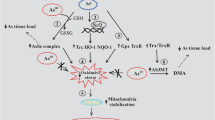
Abstract
Mercury (Hg) is a common environmental toxicant to which humans are exposed regularly through occupational and dietary means. Although selenium supplementation has been reported to prevent the toxic effects of Hg in animals, the mechanisms for this prevention are not well understood. The purpose of the current study was to determine the effects of selenium on the disposition and toxicity of Hg. Wistar rats were injected intravenously with a non-nephrotoxic dose (0.5 μmol kg−1) or a nephrotoxic dose (2.5 μmol kg−1) of HgCl2 (containing radioactive Hg) with or without co-administration of sodium selenite (Na2SeO3). Twenty-four hours after exposure, rats were euthanized, and organs were harvested. Co-administration of SeO32− with HgCl2 reduced the renal burden of Hg and the urinary excretion of Hg while increasing the amount of Hg in blood and spleen. We propose that Hg reacts with reduced selenite in the blood to form large Hg–Se complexes that are unable to be filtered at the glomerulus. Consequently, these complexes remain in the blood and are able to accumulate in blood-rich organs. These complexes, which may have fewer toxic effects than other species of Hg, may be eliminated slowly over the course of weeks to months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is recognized as a potent environmental toxicant that leads to numerous detrimental effects in a variety of organs. Humans are exposed to mercury and its related complexes primarily via occupational and/or dietary exposure. A major source of occupational exposure to Hg occurs during the processing of ore in artisanal and small-scale gold mining (ASGM) wherein miners may inhale large quantities of mercury vapor [1]. Alternatively, dietary exposure occurs following ingestion of fish and shellfish contaminated with methylmercury [2]. Following inhalation and/or ingestion of mercury compounds, mercuric ions are absorbed readily by epithelial cells in the lung and intestines [3,4,5]. Once taken up by target cells, mercuric ions can produce significant detrimental effects such as oxidative injury, disruption of signaling pathways and cellular energetics, and cytoskeletal alterations [6].
Interestingly, the toxic effects of some heavy metals, including Hg, appear to be lessened by supplementation with inorganic and/or organic forms of selenium (Se). When tin miners were given organoselenium supplements, serum markers for oxidative stress and DNA damage were found to be significantly lower than those in miners without Se supplementation [7]. Furthermore, selenite, an inorganic form of Se, has also been recognized as being protective against methylmercury (MeHg) intoxication [8] and cadmium-induced intoxication [9].
Although selenium appears to offer protection against mercury intoxication [8, 10, 11], the mechanisms involved in this process are not well understood. It has been suggested that Se has innate antioxidant properties and is able to eliminate the free radicals generated following exposure of cells and tissues to mercuric ions [12, 13]. Another possibility is that selenium ions bind to mercuric ions in a 1:1 ratio to create Hg–Se complexes that are somewhat insoluble in biological systems. Because of their lower solubility, these complexes have reduced bioavailability, which consequently lessens Hg accumulation in organs and cellular intoxication [12]. Similarly, the biomineralization of Hg with Se can lead to large inorganic compounds that may be unable to be taken up into cells due to their large size [12]. It is well-known that mercuric ions have a strong affinity for thiol (-S) groups [14], and thus, it could be argued that mercuric ions may bind preferentially to free thiols rather than to Se. However, binding studies have shown that mercuric ions have a much stronger affinity for Se than for thiols. The formation constant for methylmercury-Se complexes is estimated to be 0.1–1.2 log K units greater than that of methylmercury-thiol complexes [15]. This affinity is illustrated by studies in rats showing that Se is able to remove mercuric ions bound to metallothionein (MT), a major thiol-containing molecule [16]. Based on these studies, we hypothesize that, in the presence of Se, mercuric ions bind preferentially to Se to form Hg–Se complexes that may lessen Hg toxicity by reducing uptake into target cells.
A recent study in humans suggested that supplementation with organic selenium may be protective against Hg intoxication because it enhances excretion of Hg [17]. Interestingly, this excretion does not occur until 15–30 days following exposure [17]. Little is known about how Se alters the disposition of inorganic forms of Hg within target organs during the initial period after exposure. Additionally, previous studies have focused on humans and animals exposed to MeHg [8]. However, it is important to note that once MeHg is ingested, it is gradually biotransformed to Hg2+ in blood and tissues [18,19,20]. Furthermore, it should be noted that organic selenium, selenite, and selenate are converted within in the body to selenide, which may form complexes with mercuric ions. In addition, the binding of selenium to methylmercury has been shown to lead to demethylation, which results in the formation of inorganic mercury complexes with Se [12, 21]. Considering that cells are ultimately exposed to Hg2+ complexes, it is important to understand how Se alters the disposition and toxicity of this form of Hg. Therefore, the present study was designed to test the hypothesis that co-administration of sodium selenite with inorganic mercury (Hg2+) significantly alters the disposition of Hg in target organs and consequently reduces the toxicity of Hg.
