Abstract
The present study aims to investigate the hepatoprotective effects of selenium on toxicity induced by ‘Désormone Lourd’ based on 2,4-dichlorophenoxyacetic acid in Wistar rats. Male Wistar rats were divided into four groups and were treated orally. The (C) group was used as a control, while the test groups were treated with Se (0.2 mg/kg b.w.), 2,4-D (5 mg/kg b.w.) or both (2,4-D + Se) for 4 weeks. Our results showed that chronic treatment with 2,4-D resulted in hepatotoxicity, as revealed by an increase in liver function markers Aminotransferases (ALT, AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and total bilirubin (TB), along with reduced total protein content and albumin. An overall pro-oxidant effect was associated with a decrease in the reduced glutathione (GSH) content and the enzymatic activity of glutathione-S-transferase (GST), catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx), and an increase in malondialdehyde (MDA) and protein carbonyl levels (PCO). Microscopic observation of liver in 2,4-D-treated rats reveals lesions, which results in perivascular inflammatory infiltration around the vessel, sinusoidal dilatation and vacuolization of hepatocytes. However, selenium supplementation in 2,4-D-treated rats elicited a reduction in the toxic effects of the pesticide by improving the studied parameters, which was confirmed by the histological study of the liver. Selenium appears to have a promising prophylactic effect through its effective anti-radical action against the hepatotoxic effects of 2,4-D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenoxy herbicides form a family of agrochemical substances that have been used since the 1950s in various cultures, including silviculture. One hundred of these derivatives are still present on the world market as a result of agricultural and commercial exploitation [1, 2]. This is the case for 2,4-D (2,4-dichlorophenoxyacetic acid), a chemical analogous to the natural systemic selective auxin indole-3-acetic acid (IAA) [3], which is marketed in forms including free acids, esters and/or salts. These various forms present similar toxicity, according to the results of more than 60 studies undertaken by the ‘Industry Task Force II’ group [4]. The 2,4-D molecule is the active adjuvant of the ‘Désormone Lourd’, a commercial formula containing esterified 2,4-D, and the 2,4-D is used as a selective herbicide to treat cereals, grass and grassland areas (Fig. 1). As the most widely used form of 2,4-D worldwide [5, 6], including in Algeria, this remains the herbicide most commonly used by farmers due to low cost and low-dose efficacy. However, it represents a risk to health and the environment [7]. It was reported that 2,4-D is more likely to affect the liver [8], kidneys [9], lipid profiles [10], the nervous system [11], reproduction [12] and blood [13].
Recent studies indicate that exposure to 2,4-D promotes oxidative stress via the generation of free radicals and induces lipid peroxidation in mammalian tissues [14, 15]. Recently, Schreinemachers [16] has reported that human exposure to 2,4-D causes an alteration of lipid and carbohydrate metabolism. The latter is a potential risk factor for myocardial infarction and the development of type 2 diabetes. In addition, the work of Bors et al. [17] and Bukowska et al. [18] on human erythrocytes have shown an alteration of certain antioxidant enzymes such as erythrocyte catalase following exposure to 2,4-D. Superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione S-transferase enzymes and non-protein elements such as reduced glutathione (GSH) are part of the first line of defence in mammalian tissues [19]. Reactive oxygen species (ROS) are likely to deplete the activity of these systems; however, antioxidant molecules such as vitamins E and C, and trace elements such as selenium and zinc, are essential to strengthen the body’s antioxidant defences and prevent oxidative damage [20, 21]. Selenium is a highly important micronutrient in human nutrition [22], known for its antioxidant properties [23, 24], and it functions through the expression of selenoproteins [25]. Its biological effects are directly related to its integration into the structures of different proteins in the form of a cofactor or amino acid [26], including glutathione peroxidase (GPx), seroprotein P (seP) and thioredoxin reductase (TrxR) [27]. Selenium is important in several biochemical and physiological processes, including the biosynthesis of coenzyme Q, regulation of ionic flows through membranes, maintenance of keratinal integrity and stimulation of antibody synthesis [28]. It plays a major role in metabolic disorders including hyperlipidaemia, hyperglycaemia and hyperphenylalaninaemia due to its antioxidant properties [29]. Furthermore, it participates in the protection and repair of DNA [30] and can prevent atherosclerosis and cancer [31,32,33].
