, since the highest recoveries were obtained for each metal ion at pH 5.0, this sample pH value was selected as the optimum condition for the column SPE procedure. Therefore, it was concluded that the Cu(II), Co(II), and Ni(II) ions can be simultaneously determined at pH 5.0 with the highest recoveries.
Effective complexation depends on the concentration of DMMDTC as well. In the presence of DMMDTC, formation of hydrophobic complexes of metal ions plays a key role. In this circumstance, Cu(II), Co(II), and Ni(II) are adsorbed on XAD-4 either through van der Waals forces or hydrophobic interaction. Therefore, with the increase of DMMDTC concentration, van der Waals forces or hydrophobic interactions become more important, which is the main cause of very good recoveries for Cu(II), Co(II), and Ni(II) at pH interval of 4.0–6.0. Therefore, the effect of the volume of 0.1% DMMDTC on the quantitative recoveries of the Cu(II), Co(II), and Ni(II) ions was studied in the 0.05–8-mL wide range as given in Fig. 2b. The results indicated that the sorption efficiency was DMMDTC concentration-dependent and the recoveries of Cu(II), Co(II), and Ni(II) ions increased with DMMDTC’s volume of up to 0.5 mL. Finally, all the experiments were carried out by using 1 mL vol of 0.1% DMMDTC.
Effect of Eluent Type and Concentration
A series of different elution solutions were applied for the elution of metal-DMMDTC complexes from AXAD-4. The results obtained are shown in Fig. 3. It was found that 0.5 mol L−1 of HNO3 (in acetone) was sufficient to have efficiencies of greater than 95% for all the analyte ions, among the elution solutions. Therefore, 0.5 mol L−1 of HNO3 (in acetone) solution was selected and employed as an eluent for all the experiments.
Effects of Flow Rates of Sample and Eluent Solutions
In solid-phase extraction studies, flow rates of the sample and the eluent solutions are important factors for the quantitative adsorption and desorption of metal chelates. The retention of Cu(II), Co(II), and Ni(II) complexes with DMMDTC on AXAD-4 adsorbent was not affected by sample flow rates in the range of 3.7–7.5 mL min−1 for all metal ions (Fig. 4a). Thus, the experiments were performed at a sample flow rate of 7.5 mL min−1 which was the maximum flow rate attainable by the peristaltic pump used. High flow rates of a sample solution are advantageous because of saving analysis time. Elution flow rate was also investigated in the flow rate ranges of 0.5–8.5 mL min−1 under constant conditions. All further studies were performed at the eluent flow rates of 5.0 mL min−1 with over 95% recoveries (Fig. 4b).
Effect of Sample and Eluent Volume on Preconcentration
For the assessment of the highest enrichment factors, the maximum applicable sample volume must be defined especially in environmental water samples containing very low concentrations of metal ions. The enrichment factors were studied by a proposed column procedure by using the subsequent increased volumes in the range of 50–1200 mL of Cu(II), Co(II), and Ni (II) solutions and keeping the total amount of loaded metal ions constant as 10 μg. The results obtained are presented in Fig. 5. The metal ion adsorptions were quantitative (≥ 95) until 800, 1000, and 200 mL sample volume for Cu(II), Co(II), and Ni(II), respectively. The eluent volume was also studied in a column procedure. The results showed that 8 mL of 0.5 mol L−1 of HNO3 (in acetone) elution solution volume was sufficient for obtaining the recovery of more than 95% (Fig. 6). The preconcentration factor was calculated as the ratio of the highest sample volume (800, 1000, and 200 mL) to the final volume (10 mL) and found to be 80, 100, and 20 for Cu(II), Co(II), and Ni (II), respectively.
Interferences
Some common coexisting ions, including various cations and anions in environmental samples, have been considered as having a potential interfering effect on the preconcentration of Cu(II), Co(II), and Ni(II). In this study, the recoveries of Cu(II), Co(II), and Ni(II) were investigated in the presence of some foreign ions including Cl−, NO3−, Na+, CO32−, SO42−, I−, Ca2+, Mg2+, PO43−, and Fe2+. The above ions, at various concentrations, were added separately to the sample solution containing 10-μg amounts of analyte. No significant effect on SPE method was observed. Therefore, determination of the analytes with recoveries higher than 90% was achieved with the proposed method as given in Table 2.