Methods
Animals
Male and female Wistar rats weighing approximately 300 g were obtained from our breeder colony in the Mercer University School of Medicine. All animals were provided a commercial laboratory diet (Tekland 6% rat diet, Harlan Laboratories) and water ad libitum throughout all aspects of experimentation. The animal protocol for the current study was reviewed and approved by the Institutional Animal Care and Use Committee. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Intravenous Injections
Rats of both sexes were divided randomly into four groups, with each group containing four rats. Rats in group A were injected intravenously (i.v.) with a non-nephrotoxic dose of HgCl2 (0.5 μmol kg−1 2 mL 0.9% NaCl). This group was considered to be the “control.” Rats in group B were injected i.v. with a mixture of 0.5 μmol kg−1 HgCl2 and 5 mg kg−1 mL sodium selenite (Na2SeO3) [22]. Rats in group C were injected with a nephrotoxic dose of HgCl2 (2.5 μmol kg−1 2 mL 0.9% NaCl. Rats in group D were injected with a mixture of 2.5 μmol kg−1 HgCl2 and 5 mg kg−1 mL sodium selenite. Each set of injections contained 1 μCi of radioactive mercury ([203Hg2+]) per rat. For readability, sodium selenite will be referred to hereafter as “selenite” (SeO32−).
At the time of injection, each animal was anesthetized with 2–5% isoflurane, and a small incision was made in the skin in the mid-ventral region of the thigh to expose the femoral vein and artery. The dose of HgCl2 was administered into the femoral vein, and then the wound was closed with two 9-mm wound clips. Subsequently, animals were housed individually in plastic metabolic cages.
Radioactive Hg [203Hg2+]
Radioactive Hg [203Hg2+] was produced by neutron activation of mercuric oxide (enriched with Hg202) at the Missouri University Research Reactor (MURR) facility as described previously [23, 24]. A 3-mg sample of mercuric oxide was irradiated for 4 weeks at MURR. Following irradiation, the sample was dissolved in 1 mL of 1 N HCl, and the activity was measured using a Fluka ion chamber. The specific activities ranged from 10 to 15 mCi/mg.
Collection of Tissues and Organs
At the time of euthanasia, rats were anesthetized with an intraperitoneal (i.p.) injection of ketamine and xylazine (70/30 mg kg−1). A 3-mL sample of blood was obtained from the inferior vena cava, and a 1-mL sample was placed in a polystyrene tube for estimation of [203Hg2+] content. Approximately 0.5 mL of blood was placed in a blood separation tube in order to separate plasma from the cellular contents of blood. Serum creatinine levels were determined using the QuantiChrome assay kit from BioAssay. Total blood volume was estimated to be 6% of body weight [25].
Right and left kidneys were then removed, and each kidney was trimmed of fat and fascia, weighed and cut in half along the mid-transverse plane. One-half of the right kidney was placed in fixative (40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH) in preparation for histological analyses. A 3-mm transverse slice of the left kidney was utilized to obtain samples of cortex, outer stripe of outer medulla (OSOM), inner stripe of outer medulla (ISOM) and inner medulla. Each zone of the kidney was weighed and placed in a separate polystyrene tube for estimation of [203Hg2+] content. The liver was then excised, weighed, and a 1-g section was removed for determination of [203Hg2+] content.
Urine and feces were collected 24 h after injection with HgCl2. Urine from each animal was vortexed, and a 1-mL sample was weighed and placed in a polystyrene tube for estimation of [203Hg2+] content. Feces excreted by each animal were collected and counted to determine accurately the total fecal content of [203Hg2+]. The content of [203Hg2+] in each sample was determined by counting in a Wallac Wizard 3 automatic gamma counter (Perkin Elmer). Samples of a known concentration of [203Hg2+] were counted and used to calculate the concentration of Hg in each tissue sample.