Recent studies have shown that the antioxidant and hepatoprotective activities of this trace element are effective against a wide range of xenobiotics [34,35,36,37,38]. The aim of this study is to attempt to thwart the effect of 2,4-D subchronic toxicity through selenium supplementation in Wistar rats.
Materials and Methods
Chemicals and Reagents
The commercial formulation of 2,4-D used Désormone Lourd EC. It was dosed at 600 g of 2,4-D acid (butyl glycol ester) per litre of commercial solution, as available in Algeria, with register number R.0947009, and were prepared by the addition of appropriate volumes of distilled water.
Sodium selenite (Na2SeO3) was purchased from Sigma Chemical Co. (St Louis, France), and reduced glutathione (GSH), DTNB [5,5′-dithiobis-2-nitrobenzoic acid] and thiobarbituric acid (TBA) were purchased from Sigma Chemical Co. (St. Louis, France). All other chemicals were of analytical grade.
Animals
In our study, we used 24 Wistar male rats from the Pasteur Institute (Algiers, Algeria), aged 8 weeks, with an average weight of 220 ± 10 g. Rats were kept in an animal facility at a temperature of 22 ± 1 °C, with a photoperiod of 12 h/12 h and 50–55% relative humidity of the air. A standard pellet diet (ONAB; Bejaia, Algeria) and clean tap water were given ad libitum throughout the study. After 2 weeks of adaptation, rats were randomly divided into control and test groups.
Experimental Protocol
All experimental procedures were conducted according to the International Guidelines for Laboratory Animal Care and Use (Council of European Communities) [39] and were approved by the University Ethics Committee.
Rats were treated as follows:
-
Group I (control): rats received 1 mL of distilled water via oral gavage.
-
Group II (selenium): rats were treated with Se administered by oral gavage at 0.2 mg/kg/b.w./day for 4 weeks.
-
Group III (2,4-D): rats were treated with 2,4-D administered by oral gavage at 5 mg/kg/b.w./day for 4 weeks.
-
Group VI (2,4-D + Se): rats were treated with 2,4-D at a dose of (5 mg/kg/b.w./day) and Se (0.2 mg/kg/b.w./day) for 4 weeks. All chemicals were dissolved in distilled water.
The dose of selenium used was chosen in a way that has been suggested to provide protection against the oxidative stress induced in several tissues [35] Additionally, the dose of the pesticide was based on the previous study of Nakbi et al. [40], in which (5 mg/kg/b.w.) of 2,4-D caused oxidative damage.
Treatment continued for 4 weeks, and the weight of rats was recorded daily in the morning at the same time. At the end of the treatment period, rats were sacrificed by cervical decapitation, blood samples were immediately collected in polyethylene heparin and plain labelled tubes and these samples were centrifuged at 2500×g for 15 min at 4 °C. The livers were carefully removed, rinsed with a 0.9% NaCl solution and weighed. Plasma and liver were stored at − 20 °C.
Preparation of Liver Homogenate
One gram of the liver was homogenized in 3 mL of phosphate buffer (1:3 weight/volume PBS, pH 7.4), followed by a centrifugation of the cell suspension (9000×g for 15 min at 4 °C), and the resulting supernatant was used to determine the reactive substances of thiobarbituric acid (TBA), reduced glutathione (GSH), protein carbonyl (PCO) and protein levels and to measure GPx, GST and CAT enzymatic activity.
Biochemical Assays
The different biochemical parameters were measured by the spectrophotometric method using ready-to-use kits. Spinreact boxes were used for the determination of total protein (TP), albumin (Alb), total bilirubin (TB) and serum enzyme activities of aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyltransferase (γ-GT) and lactate dehydrogenase (LDH).
Protein Assay
Protein concentrations were measured spectrophotometrically at 595 nm, according to the method of Bradford [41], using bovine serum albumin as standard.
Determination of Lipid Peroxidation
Lipid peroxidation levels of hepatic homogenates were estimated by measuring the formation of the substances reacting with thiobarbituric acid (TBARS) using the Buege and Aust method [42]. The absorbance of the TBA-MDA complex was determined at 532 nm.