Analytical Performance
Analytical characteristics of the present SPE/UV–VIS method were evaluated for the determination of Cu(II), Co(II), and Ni(II) ions. The detection limits (LODs) were defined as the concentration corresponding to three times the standard deviation of ten runs of the blank solution with optimized preconcentration method and calculated as 11.2, 26.1, and 1.37 μg L−1, respectively, for Cu(II), Co(II), and Ni(II) ions. The relative standard deviations (RSDs) were in the range of 3.6–4.8% (n = 5, C = 0.5 μg mL−1). The resulting calibration graphs were linear over the concentration ranges of 0.1–3 μg mL−1 for Cu(II) (R2 = 0.993), 0.02–4 μg mL−1 for Co(II) (R2 = 0.998), and 0.01–1.5 μg mL−1 for Ni(II) (R2 = 0.992), respectively.
Application
The method was applied to Standard Reference Materials, namely, TMDA-70.2 Ontario Lake and BCR-715 Waste Water. The results are presented in Table 3. The results are strongly supported by the certified values for the analytes.
The proposed SPE/UV–VIS method for the analyte ions was applied to pharmaceutical and fertilizer samples. These samples were digested by using the procedure given in “Preparation of Water, Fertilizer, and Pharmaceutical Samples” and extracted by using the SPE procedure given in “Proposed Method”. The results are given in Table 4. The observed recoveries of analytes were higher than 95% for pharmaceutical and fertilizer samples. The proposed method was also applied to the preconcentration and determination of Cu(II), Co(II), and Ni(II) content of lake, irrigation, and tap water samples. The 250-mL water samples were treated according to the proposed procedure (Table 5) and it was observed that the method can be used reliably for the analysis of water samples.
Conclusion
A simple, fast, and reliable preconcentration/separation procedure was proposed as a method to determine the Cu(II), Co(II), and Ni(II) ions on Amberlite XAD-4 resin with DMMDTC complexes. The reusability of AXAD-4 was as high as or greater than 100 cycles with over 90% recovery. Developed SPE method achieved the complete removal of chemical interferences in real samples prior to the UV–VIS determination of trace metal ions. In addition, proposed SPE method needs lower time in comparison with the most of the other methods with high sample flow rates and can be combined with atomic spectroscopic methods such as ICP-MS, AAS, ICP-OES, and other electroanalytical techniques as offline or online mode. Many analytical procedures such as polarography [28], voltammetry [29], and ion selective electrode [30, 31] have been suggested for Cu(II), Co(II), and Ni(II) determinations in environmental samples [32, 33]. Many of these methods need more care for sensor preparation, are complicated and time-consuming, and have lower repeatability. However, the spectrophotometric method still has the advantages of the simplicity of procedures and the common availability of the instrumentation and requires no expensive or problematical equipment. For these reasons, UV–VIS spectrophotometry was used for rapid, relatively low cost, and simple determination of the preconcentrated DMMDTC analytes.
The Cu(II), Co(II), and Ni(II) could be determined simultaneously by using UV–VIS spectrophotometry with the detection based on the colored metal-DMMDTC complex formation. Further applications are to be anticipated. All that is necessary is a chemical system in which a selected analyte is exhaustively extracted from XAD-4 resin in a form allowing spectroscopic measurement. The study herein also provided further insight into the SPE mechanism in that DMMDTC–analyte ion complexes must also be pretreated by a nonionic surfactant Triton X-100 in order for the efficient and stable measurement solution to occur. The recoveries of analyte ions were efficiently quantitative and were unaffected by matrix media. The proposed method was successfully applied for the determination of the very low levels of Cu(II), Co(II), and Ni(II) contents in pharmaceutical and environmental samples. Comparison of different extraction techniques for the spectrophotometric determination of Cu(II), Co(II), and Ni(II) is given in Table 6.
As a result, present study developed a simple, efficient, and reliable method for the sensitive determination of Cu(II), Co(II), and Ni(II) ions in the real samples by using DMMDTC/AXAD-4 SPE system with UV–VIS spectrophotometer.