Histological Analyses
Following fixation, kidneys were washed twice with normal saline and placed in 70% ethanol. Tissues were processed in a Tissue-Tek VIP processor using the following sequence: 95% ethanol for 30 min (twice); 100% ethanol for 30 min (twice); 100% xylene (twice). Tissue was subsequently embedded in POLY/Fin paraffin (Fisher). Five-micrometer sections were cut using a Leitz 1512 microtome and were subsequently mounted on glass slides. Sections were stained with hematoxylin and eosin (H&E) and were viewed using an Olympus IX70 microscope. Images were captured with a Jenoptix Progress C12 digital camera.
Data Analyses
Data were analyzed using a one-way ANOVA followed by Tukey’s multiple comparison test. A p value of < 0.05 was chosen a priori to represent statistical significance. Data are presented as mean ± standard error.
Results
Amount of Hg in Kidney
When rats were exposed to HgCl2 and selenite, the amount of Hg detected in the total renal mass was significantly lower than that in the total renal mass of rats exposed to the corresponding dose of HgCl2 alone (Fig. 1). In rats exposed to 0.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in the total renal mass was reduced by 80% compared with that in rats exposed to HgCl2 alone. When rats were exposed to 2.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in the total renal mass was reduced by approximately 92% compared with that in rats exposed to HgCl2 alone. The amount of Hg that was detected in the total renal mass of rats exposed to a non-nephrotoxic dose of HgCl2 (0.5 μmol kg−1) was significantly lower than that of rats exposed to a nephrotoxic dose of HgCl2 (2.5 μmol kg−1). The amount of Hg detected in the total renal mass of rats exposed to 0.5 μmol kg−1 HgCl2 and selenite was not significantly different than that of rats exposed to 2.5 μmol kg−1 HgCl2 and selenite.
Amount of Hg in total renal mass of Wistar rats exposed to HgCl2 or HgCl2 and selenium (Se). Rats were injected intravenously with 0.5 μmol HgCl2 kg−1 2 mL−1 or 2.5 μmol HgCl2 kg−1 2 mL−1 in the presence or absence of 5 mg kg−1 mL sodium selenite. Asterisk symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to 0.5 μmol HgCl2. Plus symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to the same dose of HgCl2 without Se (control)
In rats exposed to either concentration of HgCl2, the accumulation of Hg (nmol g−1) in the cortex was significantly greater than that in corresponding rats exposed to HgCl2 and selenite (Fig. 2a). In addition, the amount of Hg in the renal cortex of rats exposed to 0.5 μmol kg−1 HgCl2 was significantly lower than that of rats exposed to 2.5 μmol kg−1 HgCl2. The amount of Hg in the renal cortex of rats exposed to 0.5 μmol kg−1 HgCl2 and selenite was not significantly different from that of rats exposed to 2.5 μmol kg−1 HgCl2 and selenite.
Amount of Hg in the cortex (a) and outer stripe of the outer medulla (b) of Wistar rats exposed to HgCl2 or HgCl2 and selenium (Se). Rats were injected intravenously with 0.5 μmol HgCl2 kg−1 2 mL−1 or 2.5 μmol HgCl2 kg−1 2 mL−1 in the presence or absence of 5 mg kg−1 mL sodium selenite. Asterisk symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to 0.5 μmol HgCl2. Plus symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to the same dose of HgCl2 without Se (control)
When rats were exposed to either dose of HgCl2, the accumulation of Hg (% administered dose g−1) in the outer stripe of the outer medulla (OSOM) was significantly greater than that in corresponding rats exposed to HgCl2 and selenite (Fig. 2b). The amount of Hg in the OSOM of rats exposed to 0.5 μmol kg−1 HgCl2 was significantly lower than that of rats exposed to 2.5 μmol kg−1 HgCl2. The amount of Hg in the OSOM of rats exposed to 0.5 μmol kg−1 HgCl2 and selenite was not significantly different than that of rats exposed to 2.5 μmol kg−1 HgCl2 and selenite.
The amount of Hg accumulation in the inner stripe of the outer medulla and the inner medulla was minimal in all groups of rats (data not shown).