Determination of Reduced Glutathione
Liver glutathione (GSH) levels were estimated using a colorimetric method as mentioned by Ellman (1959) and modified by Jollow et al. [43]. based on the development of a yellow colour when DTNB (5,5-dithio-bis-2-nitrobenzoic acid) is added to compounds containing sulphhydryl groups. The absorbance was read at 412 nm, and the total GSH contents is expressed as μmol GSH/mg protein.
Determination of Protein Carbonyl Levels
Protein carbonyl groups were measured in liver homogenate by using the method of Levine et al. [44], the principle of which is based on the carbonyl group reaction with 2,4-dinitrophenylhydrazine (DNPH), thus leading to the formation of a stable 2,4-hydrazone (DNP) product, and the absorbance of the solution was measured at 370 nm.
Determination of Activities of Antioxidant Enzymes
The activity of glutathione peroxidase (GPx) (EC 1.11.1.9) was measured at 420 nm, according to the Flohe and Günzler technique [45]. The activity of superoxide dismutase (E.C.1.15.1.1) was determined using the Beyer and Fridovich method [46]. Activity was evaluated by measuring the ability to inhibit the photo reduction of nitro blue tetrazolium (NBT). Catalase (CAT) (EC 1.11.1.6) activity was measured using the Aebi method [47], and this result is based on the enzyme’s ability to induce the disappearance of hydrogen peroxide by reducing the absorption capacity at 240 nm for 1 min. The glutathione S-transferase (GST) (EC 2.5.1.18) activity was determined through the use of the method of Habig et al. [48].
Histological Study
Liver fragments were rinsed with physiological water and fixed in a Bouin solution for 24 h, and then liver portions were stained with haematoxylin and eosin (H&E) [49]. Preparations were then dried and observed with the Leica M18 optical microscope.
Statistical Analysis
The results were presented as the means ± standard error of the mean (SEM), and the comparison between different groups was performed after a variance analysis (ANOVA) followed by Tukey’s post hoc test for multiple comparisons with GraphPad Prism 7 software (Prism 7, version 7.00, GraphPad Software, California, USA). Differences were considered significant when p < 0.05.
Results
Effect of Treatment on Body Weight and Absolute and Relative Liver Weight
Body weight progressively increased throughout the study in all groups and was associated with growth retardation in 2,4-D-treated rats, although there were no significant differences between them. A significant increase in the absolute and relative weight of the liver was recorded in the group treated by 2,4-D (+ 14.52% and + 23.87%, respectively) and in the (2,4-D Se) group (+ 9.13% and + 12.84%) and in the relative weight in the (2,4-D Se) group (+ 12.84%) compared with the control group (Table 1).
Treatment Effects on Biochemical Parameters
Results observed after serum assays of biochemical parameters are illustrated in Table 2. The results indicate a major metabolic disturbance. The administration of 2,4-D in rats resulted in a very significant increase in TB, ASAT, ALP and LDH (p < 0.01) and a significant increase in γ-GT and ALAT (p < 0.05), which were accompanied by a decrease in plasma contents of total protein and albumin (p < 0.05) compared with control. However, the 2,4-D/Se combination demonstrated a relative stability of these analysed parameters with respect to TB, ASAT, LDH, ALP and LDH (p < 0.05).
Treatment Effects on Hepatic Oxidative Stress Parameters
Regarding the oxidative stress effect of 2,4-D on the hepatic protein fraction, our results showed a significant increase in carbonyl protein groups and MDA (p < 0.01), and a decrease in GSH(p < 0.05), in the 2,4-D-treated group compared with the control group. However, supplementation with Se appears to attenuate the oxidative effect of 2,4-D, resulting in a significant decrease in lipid peroxidation and protein carbonyl (p < 0.05) and an increase in the GSH content (Table 3)
Treatment Effects on Antioxidant Enzyme Activities in Liver
The oral administration of 2,4-D for 4 weeks of treatment resulted in a highly significant decrease in SOD activity (p < 0.01) and a significant decrease in the activities of GST, GPx and CAT (p < 0.05) in liver tissues compared with the control group. In contrast, the administration of Se revealed a significant increase in GPx activity accompanied by improved SOD and GST activities (p < 0.05) (Fig. 2, Fig. 3).