References
Chu Z, Fan X, Wang W, Huang WC (2019) Quantitative evaluation of heavy metals’ pollution hazards and estimation of heavy metals’ environmental costs in leachate during food waste composting. Waste Manag 84:119–128
Zhang T, Xu W, Lin X, Yan H, Ma M, He Z (2019) Assessment of heavy metals pollution of soybean grains in North Anhui of China. Sci. Total Environ 646:914–922
Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385
Kazantzi V, Drosaki E, Skok A, Vishnikin AB, Anthemidis A (2019) Evaluation of polypropylene and polyethylene as sorbent packing materials in on-line preconcentration columns for trace Pb (II) and Cd (II) determination by FAAS. Microchem J 148:514–520
Elci L, Kartal AA, Soylak M (2008) Solid phase extraction method for the determination of iron, lead and chromium by atomic absorption spectrometry using Amberite XAD-2000 column in various water samples. J Hazard Mater 153:454–461
Ozcan SG, Satiroglu N, Soylak M (2010) Column solid phase extraction of iron (III), copper (II), manganese (II) and lead (II) ions food and water samples on multi-walled carbon nanotubes. Food Chem Toxicol 48:2401–2406
Soylak M, Elci L, Dogan M (2001) Solid phase extraction of trace metal ions with Amberlite XAD resins prior to atomic absorption spectrometric analysis. J Trace Microprobe Tech 19:329–344
Memon NA, Memon FN, Kara HK, Kara H, Sherazi ST, Memon AA, Leghari MK (2019) Selective online solid-phase extraction of copper using p-morpholino-methylcalix [4] arene appended silica-based column. Sep Sci Technol 0:1–6
Borsagli FGLM, Borsagli A (2019) Chemically modified chitosan bio-sorbents for the competitive complexation of heavy metals ions: a potential model for the treatment of wastewaters and industrial spills. J Polym Environ 27:1542–1556
Elci L, Soylak M, Uzun A, Büyükpatır E, Doğan M (2000) Determination of trace impurities in some nickel compounds by flame atomic absorption spectrometry after solid phase extraction using Amberlite XAD-16 resin. Fresenius J Anal Chem 368:358–361
Zhou Q, Xing A, Zhao K (2014) Simultaneous determination of nickel, cobalt and mercury ions in water samples by solid phase extraction using multiwalled carbon nanotubes as adsorbent after chelating with sodium diethyldithiocarbamate prior to high performance liquid chromatography. J Chromatogr A 1360:76–81
Duran C, Senturk HB, Elci L, Soylak M, Tufekci M (2009) Simultaneous preconcentration of Co (II), Ni (II), Cu (II), and Cd (II) from environmental samples on Amberlite XAD-2000 column and determination by FAAS. J Hazard Mater 162:292–299
Khalil TE, Elbadawy HA, El-Dissouky A (2018) Synthesis, characterization and physicochemical studies of new chelating resin 1, 8-(3, 6-dithiaoctyl)-4-polyvinylbenzenesulphonate (dpvbs) and its metallopolymer Cu (II), Ni (II), Co (II) and Fe (III) complexes. J Mol Struct 1154:100–113
Otero-Romaní J, Moreda-Piñeiro A, Bermejo-Barrera A, Bermejo-Barrera P (2005) Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination. Anal Chim Acta 536:213–218
Tuzen M, Narin I, Soylak M, Elci L (2004) XAD-4/PAN solid phase extraction system for atomic absorption spectrometric determinations of some trace metals in environmental samples. Anal Lett 37:473–489
Dos Santos EJ, Herrmann AB, Ribeiro AS, Curtius AJ (2005) Determination of Cd in biological samples by flame AAS following on-line preconcentration by complexation with O, O-diethyldithiophosphate and solid phase extraction with Amberlite XAD-4. Talanta 65:593–597
Rajesh N, Jalan RK, Hotwany P (2008) Solid phase extraction of chromium (VI) from aqueous solutions by adsorption of its diphenylcarbazide complex on an Amberlite XAD-4 resin column. J Hazard Mater 150:723–727
Rogachev I, Gusis V, Gusis A, Cortina JL, Gressel J, Warshawsky A (1999) Spectrophotometric determination of copper complexation properties of new amphiphilic dithiocarbamates. React Funct Polym 42:243–254
San Andres MP, Marina ML, Vera S (1995) Spectrophotometric determination of copper (II), nickel (II), and cobalt (II) as complexes with sodium diethyldithiocarbamate in the anionic micellar media of dodecylsulfate salts. Analyst 120:255–259
Rudnev A, Spivakov B, Timerbaev A (2000) Solid-phase extraction and subsequent capillary zone electrophoresis of trace metal ions as soluble dithiocarbamate complexes. Chromatographia 52:99–102