Histological Analysis of Kidney
When rats were exposed to 0.5 μmol kg−1 HgCl2, there was no detectable renal injury (Fig. 3a). Similarly, when rats were exposed to 0.5 μmol kg−1 HgCl2 with SeO32−, abnormal histological alterations were not detected (Fig. 3b). In contrast, when rats were exposed to 2.5 μmol kg−1 HgCl2, significant toxicological changes were observed histologically (Fig. 3c). These changes were predominantly localized to the OSOM and included swelling of tubular epithelial cells, sloughing of tubular epithelial cells, occlusion of the tubular lumen, and the presence of pyknotic nuclei in tubular epithelial cells. Interestingly, when rats were exposed to 2.5 μmol kg−1 HgCl2 and selenite, Hg-induced histological alterations were not observed (Fig. 3d).
Histological analyses of kidneys from Wistar rats exposed to HgCl2 or HgCl2 and selenium (Se). Panel A shows the corticomedullary junction in a representative section of kidney from rats exposed to 0.5 μmol HgCl2. Panel B shows a section of kidney from rats injected intravenously with 0.5 μmol kg−1 HgCl2 and 5 mg kg−1 mL sodium selenite. Panel C shows a section of kidney from rats exposed to 2.5 μmol HgCl2 kg−1. Numerous tubules exhibited signs of injury (asterisk symbol) including sloughing off of tubular cells, swelling, and pyknotic nuclei (arrows). Panel D shows a section of kidney from rats exposed to 2.5 μmol HgCl2 kg−1 and 5 mg kg−1 sodium selenite. Bar = 50 μm
Amount of Hg in the Spleen, Liver, and Blood
When the amount of Hg in the spleen was measured (Fig. 4), it was found that the accumulation of Hg in rats exposed to 0.5 μmol kg−1 HgCl2 was significantly greater than those exposed to 0.5 μmol kg−1 HgCl2 and selenite. When rats were exposed to 2.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in the spleen was fourfold greater than that in spleens of rats exposed to 2.5 μmol kg−1 HgCl2 only. The accumulation of Hg in spleens of rats were exposed to 2.5 μmol kg−1 HgCl2 (with and without selenite) was significantly greater than that in spleens of corresponding rats exposed to the 0.5 μmol dose of HgCl2.
Amount of Hg in the spleen of Wistar rats exposed to HgCl2 or HgCl2 and Selenium (Se). Rats were injected intravenously with 0.5 μmol HgCl2 kg−1 2 mL−1 or 2.5 μmol HgCl2 kg−1 2 mL−1 in the presence or absence of 5 mg kg−1 mL sodium selenite. Asterisk symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to 0.5 μmol HgCl2. Plus symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to the same dose of HgCl2 without Se (control)
Interestingly, co-exposure of Hg and selenite enhanced the accumulation of Hg in the liver (Fig. 5). When rats were exposed to 0.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in the liver was significantly greater than that of rats exposed to 0.5 μmol kg−1 HgCl2 alone. Similarly, when rats were exposed to 2.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in the liver was significantly greater than that of rats exposed to 2.5 μmol kg−1 HgCl2 alone. The hepatic burden of Hg was significantly greater in rats exposed to 2.5 μmol kg−1 HgCl2 (with or without selenite) than in corresponding rats exposed to 0.5 μmol kg−1 HgCl2.
Amount of Hg in the liver of Wistar rats exposed to HgCl2 or HgCl2 and selenium (Se). Rats were injected intravenously with 0.5 μmol HgCl2 kg−1 2 mL−1 or 2.5 μmol HgCl2 kg−1 2 mL−1 in the presence or absence of 5 mg kg−1 mL sodium selenite. Asterisk symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to 0.5 μmol HgCl2. Plus symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to the same dose of HgCl2 without Se (control)
A large percentage of the administered dose of Hg was found in the blood (Fig. 6). Co-administration of selenite with HgCl2 led to a significant increase in the amount of Hg in blood. When rats were exposed to 0.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in blood increased 10-fold. Similarly, when rats were exposed to 2.5 μmol kg−1 HgCl2 and selenite, the amount of Hg in blood increased approximately 12-fold. As expected, the amount of Hg in blood of rats exposed to 2.5 μmol kg−1 HgCl2 (with and without selenite) was greater than that in blood of rats exposed to the 0.5 μmol dose of HgCl2.