Enzymatic activity of GST (nmoles C-DNB conjugate formed/min/mg protein) (a) and CAT (nmol H2O2/min/mg protein) (b) in the liver in control and treated rats (Se; 2,4-D; 2,4-D + Se) for 4 weeks. Values are given as the mean ± SEM of 6 rats. Selenium (Se); 2,4-dichlorophenoxyacetic acid (2,4-D); glutathione S-transferase (GST); catalase (CAT). Significant difference compared with the control group (*p < 0.05). Significant difference compared with the 2,4-D-treated group (#p < 0.05, ##p < 0.01)
Enzymatic activity of SOD (U/mg prot) (c) and GPx (μmol GSH/mg prot) (d) in the liver in control and treated rats (Se; 2,4-D; 2,4-D + Se) for 4 weeks. Values are given as the mean ± SEM of 6 rats. Selenium (Se); 2,4-dichlorophenoxyacetic acid (2,4–D); superoxide dismutase (SOD); glutathione peroxidase (GPx). Significant difference compared with the control group (**p < 0.01, *p < 0.05). Significant difference compared with the 2,4-D-treated group (#p < 0.05, ##p < 0.01)
Histological Study
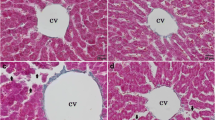
Representative photomicrographs of the control and 2,4-D-treated rat liver sections are presented in Fig. 4.
Representative haematoxylin eosin–stained liver sections from control Wistar rat (a, b) or 2,4-D (c, d, e, f, g, h) and selenium treatment (i, j) during 30 days. Staining was performed to detect fibrotic areas (indicated by red arrows); arrows: mononuclear cell infiltrates in the portal system (PS), If: hepatic cells inflammation, lipids vacuoles Asterix: i and j show normal hepatic structure (NHpS). Sections were observed at 400-fold magnification (scale bars 50 μm)
The livers of control rats show normal hepatic cells and a normal lobular architecture marked by the presence of hepatic (H) pores separated by sinusoids (S) (Fig. 4a, b). However, in 2,4-D-treated animals (Fig. 4c–h), the severe alterations in the architecture of hepatic tissue are shown, represented by mononuclear cell infiltration with vacuole degeneration (DV) around a dilated centrilobular vein (VC) and hepatic sinusoid congestion. Furthermore, the cytoplasm appeared to be extensively vacuolated and contained large numbers of lipid droplets (Fig. 4h). In the sections of 2,4-D selenium–treated rats, a normal architecture of hepatic cells was shown, and we noted a decrease in both the intensity of inflammation and the size of the hepatic vein (Fig. 4i, j).
Discussion
‘Désormone Lourd’ based on 2,4-D is an herbicide widely used in agriculture in Algeria, particularly to control the development of adventitious cereal crops (soft wheat, durum wheat, barley and oats).
The toxicity of 2,4-D has been the subject of several experimental studies. These studies have shown that 2,4-D inhibited the antioxidant enzyme system in a dose-dependent manner and, thereby, promotes the occurrence of liver damage [8, 15]. The present study was able to focus for the first time on the protective effect of sodium selenite supplementation on 2,4-D hepatotoxicity in rats. In toxicology studies, the estimation of organ weights, primarily the liver, appears to be a good indicator to assess the harmful effects of xenobiotics [50, 51]. The present study revealed a significant increase in relative and absolute liver weight. This hypertrophy could be explained by enhanced solicitation of detoxification mechanisms in response to the toxic effects of the herbicide. In this sense, Maronpot et al. [52] and Sharma et al. [53] reported that pesticide ingestion in rats is accompanied by an increase in hepatic metabolism and renal excretion, resulting in an increase in relative liver and kidney mass. In addition, our results are consistent with those of Nakbi et al. [40], which show that the administration of 2,4-D at 5 mg/kg body weight for 4 weeks in rats significantly increased liver weight.
