Abstract
As the understanding of the pathways involved in such effect are quite limited, we investigated the gene pathways that modulate lipid metabolism in layers and the fatty acid profiles of the yolk of layers that were challenged with dietary vanadium (V) and supplemented with epigallo-catechin-3-gallate (EGCG). For this purpose, a total of 120 hens were divided into four groups which were fed the following experimental diets for a period of 8 weeks: control (basal diet), V10 (control + 10 mg/kg V), EGCG130 (V10 + 130 mg/kg EGCG), and EGCG217 (V10 + 217 mg/kg EGCG). Blood total cholesterol, triglyceride, glucose, and very low-density lipoprotein-cholesterol concentration were lower in V10, EGCG130, and EGCG217 groups compared to the control group, while total cholesterol and triglyceride content in blood were lower in the EGCG217 group than in V10 group (P < 0.05). Hens consumed V10 diet had the highest triglyceride content in liver among treatments, whereas EGCG130 and EGCG217 groups had lower values when compared to those observed in the control group (P < 0.01). Dietary inclusion of V increased yolk polyunsaturated fatty acid (PUFA) and total unsaturated fatty acid (UFA) content compared to the control group (P < 0.05), whereas the addition of either 130 or 217 mg/kg EGCG in V containing diet resulted in similar yolk PUFA and UFA contents with those observed in the control group. Treatment with V alone upregulated the expression of hepatic fatty acid synthase (FAS) and sterol-regulator element-binding protein 1 (SREBP1), while EGCG downregulated FAS and SREBP1 expressions in contrast to V10 treatments (P < 0.01). Liver gene expression peroxisome proliferator-activated receptor gamma (PPARγ) was lower in the V10 than in the control group while EGCG inclusion groups upregulated their expression (P < 0.05). In conclusion, the data gathered in this study indicate that dietary V and EGCG alter the layers’ lipid metabolism and fat deposition pattern in egg yolk, which might be associated with their modulatory effect on lipogenesis-related gene (FAS, SREBP1, and PPARγ) expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vanadium (V) is a transition metal which enhances the effect of insulin; indeed, this element can increase the activity of hepatocytes and myocytes in response to insulin administration [1, 2]. The biological action of V on improving glucose homeostasis and preserving insulin reserves has been studied extensively; however, only a relative small number of studies have investigated the effect of V on lipid metabolism. It has been demonstrated that V can reduce blood total cholesterol and triglyceride levels in rats [3, 4], and increase high-density lipoprotein-cholesterol (HDL-C) in humans [5]. It seems that V can activate enzymes involved in glycolysis and glycogenesis as well as lipid metabolism. Sterol regulatory element-binding proteins (SREBPs) are considered as master regulator of lipid homeostasis by controlling the expression enzymes required for fatty acid, triacylglycerol, and cholesterol [6]. There are three isomers of SREBPs, including SREBP1a, SREBP1c, and SREBP2, while the SREBP1c mainly regulates fatty acid synthesis, and in particular, is expressed remarkably in the liver and adipocytes and is regulated by nutritional state [6]. Fatty acid synthase (FAS) catalyzes the last step in the fatty acid biosynthetic pathway, and is a key determinant of the maximal capacity of a tissue to synthesize fatty acids by the de novo pathway [7]. Peroxisome proliferator activator receptor γ (PPARγ) is a key regulator of adipocyte differentiation that orchestrates the expression of adipogenic and lipogenic genes [8, 9]. It has been reported that V can modulate lipid metabolism by alternation the expression of FAS, PPARγ, and SREBP1c in mouse model [10,11,12,13,14]. However, the literature about the effect of V on yolk fatty acid profiles and lipid metabolism in layers is limited.