Amount of Hg in the blood of Wistar rats exposed to HgCl2 or HgCl2 and selenium (Se). Rats were injected intravenously with 0.5 μmol HgCl2 kg−1 2 mL−1 or 2.5 μmol HgCl2 kg−1 2 mL−1 in the presence or absence of 5 mg kg−1 mL sodium selenite. Asterisk symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to 0.5 μmol HgCl2. Plus symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to the same dose of HgCl2 without Se (control)
Serum Creatinine Levels
Serum creatinine levels are shown in Table 1. Serum creatinine was significantly greater in rats exposed to 2.5 μmol HgCl2 than in corresponding rats exposed to 0.5 μmol HgCl2. There was no significant difference in serum creatinine between rats exposed to 0.5 μmol HgCl2 and those exposed to 0.5 μmol HgCl2 + selenite. In contrast, serum creatinine levels were significantly lower in rats exposed to 2.5 μmol HgCl2 + selenite than in corresponding rats exposed to 2.5 μmol HgCl2 alone.
Amount of Hg in Urine and Feces
Interestingly, co-administration of selenite with HgCl2 did not enhance the urinary excretion of Hg (Fig. 7a). In fact, the urinary excretion of Hg in the presence of selenite was minimal following exposure to either dose of HgCl2. The amount of Hg in urine of rats exposed to 0.5 μmol kg−1 HgCl2 and selenite was significantly lower than that of rats exposed to the corresponding dose of HgCl2 alone. The amount of Hg in urine of rats exposed to 2.5 μmol kg−1 HgCl2 and selenite appeared to be lower than that in urine of rats exposed to the corresponding dose of HgCl2 alone; however, the difference observed was not statistically significant due to high variability within the group of samples from rats exposed to 2.5 μmol kg−1 HgCl2.
Amount of Hg in urine (a) and feces (b) of Wistar rats exposed to HgCl2 or HgCl2 and selenium (Se). Rats were injected intravenously with 0.5 μmol HgCl2 kg−1 2 mL−1 or 2.5 μmol HgCl2 kg−1 2 mL−1 in the presence or absence of 5 mg kg−1 mL sodium selenite. Asterisk symbol indicates significantly different values (p < 0.05) from the mean of the corresponding group of rats exposed to 0.5 μmol HgCl2. Plus symbol indicates significantly different (p < 0.05) from the mean of the corresponding group of rats exposed to the same dose of HgCl2 without Se (control)
Fecal excretion of Hg (Fig. 7b) differed from that in urine. The amount of Hg excreted in feces of animals exposed to 0.5 μmol kg−1 HgCl2 was not significantly different from that of animals exposed to 0.5 μmol kg−1 HgCl2 with selenite. Similarly, the amount of Hg excreted in feces of animals exposed to 2.5 μmol kg−1 HgCl2 was not significantly different from that of animals exposed to 2.5 μmol kg−1 HgCl2 with selenite. Due to high variability within sample sets, the fecal excretion of Hg in rats exposed to 2.5 μmol kg−1 HgCl2 (with or without selenite) was not significantly different than that of rats exposed to 0.5 μmol kg−1 HgCl2.
Discussion
Published studies have suggested that dietary supplementation with selenium may offer protection from mercury-induced intoxication [17, 26]. One potential mechanism for this protection relates to the role of selenium as a component of glutathione peroxidase (GPx). Excess selenium is thought to drive the production of GPx, which in turn could protect cells and organs against oxidative damage [10]. Alternatively, since selenium has a very high binding affinity for Hg, selenium supplementation may lead to the formation of mercury-selenide compounds. Owing to the insoluble nature of these compounds, they tend to accumulate in the tissue or organ in which they were formed.
In the current study, co-administration of Hg with selenite led to a shift in the disposition of Hg. The amount of Hg detected in blood increased by 10-fold following co-administration with selenite. This finding suggests that selenite prevents the uptake of Hg into cells and organs by trapping it within the blood. Several studies have suggested that when Se and Hg are coadministered, they react to produce a high-molecular weight complex containing 1:1 Hg:Se [16, 27]. Interestingly, when Hg and selenite are combined in the presence of glutathione, the resulting complex is quite large and highly water-soluble [28]. The size of the complex may lead to its inability to be filtered at the glomerulus and excreted in urine, preventing its uptake into cells regardless of solubility.