Likewise, Tayeb et al. [54] showed that the administration of 2,4-D at doses of 15.75 and 150 mg/kg for 28 days in male rats resulted in a significant increase in relative and absolute liver weight. These weight variations correlate with liver tissue alterations. Sinusoidal spaces, centrilobular vein congestion and cellular necrosis were observed in rats receiving 2,4-D. Our results are consistent with those of Nakbi et al. [10], which showed that exposure to 2,4-D at 5 mg/kg body weight for 4 weeks generates damage from vascularization and stimulates hepatocyte necrosis. Similarly, Mountassif et al. [55] and Deshmukh and Ramteke [13] have noted severe alterations in liver architecture accompanied by cell necrosis. These changes in biometric and histopathological parameters correlate with the alteration in responses of hepatic enzyme markers to the toxicity of herbicide. In fact, the administration of 2,4-D at 5 mg/kg body weight for 4 weeks in rats resulted in a solicitation of transaminases (AST, ALT), γ-GT, PAL, LDH and bilirubin, with a decrease in total protein levels, especially albumin. These biochemical changes highlight a cytolytic effect induced by 2,4-D. In this sense, Celik et al. [56] showed that this herbicide could alter membrane permeability and leak enzymes to the plasma. In addition, Al-Baroudi et al. [57] noted an increase in ALAT activity, a marker of membrane hepatocytic cytolysis.
This 2,4-D toxicity appears to be mediated by its pro-oxidant effect, thus favouring the hyperproduction of free radicals. The latter could interact with biological molecules such as membrane and mitochondrial proteins, lipids and DNA, thereby causing a malfunction of cellular metabolism. Indeed [58], our results related to the indicator parameters of oxidative stress state, namely the concentrations of MDA, protein carbonyl and hepatic GSH content, in addition to the quality of the responses of antioxidant enzymes, catalase, SOD, GPx and GST, clearly support this hypothesis. Our results distinctly show that significant increases in lipoperoxide and carbonyl protein groups in rats treated with 2,4-D at 5 mg/kg for 4 weeks are associated with decreases in hepatic GSH concentrations, catalase activity, SOD, GPx and GST. This response profile of 2,4-D supports an imbalance between oxidants (free radicals) and antioxidants, which is the origin of the installation of OS. Several recent studies have found high levels of MDA in 2,4-D-treated rats, similar to our results [40]. These rats are able to oxidize lipids by generating different aldehydes such as MDA, a toxic compound that could alter the fluidity and function of membranes [59]. In a recent study on mouse hepatocytes, Dakhakhni et al. [15] indicate that 2,4-D causes a deterioration in lipid polarity which makes them vulnerable to free radical attacks.
In our work, the administration of selenium at 0.2 mg/kg appears to exert a remedial and even protective effect against the toxicity of 2,4-D at the hepatic level: this is revealed by the restoration of serum enzyme markers, transaminases, ALP and LDH, as well as by the restoration of protein levels, albumin and tissue architecture. This prophylactic effect of Se has also been reported by Ben Amara et al. [60] and Djeffal et al. [21] against the respective toxicities of dimethoate and methomyl. Se supplementation could modulate the expression of certain selenoproteins such as glutathione peroxidase 1, methionine-R-sulfoxide reductase 1 (MsrB1), selenoprotein S and selenoprotein P, which indirectly results in the decrease in intracellular ROS [61].
The administration of Se to 2,4-D-treated rats has also been proven able to reduce MDA and protein carbonyl levels, reinforcing the important role of Se as a shield against lipid and protein oxidation [60, 61]. El-Demerdash and Nasr [35] showed that selenium supplementation in the form of sodium selenite (200 μg/kg/day) significantly reduced serum MDA in rats treated with an organophosphorous pesticide, diazinon.
This antioxidant effect of Se is likely due to the modulation of the body’s antioxidant capacities relating to the solicitation of certain enzymes. In fact, the administration of Se in the 2,4-D-treated group led to a considerable improvement in the enzyme activities of GPx, CAT, GST and SOD. As a cofactor of many antioxidant enzymes, such as glutathione peroxidase GPx and thioredoxin reductase, Se can prevent the formation of free radicals by increasing the activity of these enzymes within the target tissues [34, 37, 38, 62]. The recent study of Xia et al. [63] on hepatopancreas, gills and haemocytes of Anodonta woodiana, a freshwater bivalve exposed to 2,4-DCP, 2,4,6-TCP and PCP, has highlighted the crucial role of the selenium-dependent glutathione peroxidase in suppressing oxidative stress.