Green tea consumption has been associated with increased antioxidant activity, improved lipid metabolism, and reduced body fat accumulation [13, 15, 16]. Epigallocatechin gallate (EGCG) has been proposed as the major active compound in green tea because of its potency and relative quantity, constituting more than 50% of polyphenols [17, 18]. Recent studies have reported that EGCG reduced the concentration of plasma total cholesterol, triglycerides, LDL cholesterol, and body fat mass in rodents and laying hens [19, 20]. EGCG’s effect on adipocyte differentiation seems to be accompanied by downregulation of the expression of PPARγ, SREBP1c, and FAS at the messenger RNA (mRNA) and protein levels and activation of AMPK, a suppressor of PPARγ expression [9, 21, 22]. There is no research about the effect of EGCG on lipid metabolism and the fatty acid composition of egg yolk of laying hens in V-containing diets.
Therefore, the aim of this study was to investigate the effect of dietary EGCG supplementation on lipid metabolism and the fatty acid composition of egg yolk in laying hens exposed to V-containing diets.
Materials and Methods
Birds and Experimental Design
The experiment was carried out at the Animal Nutrition Experimental Station of Sichuan Agricultural University, in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China. A total of 120 Lohmann laying hens (67-week-old) were used in a single-factor randomized block design. The birds were divided into four treatment groups: control (basal diet), V10 (control + 10 mg/kg V), EGCG130 (V10 + 130 mg/kg EGCG), and EGCG217 (V10 + 217 mg/kg EGCG); dietary treatments were applied for a total of 8 weeks. There were six replicates with five hens per replicate. Birds were housed individually in stainless steel cages (38.1-cm width × 25 length × 40 height) and room environment was controlled at 22 °C by a daily lighting schedule of 16-h light and 8-h dark. Hens were allowed ad libitum access to experimental diets and water.
The basal diet (Table 1) was formulated according to recommendations of the manual of the breeder for Lohmann layers and to meet or exceed the requirement of the NRC (NRC 1994). Vanadium (added in the form of ammonium metavanadate) and epigallo-catechin-3-gallate (EGCG) with 98% purity were purchased from Sigma (St. Louis, MO, USA). And the dry matter, crude protein, calcium, and phosphorus of basal diets were determined using the methods of AOAC. The V of experimental diets were also determined by ICP-MS (7500a, Agilent Technologies Inc., CA, USA). The analysis V content in the experimental diet were 0.12 (control), 10.56 (V10), 10.71 (EGCG130), and 10.67 mg/kg (EGCG217), respectively.
Sample Collection
At the end of the experiment, 24 laying hens (1 hen/replicate, 6 replicates/treatment) were chosen to collect blood and sacrificed by cervical dislocation. Blood sample were taken from wing vein and serum were obtained after centrifugation at 3000×g for 10 min at 4 °C, and then, sera were stored at − 20 °C until further analysis. After slaughter, livers were quickly removed, snap frozen in liquid nitrogen and at − 80 °C until assay.
Triglycerides in Liver and Serum Lipid Markers
Liver triglycerides, serum total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very low density lipoprotein cholesterol (VLDL-C) were measured using reagent kits, which purchased from Nanjing Jiancheng Bioengineering Institute of China (Nanjing, China).
Extraction and Analysis of Fatty Acid Profiles in Egg Yolk
A total of 0.5 g each of the fresh yolk was homogenized with a Polytron for 5 to 10 s at high speed. Butylated hydroxyanisole in 98% ethanol was added prior to homogenization. The homogenizer was filtered through a Whatman 1 filter paper into a 100-mL graduated cylinder and 5 mL of 0.88% sodium chloride solution was added, stoppered, and mixed. After phase separation, the volume of lipid layer was recorded, and the top layer was completely siphoned off. Total lipids were converted to fatty acid methyl esters (FAME) using a mixture of boron-trifluoride, hexane, and methanol (35:20:45, v/v/v). The FAME were separated and quantified by an automated gas chromatograph equipped with an autosampler and flame ionization detector, using a 30 m × 0.32-mm-inside-diameter fused silica capillary column. A Shimadzu EZChrom chromatography (2010 type) data system was used to integrate peak areas. The calibration and identification of fatty acid peaks were carried out by comparison with retention times of known authentic standards. Fatty acid composition is expressed as weight percentages.