One of the primary sites of mercury accumulation and intoxication is the kidney. Interestingly, in the presence of selenite, the renal accumulation of Hg was reduced by 80–90%. In biological systems, selenite is converted to selenide; therefore, we propose that following exposure to Hg and selenite, Hg-selenide complexes are formed in blood. These complexes are likely too large to be filtered at the glomerular basement membrane and thus the uptake of mercuric ions into renal proximal tubular cells is reduced significantly. This idea is supported by current data showing a decrease in the renal accumulation of Hg and a concomitant increase in the amount of Hg in blood. In addition, previous studies have suggested that large Hg-selenide complexes are formed readily in biological systems [29, 30]. Analyses of muscle from oceanic mammals exposed to MeHg found that MeHg is demethylated in muscle and mercuric ions are bound subsequently to selenium to form inert HgSe [31]. Similarly, studies in pilot whales have shown that Hg and Se initially form nanoparticles, which likely serve as sites of nucleation for larger Hg-Se complexes. The large size of these complexes prevents their uptake into cells [32]. Hg-selenide complexes that remain in the blood may bind to selenoprotein P (SelP) to create a high-molecular-weight complex that is unable to be taken up by target cells [33].
Histological analyses of the kidney show that co-administration of selenite with HgCl2 prevented most all of the toxic effects induced by exposure to a nephrotoxic dose of HgCl2. In animals exposed to HgCl2 alone, significant injury was observed in the kidney, yet in the presence of selenite, very little renal injury was observed. These findings correspond well to the amount of Hg accumulation in the kidney. In addition, these findings also correspond to measurements of renal function (Table 1). Plasma creatinine levels, which are reliable indicators of renal function, were significantly greater when rats were exposed to a nephrotoxic dose of HgCl2 (2.5 μmol) than when rats were exposed to a non-nephrotoxic dose of HgCl2 (0.5 μmol). It is important to note that serum creatinine levels in rats exposed to 2.5 μmol HgCl2+selenite were significantly lower than those of rats exposed to 2.5-μmol HgCl2 alone. These data suggest that the addition of selenite protects the kidney from injury. Co-administration of selenite with HgCl2 almost completely eliminated urinary excretion of Hg, suggesting that Hg-selenide complexes are not filtered by the glomerulus and/or secreted by the renal tubules. We are currently exploring potential modes of Hg sequestration following selenite reduction.
Interestingly, co-administration of selenite with HgCl2 enhanced uptake of mercuric ions in the spleen. This finding is likely related to the open circulatory system that exits in the mammalian spleen [34, 35]. Considering that Hg-selenide complexes remain in blood, they can be delivered to other organs, particularly those with high blood flow. Splenic blood flow is approximately 0.71 mL min−1 g−1 [36], and given the estimated blood volume of the rats used in this study, we predict that the entire volume of blood circulated through the spleen approximately every 25–30 min. The repeated circulation through the spleen facilitates delivery of Hg-selenide complexes into the red pulp of the spleen via open circulation. We suggest that once these complexes enter the red pulp, they are unable (due to their size) to re-enter the splenic sinuses for exit from the spleen. It should also be considered that accumulation of Hg-selenide complexes may lead to nucleation and subsequent enhanced accumulation of Hg.
In the liver, co-administration of selenite with HgCl2 enhanced the accumulation of Hg. The hepatic accumulation is likely related directly to the concentration of the Hg-selenide complex in the blood. Venous blood flow to the liver in rats has been reported to be approximately 1.5 ± 0.09 mL min−1 g−1 liver [37], indicating that large amounts of Hg-selenide complexes may be delivered to the liver in a short period of time. It is important to note that sinusoids within the liver have a discontinuous endothelium, meaning that there are fenestrae in the endothelium to allow large molecules, such as albumin, to easily cross the sinusoidal wall [38]. Once Hg-Se complexes exit the sinusoid, they are presented to the plasma membrane of the hepatocyte, where they may be taken up by hepatocytes. Endocytosis along the sinusoidal membrane of hepatocytes occurs frequently [39], and thus, this mechanism is a probable route of Hg-Se uptake into cells. It is unclear if Hg is able to be removed from the Hg-Se complex within the intracellular compartment of hepatocytes; however, it is important to recognize that hepatocytes contain high intracellular concentrations of glutathione (GSH) [40] and metallothionein (MT) [41]. In addition, exposure to Hg has been shown to increase MT levels in hepatocytes [42]. The high intracellular concentrations of GSH and MT may drive the binding of Hg toward GSH and MT rather than Se. Binding of Hg to GSH or MT would facilitate the transport of mercuric ions out of hepatocytes over time for subsequent excretion. Hg complexes exported out of hepatocytes at the canalicular membrane would be exported into bile for fecal excretion. However, since fecal excretion of Hg was not altered significantly following co-administration with selenite, we propose that fecal elimination is not a major route of Hg excretion under these conditions. The role of hepatoenteral circulation should also be considered as a possible mechanism for movement of Hg–Se complexes. We currently do not have evidence that this pathway plays a role in the intestinal handling of Hg–Se complexes. Therefore, we propose that Hg-selenide complexes taken up by hepatocytes are converted slowly to Hg-thiol complexes (e.g., Hg-GSH2) that can be transported out of hepatocytes at the sinusoidal membrane into blood for eventual filtration and elimination by the kidney.