Conclusion
Based on the data from the present study, chronic exposure to 2,4-D caused hepatotoxicity in rats, resulting in increased lipid peroxidation and increased free radical production that impaired the hepatic antioxidant defence. Selenium supplementation improves the ability of enzymatic antioxidants. However, the precise mechanism of protection cannot be verified from the results of this study so practical investigations are needed to determine the precise mechanisms of its action.
References
Grube A, Donaldson D, Kiely T, Wu L, Kiely T (2011) Pesticides industry sales and usage: 2006 and 2007 market estimates. U.S. Environmental Protection Agency 1–41 https://doi.org/https://www.epa.gov/sites/production/files/2015-10/documents/market_estimates2007.pdf
Fenner K, Canonica S, Wackett LP, Elsner M (2013) Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 341:752–758
Grossmann K (2010) Auxin herbicides: current status of mechanism and mode of action. Pest Manag Sci 66:113–120
Bus JS, Hammond LE (2007) Regulatory progress, toxicology, and public concerns with 2,4-D: where do we stand after two decades. Crop Prot 26:266–269
Peterson MA, McMaster SA, Riechers DE, Skelton J, Stahlman PW (2016) 2,4-D past, present, and future: a review. Weed Technol 30(2):303–345
Smith AM, Smith MT, La Merrill MA, Liaw J, Steinmaus C (2017) 2, 4-Dichlorophenoxyacetic acid (2, 4-D) and risk of non-Hodgkin lymphoma: a meta-analysis accounting for exposure levels. Ann Epidemiol 27(4):281–289
Islam F, Wang J, Farooq MA, Khan MS, Xu L, Zhu J, Zhou W (2018) Potential impact of the herbicide 2, 4-dichlorophenoxyacetic acid on human and ecosystems. Environ Int 111:332–351
Tayeb W, Nakbi A, Cheraief I, Miled A, Hammami M (2013) Alteration of lipid status and lipid metabolism, induction of oxidative stress and lipid peroxidation by 2, 4-dichlorophenoxyacetic herbicide in rat liver. Toxicol Mech Methods 23(6):449–458
Troudi A, Soudani N, Samet AM, Amara IB, Zeghal N (2011) 2,4-Dichlorophenoxyacetic acid effects on nephrotoxicity in rats during late pregnancy and early postnatal periods. Ecotoxicol Environ Saf 74(8):2316–2323
Nakbi A, Tayeb W, Dabbou S, Chargui I, Issaoui M, Zakhama A, Miled A, Hammami M (2012) Hypolipidemic and antioxidant activities of virgin olive oil and its fractions in 2,4-diclorophenoxyacetic acid-treated rats. Nutrition 28:81–91
Amel N, Wafa T, Samia D, Yousra B, Issam C, Cheraif I, Mohamed H (2016) Extra virgin olive oil modulates brain docosahexaenoic acid level and oxidative damage caused by 2, 4-Dichlorophenoxyacetic acid in rats. J Food Sci Technol 53(3):1454–1464
Marouani N, Tebourbi O, Cherif D, Hallegue D, Yacoubi MT, Sakly M, Rhouma KB (2017) Effects of oral administration of 2, 4-dichlorophenoxyacetic acid (2, 4-D) on reproductive parameters in male Wistar rats. Environ Sci Pollut Res 24(1):519–526
Deshmukh US, Ramteke PM (2017) Hematological, biochemical alterations, and changes in histological architecture of some tissue of male Wistar rats exposed to 2, 4-D- herbicide. Eur J Environ Ecol 4:17–21
Wafa T, Amel N, Issam C, Imed C, Abdelhedi M, Mohamed H (2011) Subacute effects of 2, 4-dichlorophenoxyacetic herbicide on antioxidant defense system and lipid peroxidation in rat erythrocytes. Pestic Biochem Physiol 99(3):256–264
Dakhakhni TH, Raouf GA, Qusti SY (2016) Evaluation of the toxic effect of the herbicide 2, 4-D on rat hepatocytes: an FT-IR spectroscopic study. Eur Biophys J 45:311–320
Schreinemachers DM (2010) Perturbation of lipids and glucose metabolism associated with previous 2, 4-D exposure: a cross-sectional study of NHANES III data, 1988-1994. Environ Health 9(1):11