RT-PCR Analysis
Total RNA was extracted using TRIzol® reagent (Invitrogen, Canada) according to the manufacturer’s instruction, and the contraction of RNA for every sample was measured by a Spectrophotometer (NanoDrop 2000, Thermo, USA). For each sample, a total of 600 ng RNA was reverse-transcribed in a total volume of 20 μl of single-stranded complementary DNA (cDNA) with PrimeScript RT Reagent Kit (TaKaRa, Kusatsu, Shiga, Japan) by using T100™ Thermal Cycler (Bio-Rad, USA). The cDNA, forward, and reverse primers (Table 2) combined with SYBR® Premix Ex Taq II (TaKaRa, Kusatsu, Shiga, Japan) according to manufacturer’s instructions and measured Ct values with Standard Real-Time PCR System (ABI 7900HT, Applied Biosystems, USA). PCR reaction program: 94 °C 5 min; 94 °C 50 s, 60 °C 30 s, 72 °C 1 min, 40 cycles; and 72 °C 10 min. A relative quantification of gene expression was calculated after using 2–ΔΔCt method, and the normalization of β-actin transcript (endogenous control) acted as a calibrator.
Statistical Analysis
The data were expressed as mean ± SD, and the analysis of variance was performed using the GLM program in SAS 9.2. When an effect was significant (P < 0.05), means were compared by Turkey’s multiple comparison tests to determine specific differences among means.
Results
Effect of EGCG on Serum Lipid Markers and Liver Triglyceride Content
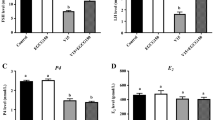
Serum total cholesterol, glucose, triglyceride, and VLDL-C contents were lower in V10, EGCG130, and EGCG217 treatments than that in the control treatment (P < 0.05; Fig. 1). Layers exhibited lower total cholesterol and triglyceride content in blood in response to 217-mg/kg EGCG supplementation with V10 diet (P < 0.05). Hens consumed V10 diet had the highest triglyceride content among treatments, whereas EGCG130 and EGCG217 groups had lower values when compared to those observed in the control group (Fig. 2; P < 0.01). There were no significant changes in serum HDL-C and LDL-C levels among different treatments (P > 0.05).
The effect of epigallo-catechin-3-gallate on blood characteristics in laying hens exposed to vanadium. V10 10 mg/kg vanadium, EGCG130 V10 + 130 mg/kg epigallo-catechin-3-gallate, EGCG217 V10 + 217 mg/kg epigallo-catechin-3-gallate, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, VLDL very low density lipoprotein cholesterol, GLU glucose
Effect of EGCG on Yolk Fatty Acid Profiles
Palmitic acid (C16:0) content was lower (P = 0.05) in the V10, EGCG130, and EGCG217 groups when compared to the control group (Table 3). Dietary inclusion of V increased polyunsaturated fatty acid (PUFA) and total unsaturated fatty acid (UFA) content in yolk compared to the control group (P < 0.05), whereas the addition of 130 or 217 mg/kg EGCG resulted in similar PUFA and UFA content with the control treatment. No differences in the egg yolk content of other fatty acids were observed among the treatment groups.
Effect of EGCG on Lipid Metabolism-Related Gene Expression
As shown in Fig. 3, treatment with V alone increased the mRNA expression of FAS and SREBP1, while EGCG217 downregulated FAS and SREBP1 expressions in contrast to V10 and EGCG130 treatment (P < 0.05). The relative mRNA expressions of PPARγ in the V10 were lower than in the control group, whereas EGCG inclusion groups upregulated their expression (P < 0.01).