In summary, the current study shows that co-administration of selenite with HgCl2 in an experimental mammalian model reduces mercury toxicity by altering the disposition of Hg in the body. Moreover, the current study also shows that this re-distribution of mercuric ions reduces intoxication of target organs, such as the kidney. Selenite appears to form large complexes with Hg in blood. These complexes may remain in blood due to low solubility and bioavailability, or they may bind to the plasma protein, selenoprotein P to create a larger, less available complex. The resulting high-molecular-weight complex is renoprotective because it is unable to be filtered at the glomerulus and taken up by renal tubules. Consequently, Hg-selenide complexes remain in blood and accumulate in organs, such as the spleen and liver, with high blood flow. Future studies assessing mechanisms of elimination are clearly needed to understand fully how selenium reduces mercury toxicity.
References
Gibb H, O'Leary KG (2014) Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ Health Perspect 122:667–672
EPA (2018) How people are exposed to mercury. US Environmental Protection Agency, Washington, D.C.
Asano S, Eto K, Kurisaki E, Gunji H, Hiraiwa K, Sato M, Sato H, Hasuike M, Hagiwara N, Wakasa H (2000) Review article: acute inorganic mercury vapor inhalation poisoning. Pathol Int 50:169–174
Vazquez M, Calatayud M, Velez D, Devesa V (2013) Intestinal transport of methylmercury and inorganic mercury in various models of Caco-2 and HT29-MTX cells. Toxicology 311:147–153
Vazquez M, Velez D, Devesa V, Puig S (2015) Participation of divalent cation transporter DMT1 in the uptake of inorganic mercury. Toxicology 331:119–124
Spiller HA (2018) Rethinking mercury: the role of selenium in the pathophysiology of mercury toxicity. Clin Toxicol (Phila) 56:313–326
Yu SY, Mao BL, Xiao P, Yu WP, Wang YL, Huang CZ, Chen WQ, Xuan XZ (1990) Intervention trial with selenium for the prevention of lung cancer among tin miners in Yunnan, China. A pilot study. Biol Trace Elem Res 24:105–108
Ralston NV, Raymond LJ (2010) Dietary selenium's protective effects against methylmercury toxicity. Toxicology 278:112–123
Binte Hossain KF, Rahman MM, Sikder MT, Saito T, Hosokawa T, Kurasaki M (2018) Inhibitory effects of selenium on cadmium-induced cytotoxicity in PC12 cells via regulating oxidative stress and apoptosis. Food Chem Toxicol 114:180–189
Bjorklund G (2015) Selenium as an antidote in the treatment of mercury intoxication. Biometals 28:605–614
Bjorklund G, Dadar M, Mutter J, Aaseth J (2017) The toxicology of mercury: current research and emerging trends. Environ Res 159:545–554
Khan MA, Wang F (2009) Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 28:1567–1577
Chen C, Yu H, Zhao J, Li B, Qu L, Liu S, Zhang P, Chai Z (2006) The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ Health Perspect 114:297–301
Zalups RK (2000) Molecular interactions with mercury in the kidney. Pharmacol Rev 52:113–143
Arnold AP, Tan KS, Rabenstein DL (1986) Nuclear magnetic resonance studies of the solution chemistry of metal complexes. 23. Complexation of methylmercury by selenohydryl-containing amino acids and related molecules. Inorg Chem 25:2433–2437
Chen RW, Whanger PD, Fang SC (1974) Diversion of mercury binding in rat tissues by selenium: a possible mechanism of protection. Pharmacol Res Commun 6:571–579
Li YF, Dong Z, Chen C, Li B, Gao Y, Qu L, Wang T, Fu X, Zhao Y, Chai Z (2012) Organic selenium supplementation increases mercury excretion and decreases oxidative damage in long-term mercury-exposed residents from Wanshan, China. Environ Sci Technol 46:11313–11318
Norseth T, Clarkson TW (1970) Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch Environ Health 21:717–727
Norseth T, Clarkson TW (1970) Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem Pharmacol 19:2775–2783