Bors M, Bukowska B, Pilarski, R, Gulewicz, K, Oszmiański, J, Michałowicz J, & Koter-Michalak M (2011) Protective activity of the Uncaria tomentosa extracts on human erythrocytes in oxidative stress induced by 2, 4-dichlorophenol (2, 4-DCP) and catechol. Food and chemical toxicology49(9): 2202–2211
Bukowska B, Bors M, Gulewicz K, Koter-Michalak M (2012) Uncaria tomentosa extracts protect human erythrocyte catalase against damage induced by 2, 4-D-Na and its metabolites. Food Chem Toxicol 50(6):2123–2127
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54(4):287–293
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74
Djeffal A, Messarah M, Boumendjel A, Kadeche L, Feki AE (2015) Protective effects of vitamin C and selenium supplementation on methomyl-induced tissue oxidative stress in adult rats. Toxicol Ind Health 31(1):31–43
Papp LV, Holmgren A, Khanna KK (2010) Selenium and selenoproteins in health and disease. Antioxid Redox Signal 12(7):793–795
Wrobel JK, Power R, Toborek M (2016) Biological activity of selenium: revisited. IUBMB Life 68(2):97–105
Liu H, Xu H, Huang K (2017) Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 9:21–37
Ruseva B, Atanasova M, Tsvetkova R, Betova T, Mollova M, Alexandrova M, Laleva P, Dimitrova A (2015) Effect of selenium supplementation on redox status of the aortic wall in young spontaneously hypertensive rats. Oxidative Med Cell Longev 2015:1–10. https://doi.org/10.1155/2015/609053
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54
Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66(15):2457–2478
Eltayeb AA, Liu Q, Ganl L, Liu H, Xu H (2004) Antagonistic effect of scutellarin on the toxicity of selenium in rat livers. Biol Trace Elem Res 98(3):253–264
Wang N, Tan HY, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxidative Med Cell Longev 2017:1–13. https://doi.org/10.1155/2017/7478523
Favrot C, Beal D, Blouin E, Leccia MT, Roussel AM, Rachidi W (2018) Age-dependent protective effect of selenium against UVA irradiation in primary human keratinocytes and the associated DNA repair signature. Oxidative Med Cell Longev 2018:1–9. https://doi.org/10.1155/2018/5895439
Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci 39(3):112–120
Rayman M (2017) Selenium Intake and Status in Health & Disease. Free Radic Biol Med 112:5
Liu H, Xu H, Huang K (2017) Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 9:21–37
Messarah M, Klibet F, Boumendjel A, Abdennour C, Bouzerna N, Boulakoud MS, El Feki A (2012) Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp Toxicol Pathol 64(3):167–174
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Biol 28:89–93
Ben-Saad H, Kammoun I, Boudawara T, Zeghal KM, Hakim A, Amara IB (2017) Effects of selenium on tebuconazole-induced hepatotoxicity in adult rats. Res Rev Biosci 12(2):117
Ansar S, Alshehri SM, Abudawood M, Hamed SS, Ahamad T (2017) Antioxidant and hepatoprotective role of selenium against silver nanoparticles. Int J Nanomedicine 12:7789–7797
Mansour SA, Mohamed RI, Ali AR (2017) Ameliorating effect of selenium against deltamethrin induced hepato-renal dysfunction and oxidative stress to pregnant rats and their offspring. Journal of Toxicology and Pharmacology1(002)
Council of European Communities (1986) Council instructions about the protection of living animals used in scientific investigations. Off J Eur Commun (JO86/609/CEE) L358:1–18
Nakbi A, Tayeb W, Grissa A, Issaoui M, Dabbou S, Chargui I, Ellouz M, Miled A, Hammami M (2010) Effects of olive oil and its fractions on oxidative stress and the liver’s fatty acid composition in 2, 4-Dichlorophenoxyacetic acid-treated rats. Nutr Metab 7(1):80
Bradford M (1976) A rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1984) Microsomal lipid peroxidation. Methods Enzymol 105:302–310
Jollow DJ, Mitchel JR, Zamppaglione Z, Gillette JR (1974) Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolites. Pharmacology 11:51–57