Effect of vanadium and epigallo-catechin-3-gallate on lipid metabolism-related gene expression in liver. V10 10 mg/kg vanadium, EGCG130 V10 + 130 mg/kg epigallo-catechin-3-gallate, EGCG217 V10 + 217 mg/kg epigallo-catechin-3-gallate, FAS fatty acid synthase, ACAT2 acyl-coenzyme-A cholesterol acyltransferase 2, VLDLR very low density lipoprotein receptor, SREBP1 sterol-regulator element-binding protein 1, PPARγ peroxisome proliferator-activated receptor-γ, AMPKα1 AMP-activated protein kinase α1, LDLR low-density lipoprotein receptor, ACC acetyl-CoA carboxyla
Discussion
In this study, total serum glucose and triglyceride concentration was decreased by dietary V or EGCG, but their liver contents were increased by V; this effect was reversed by EGCG supplementation in liver. Since the V was used as the insulin-mimic drugs to control the blood glucose levels in the past several years [1], it is not surprisingly that the glucose levels of layers were decreased by V treatment. Triglycerides are considered effective energy donors, and the liver is the main organ responsible for triglyceride metabolism [23]. This result is in agreement with the previous studies, who observed that vanadium can reduce blood triglyceride concentration in rat studies [5, 24]. Moreover, we find that V decreased triglyceride content in the blood but increased its content in the liver, and this result was in consistent with that of Imura et al. [24], who also found that vanadium led to lipid accumulation in liver in a dose-dependent pattern. And an excess amount of lipid accumulation in hepatocytes may cause by uptake of serum triglyceride and impaired lipid metabolism, which may explain the lower triglycerides in serum. Similarly, as predominant component of green tea, EGCG was reported to be able to reduce blood triglyceride levels [25, 26]. In this experiment, supplementation of 217 mg/kg EGCG in vanadium-containing diet led to a reduction in cholesterol and VLDL-C content in blood, as well as total triglyceride both in blood and liver. Similarly, it has shown that EGCG can reduce total cholesterol and low-density lipoprotein plasma levels in rats [27]. Also, it has been reported that the addition of EGCG can reduce hepatic total cholesterol, triglycerides, and LDL-C content, and increase serum HDL-C concentration in laying hens [28]. Overall, our findings suggest that ECGG supplementation can reverse the dietary vanadium increase in liver triglyceride content. Therefore, to further investigate the interaction between dietary EGCG and vanadium in laying hens, we examined the changes in some of the key gene pathways that regulate lipid metabolism in the liver.
Dietary vanadium and EGCG did not result in significant modification of the egg yolk fatty acid profiles with the exception of palmitic acid which was increase by both V10 and the two EGCG groups. Egg yolk PUFA content was increased by V10 as expected; this increase was even higher in the EGCG130 and EGCG217 groups as it has been demonstrated the EGCG increase intestinal absorption of PUFAs [29], making them more available for the yolk synthesis and deposition. While vanadium decrease SFA and increased unsaturated FA, both EGCG groups tend to reverse this effect by bringing the content of these fatty acids to levels similar to those observed in the control group. The reason for why vanadium and EGCG can modulate the fatty acid profiles in egg yolk is not clear. This may be associated with the lipid metabolism of liver since the lipid in yolk was transported from the liver by lipid protein [30].
Our observations indicate that V10 increased FAS mRNA expression and SREBP1 expression, but EGCG supplementation was able to reverse this effect. FAS plays an important role in the regulation of lipid metabolism as it is the key enzyme that catalyzes the formation of long-chain fatty acids [31]. It has been reported that FAS mRNA expression is increased in the adipose tissue of obese animals [32], and that this can lead to an increased deposition of triglycerides in several organs including the liver [33]. Also, the SREBPs were key factors in controlling fatty acid and cholesterol synthesis, and activated SREBP promotes the expression of glucose and fatty acid synthesis enzymes [6]. In agreement with our results, EGCG has been reported to downregulate FAS [34, 35] and SREBP1c expression in vitro [9]. These observations could also explain the increased liver triglyceride content in the V10 group and the significant decrease in liver triglyceride content in both EGCG groups observed in this study. Therefore, it seems that dietary EGCG may alleviate the negative impact of vanadium on lipid metabolism by downregulating the FAS and SREBP1 pathways. Furthermore, PPARγ is predominantly expressed in adipose tissue, which is considered as the master regulator of adipocyte differentiation and lipogenesis [36]. While the mRNA expression level of PPARγ was decreased by V treatment, no significant changes were observed after feeding EGCG at present study. These data are consistent with the previous study which demonstrated that V downregulates PPARγ mRNA expression in T3-L1 preadipocytes [37]. This indicated that vanadium may act as general transcription modulator in lipid metabolism. On the other hand, EGCG was also observed to upregulate PPARγ mRNA expression in vitro and vitro studies [19, 38]. EGCG failed to have effect on the PPARγ expression, which is in line with the result of Wang et al. [39]. However, other studies conducted on cell levels (3T3-L1 cell) demonstrated that EGCG were able to alt protein and gene expression of PPARγ [34, 36]. The inconsistent result may be ascribed to the difference in experiment model and level of EGCG used in above experiment. Further studies are necessary to investigate the molecular mechanism of EGCG in cell level of layer.