Norseth T, Clarkson TW (1971) Intestinal transport of 203Hg-labeled methyl mercury chloride. Role of biotransformation in rats. Arch Environ Health 22:568–577
Wang X, Wang WX (2017) Selenium induces the demethylation of mercury in marine fish. Environ Pollut 231:1543–1551
Lemke M, Gorl N, Berg A, Weber H, Hennighausen G, Merkord J (2006) Influence of selenium treatment on the acute toxicity of dibutyltin dichloride in rats. Pancreatology 6:486–496
Belanger M, Westin A, Barfuss DW (2001) Some health physics aspects of working with 203Hg in university research. Health Phys 80:S28–S30
Bridges CC, Bauch C, Verrey F, Zalups RK (2004) Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J Am Soc Nephrol 15:663–673
Lee HB, Blaufox MD (1985) Blood volume in the rat. J Nucl Med 26:72–76
Li X, Yin D, Yin J, Chen Q, Wang R (2014) Dietary selenium protect against redox-mediated immune suppression induced by methylmercury exposure. Food Chem Toxicol 72:169–177
Naganuma A, Imura N (1983) Mode of in vitro interaction of mercuric mercury with selenite to form high-molecular weight substance in rabbit blood. Chem Biol Interact 43:271–282
Naganuma A, Tabata J, Imura N (1982) A reaction product from mercuric mercury, selenite and reduced glutathione. Res Commun Chem Pathol Pharmacol 38:291–299
Naganuma A, Ishii Y, Imura N (1984) Effect of administration sequence of mercuric chloride and sodium selenite on their fates and toxicities in mice. Ecotoxicol Environ Saf 8:572–580
Watanabe C (2002) Modification of mercury toxicity by selenium: practical importance? Tohoku J Exp Med 196:71–77
Sakamoto M, Itai T, Yasutake A, Iwasaki T, Yasunaga G, Fujise Y, Nakamura M, Murata K, Chan HM, Domingo JL, Marumoto M (2015) Mercury speciation and selenium in toothed-whale muscles. Environ Res 143:55–61
Gajdosechova Z, Lawan MM, Urgast DS, Raab A, Scheckel KG, Lombi E, Kopittke PM, Loeschner K, Larsen EH, Woods G, Brownlow A, Read FL, Feldmann J, Krupp EM (2016) In vivo formation of natural HgSe nanoparticles in the liver and brain of pilot whales. Sci Rep 6:34361
Yoneda S, Suzuki KT (1997) Equimolar Hg-Se complex binds to selenoprotein P. Biochem Biophys Res Commun 231:7–11
Steiniger BS (2015) Human spleen microanatomy: why mice do not suffice. Immunology 145:334–346
Jakubovsky J, Brozman M, Ruzickova M, Surmikova E, Belko I, Liska J, Polak S, Sadlonova I, Belosovic M (1990) Structural basis of the spleen in rats. Bratisl Lek Listy 91:466–478
Vaupel P, Ruppert H, Hutten H (1977) Splenic blood flow and intrasplenic flow distribution in rats. Pflugers Arch 369:193–201
Daemen MJ, Thijssen HH, van Essen H, Vervoort-Peters HT, Prinzen FW, Struyker Boudier HA, Smits JF (1989) Liver blood flow measurement in the rat. The electromagnetic versus the microsphere and the clearance methods. J Pharmacol Methods 21:287–297
Braet F, Wisse E (2002) Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol 1:1
Schroeder B, McNiven MA (2014) Importance of endocytic pathways in liver function and disease. Compr Physiol 4:1403–1417
Forman HJ, Zhang H, Rinna A (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med 30:1–12
Henriques GS, Cozzolino SMS (2001) Determination of metallothionein levels in tissues of young rats fed zinc-enriched diets. Rev Nutr, Campinas 14:163–169
Piotrowski JK, Trojanowska B, Wisniewska-Knypl JM, Bolanowska W (1974) Mercury binding in the kidney and liver of rats repeatedly exposed to mercuric chloride: induction of metallothionein by mercury and cadmium. Toxicol Appl Pharmacol 27:11–19
Funding
The current study was funded by a grant from the Navicent Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orr, S.E., George, H.S., Barnes, M.C. et al. Co-administration of Selenium with Inorganic Mercury Alters the Disposition of Mercuric Ions in Rats. Biol Trace Elem Res 195, 187–195 (2020). https://doi.org/10.1007/s12011-019-01835-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01835-y