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Flohe L, Gunzler WA (1984) Analysis of glutathione peroxidase. Methods Enzymol 105:114–121
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity. Anal Biochem 161:559–566
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Biol Chem 249(22):7130–7139
Hould R (1984) Techniques d’histopathologie et de cytopathologie. Ed Maloine 19(21):225–227
Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol 32(4):448–466
Sellers RS, Mortan D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K (2007) Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol 35(5):751–755
Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, Ward JM (2010) Hepatic enzyme induction: histopathology. Toxicol Pathol 38(5):776–795
Sharma P, Sharma A, Jasuja ND, Joshi SC (2014) Organophosphorus compounds and oxidative stress: a review. Toxicol Environ Chem 96(5):681–698
Tayeb W, Nakbi A, Trabelsi M, Attia N, Miled A, Hammami M (2010) Hepatotoxicity induced by sub-acute exposure of rats to 2,4-Dichlorophenoxyacetic acid based herbicide “Désormone lourd.”. J Hazard Mater 180(1–3):225–233
Mountassif D, Kabine M, Mounchid K, Mounaji K, Latruffe N, El Kebbaj MHS (2008) Biochemical and histological alterations of cellular metabolism from jerboa (Jaculusorientalis) by 2, 4-dichlorophenoxyacetic acid: effects on D-3-hydroxybutyrate dehydrogenase. Pestic Biochem Physiol 90(2):87–96
Celik I, Tuluce Y, Isik I (2006) Influence of subacute treatment of some plant growth regulators on serum marker enzymes and erythrocyte and tissue antioxidant defense and lipid peroxidation in rats. J Biochem Mol Toxicol 20:174–182
Al-Baroudi DA, Arafat R, El-kholy T (2014) Hepatoprotective effect of chamomile capitula extract against 2, 4-dichlorophenoxyacetic acid-induced hepatotoxicity in rats. Life Sci J 11:34–40
Bukowska B (2006) Toxicity of 2,4-dichlorophenoxyacetic acid - molecular mechanisms. Pol J Environ Stud 15:365–374
Abbassy MA, Marzouk MA, Mansour SA, Shaldam HA, Mossa AH (2014) Impact of oxidative stress and lipid peroxidation induced by lambdacyhalothrin on p450 in male rats: the ameliorating effect of zinc. J Environ Anal Toxicol 4:1–5
Ben Amara I, Soudani N, Troudi A, Bouaziz H, Boudawara T, Zeghal N (2011) Antioxidant effect of vitamin E and selenium on hepatotoxicity induced by dimethoate in female adult rats. Ecotoxicol Environ Saf 74:811–819
Zhou J, Huang K, Lei XG (2013) Selenium and diabetes—evidence from animal studies. Free Radic Biol Med 65:1548–1556
Stephen AO, James O, Ikoojo ER, Sunday AO (2016) Effects of selenium treatment on healing of acetic acid induced gastric ulcer in albino Wistar rats. Am J Biomed Res 4(1):18–22
Xia X, Hua C, Xue S, Shi B, Gui G, Zhang D, Wang X, Guo L (2016) Response of selenium dependent glutathione peroxidase in the freshwater bivalve Anodonta woodiana exposed to 2,4-dichlorophenol,2,4,6-trichlorophenol and pentachlorophenol. Fish Shellfish Immunol 55:499–509
Funding
This research is supported by the General Direction of Scientific Research and Development of Technology and Ministry of Higher Education and Scientific Research, DGRSDT-MESRS Algeria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were conducted according to the International Guidelines for Laboratory Animal Care and Use (Council of European Communities) [39] and were approved by the University Ethics Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tichati, L., Trea, F. & Ouali, K. Potential Role of Selenium Against Hepatotoxicity Induced by 2,4-Dichlorophenoxyacetic Acid in Albino Wistar Rats. Biol Trace Elem Res 194, 228–236 (2020). https://doi.org/10.1007/s12011-019-01773-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01773-9