Conclusion
In conclusion, the data gathered in this study indicate that dietary V and EGCG alter the layers’ lipid metabolism and fat deposition pattern in egg yolk, which might be associated with their modulatory effect on lipogenesis gene (FAS, SREBP1, and PPARγ) expressions.
References
Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M (2004) Vanadium-an element of atypical biological significance. Toxicol Lett 150:135–143
Francik R, Kryczyk-Koziol J, Francik S, Grybos R, Krosniak M (2017) Bis (4,4′-dimethyl-2,2′-bipyridine) oxidovanadium (IV) sulfate dehydrate: potential candidate for controlling lipid metabolism? Biomed Res Int 2017:6950516
Zychlinski L, Byczkowski JZ, Kulkarni AP (1991) Toxic effects of long-term intratracheal administration of vanadium pentoxide in rats. Arch Environ Contam Toxicol 20(3):295–298
Wasan KM, Risovic V, Yuen VG, McNeill JH (2006) Differences in plasma homocysteine levels between Zucker fatty and Zucker diabetic fatty rats following 3 weeks oral administration of organic vanadium compounds. J Trace Elem Med Biol 19(4):251–258
Zhang Y, Zhang Q, Feng CY, Ren XH, Li H, He KP, Wang FX, Zhou DL, Lan YJ (2014) Influence of vanadium on serum lipid and lipoprotein profiles: a population-based study among vanadium exposed workers. Lipids Health Dis 13:39
Eberle D, Hegarty B, Bossard P et al (2004) SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86(11):839–848
Clarke J (1993) Regulation of gene transcription by polyunsaturated fatty acids. Prog Lipid Res 32:139–149
Wakil SJ, Abu-Elheiga LA (2009) Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 50:S138–S143
Kim H, Hiraishi A, Tsuchiya K, Sakamoto K (2010) (−) epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology 62:245–255
Park SJ, Youn C, Hyun JW, You HJ (2014) The anti-obesity effect of natural vanadium-containing Jeju ground water. Biol Trace Elem Res 151:294–300
Zhang L, Huang Y, Liu F, Zhang F, Ding WJ (2016) Vanadium(IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. J Inorg Biochem 162:1–8
Wu Y, Huang M, Zhao P, Yang X (2013) Vanadyl acetylacetonate upregulates PPARγ and adiponectin expression in differentiated rat adipocytes. J Biol Inorg Chem 18:623–631
Zhao P, Yang X (2013) Vanadium compounds modulate PPARγ activity primarily by increasing PPARγ protein levels in mouse insulinoma NIT-1 cells. Metallomics 5:836–843
Chen WL, Wei HW, Chiu WZ, Kang CH, Lin TH, Hung CC, Chen MC, Shieh MS, Lee CC, Lee HM (2011) Metformin regulates hepatic lipid metabolism through activating AMP-activated protein kinase and inducing ATGL in laying hens. Eur J Pharmacol 671:107–112
Seale AP, de Jesus LA, Park M, Kim YS (2006) Vanadium and insulin increase adiponectin production in 3T3-L1 adipocytes. Pharmacol Res 54:30–38
Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ (2003) Relationship among habitual tea consumption, percentage body fat, and body fat distribution. Obes Res 11:1088–1095
Thavanesan N (2011) The putative effects of green tea on body fat: an evaluation of the evidence and a review of the potential mechanisms. Br J Nutr 106:1297–1309
Mak JC (2012) Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol 39:265–273
Chen N, Bezzina R, Hinch E, Lewandowski PA, Camerion-Smith D, Mathai ML, Jois M, Sinclair AJ, Begg DP, Wark JD, Weisinger HS, Weisinger RS (2009) Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr Res 29:784–793
Sae-Tan S, Grove KA, Kennett MJ, Lambert JD (2011) Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct 2:111–116
Chan CY, Wei L, Castro-Munozledo F, Koo WL (2011) (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci 89:779–785
Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ (2005) Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun 338:694–699
Tamura S, Shimomura I (2005) Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest 115(5):1139–1142
Imura H, Shimada A, Naota M, Morita T, Togawa M, Hasegawa T, Seko Y (2013) Vanadium toxicity in mice: possible impairment of lipid metabolism and mucosal epithelial cell necrosis in the small intestine. Toxicol Pathol 41(6):842–856
Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS (2008) The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr 138:1677–1683
Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS (2011) Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem 59:11862–11871
Raederstorff DG, Schlachter MF, Elste V, Weber P (2003) Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem 14(6):326–332
Lou HY, Lin Z, Wang YM, Lu FZ, Tan JF, Yin JF, Yang ZM (2004) Effect of dietary tea polyphenols on performances, lipid metabolism and egg quality of laying hens. J Tea Sci (Chinese) 24(2):135–140
Maestre R, Douglass JD, Kodukula S, Medina I, Storch J (2013) Alterations in the intestinal assimilation of oxidized PUFAs are ameliorated by a polyphenol-rich grape seed extract in an in vitro model and Caco-2 cells. J Nutr 143:295–301
Neijat M, Eck P, House JD (2017) Impact of dietary precursor ALA versus preformed DHA on fatty acid profiles of eggs, liver and adipose tissue and expression of genes associated with hepatic lipid metabolism in laying hens. Prostag Leukotr Ess 119:1–17
Wakil SJ, Stoops JK, Joshi VC (1983) Fatty acid synthesis and its regulation. Annu Rev Biochem 52:537–579
Roncari DAK (1990) Abnormalities of adipose cells in massive obesity. Int J Obes 14(s3):187–192
Semenknvich CF (1997) Regulation of fatty acid synthase (FAS). Prog Lipid Res 36:43–63
Wu M, Liu D, Zeng R, Xian T, Lu Y, Zeng G, Sun Z, Huang B, Huang Q (2017) Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. Eur J Pharmacol 795:134–142
Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S (2012) Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int J Obes 36:735–743
Zhang ZX, Li YB, Zhao RP (2016) Epigallocatechin Gallate attenuates β-amyloid generation and oxidative stress involvement of PPARγ in N2a/APP695 cells. Neurochem Res 42(2):468–480
Park SJ, Youn CK, Hyun JW (2013) The anti-obesity effect of natural vanadium-containing Jeju ground water. Biol Trace Elem Res 151(2):294–300
Lao WG, Tan Y, Xiao LD, JJY K, Qu XQ (2015) Comparison of cytotoxicity and the anti-Adipogenic effect of green tea polyphenols with Epigallocatechin-3-Gallate in 3T3-L1 Preadipocytes. Am J Chin Med 43(06):1177–1190
Wang SH, Wang X, Zhang L, Parnell LD, Admed B, LeRoith T, Ansah T, Zhang L, Li J, Ordovás JM, Si H, Liu D, Lai CQ (2018) Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J fj.201800554RR. https://doi.org/10.1096/fj.201800554RR
Funding
The financial supports of this project were given by the National Natural Science Foundation of China (31872792, 31402031), National Key technology Research and Development Program of the Ministry of Science and Technology of China (2014BAD13B04), and Sichuan Provincial Science and Technology Projects (13ZB0290, 2014NZ0043, 2014NZ0002, 2013NZ0054).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, L.Y., Wang, J.P., Pietro, C. et al. Effect of Epigallo-Catechin-3-Gallate on Lipid Metabolism Related Gene Expression and Yolk Fatty Acid Profiles of Laying Hens Exposed to Vanadium. Biol Trace Elem Res 190, 501–508 (2019). https://doi.org/10.1007/s12011-018-1562-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1562-0