Kocot K, Sitko R (2014) Trace and ultratrace determination of heavy metal ions by energy-dispersive X-ray fluorescence spectrometry using graphene as solid sorbent in dispersive micro solid-phase extraction. Spectrochim Acta B 94:7–13
Chandra Rao GP, Veni SS, Pratap K, Koteswara Rao Y, Seshaiah K (2006) Solid phase extraction of trace metals in seawater using morpholine dithiocarbamate-loaded Amberlite XAD-4 and determination by ICP-AES. Anal Lett 39:1009–1021
De la Calle I, Ruibal T, Lavilla I, Bendicho C (2019) Direct immersion thin-film microextraction method based on the sorption of pyrrolidine dithiocarbamate metal chelates onto graphene membranes followed by total reflection X-ray fluorescence analysis. Spectrochim Acta B 152:14–24
Erbas Z, Soylak M, Yilmaz E, Dogan M (2019) Deep eutectic solvent based liquid phase microextraction of nickel at trace level as its diethyldithiocarbamate chelate from environmental samples. Microchem J 145:745–750
Kocot K, Zawisza B, Sitko R (2012) Dispersive liquid–liquid microextraction using diethyldithiocarbamate as a chelating agent and the dried-spot technique for the determination of Fe, Co, Ni, Cu, Zn, Se and Pb by energy-dispersive X-ray fluorescence spectrometry. Spectrochim Acta B 73:79–83
Topuz B (2019) Selective solid phase extraction and preconcentration of ultra-trace inorganic mercury in water samples using 2,6-dimethyl-morpholine dithiocarbamate. Int J Environ An Ch 99:61–73
Modaihsh AS, AI-Swailem MS, Mahjoub MO (2004) Heavy metals content of commercial inorganic fertilizers used in the Kingdom of Saudi Arabia. J Agric Mar Sci 9:21–25
Omarova S, Demir S, Andac M (2018) Development of a new spectrophotometric based flow injection analysis method for the determination of copper (II). J Taibah Univ Sci 12:820–825
Zhu HQ, Du J, Li YG, Zhang TM, Cheng F (2019) Selective determination of trace cobalt in zinc electrolytes by second-derivative catalytic polarography. J Cent South Univ 26:207–218
Buffle J, Tercier-Waeber ML (2005) Voltammetric environmental trace-metal analysis and speciation: from laboratory to in situ measurements. Trends Anal Chem 24:172–191
Singh AK, Mehtab S, Saxena P (2007) A novel potentiometric membrane sensor for determination of Co2+ based on 5-amino-3-methylisothiazole. Sensors Actuators B Chem 120:455–461
Mashhadizadeh MH, Khani H, Shockravi A (2010) Used a new aza-thia-macrocycle as a suitable carrier in potentiometric sensor of copper (II). J Incl Phenom Macrocycl Chem 68:219–227
Chang HJ, Sung YH, Huang SD (1999) Determination of ultra-trace amounts of cadmium, cobalt and nickel in sea-water by electrothermal atomic absorption spectrometry with on-line preconcentration. Analyst 124:1695–1699
Ferreira SLC, Dos Santos WN, Lemos VA (2001) On-line preconcentration system for nickel determination in food samples by flame atomic absorption spectrometry. Anal Chim Acta 445:145–151
Safavi A, Abdollahi H, Nezhad MHR, Kamali R (2004) Cloud point extraction, preconcentration and simultaneous spectrophotometric determination of nickel and cobalt in water samples. Spectrochim Acta A 60:2897–2901
Reddy KJ, Kumar JR, Narayana SL, Ramachandraiah C, Thriveni T, Reddy AV (2007) N-Ethyl-3-carbazolecarboxaldehyde-3-thiosemicarbazone: a new extractive spectrophotometric reagent for the determination of copper (II) in environmental and pharmaceutical samples. Environ Monit Assess 124:309–320
Sarma LS, Kumar JR, Reddy KJ, Reddy AV (2005) Development of an extractive spectrophotometric method for the determination of copper (II) in leafy vegetable and pharmaceutical samples using pyridoxal-4-phenyl-3-thiosemicarbazone (PPT). J Agric Food Chem 53:5492–5498
Amin AS, AL-Attas AS. (2012) Study of the solid phase extraction and spectrophotometric determination of nickel using 5-(4′-chlorophenylazo)-6-hydroxypyrimidine-2, 4-dione in environmental samples. J Saudi Chem Soc 16:451–459
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Topuz, B. Simultaneous Spectrometric Determination of Cu(II), Co(II), and Ni(II) in Pharmaceutical and Environmental Samples with XAD-4/DMMDTC Solid-Phase Extraction System. Biol Trace Elem Res 194, 295–302 (2020). https://doi.org/10.1007/s12011-019-01930-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01930-0