Abstract
Lead (Pb), a heavy metal and an environmental stressor, may affect many physiological processes, including the serum index and the immune response. The aim of this study was to explore the toxic effects of Pb on the serum index and the immune response of Carassius auratus gibelio (C. gibelio) fed 0, 120, or 240 mg/kg Pb, and 109 cfu/g Bacillus subtilis (B. subtilis). After 15 and 30 days of dietary exposure, the serum indices and the immune responses of the fish were assessed. Dietary Pb exposure significantly affected various components of the serum index, including calcium, magnesium, glucose, cholesterol, total protein, glutamic-pyruvic transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH). However, sIgA activity in the gut increased significantly following B. subtilis supplementation. Notable changes were also observed in the expression levels of immune-related genes, including HSP70, IgM, HSP90, IL-1β, IL-6, and TNF-α. B. subtilis supplementation effectively attenuated the effects of dietary Pb exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a toxic heavy metal that causes adverse health effects in humans and animals. Pb is ubiquitous in the environment, occurring extensively in air, water, and soil [1]. Many studies have reported high levels of environmental Pb, even some exceeding 90 mg/kg in marine sediments [2]. At low aquatic Pb concentrations, aquatic animals, including the common carp and Carassius gibelio, are more likely to accumulate high levels of Pb in their tissues [3]. Humans are thus at risk of Pb exposure through consumption of aquatic products.
As a non-essential biological element, Pb is widely toxic, affecting the hematopoietic system and the immune response, causing oxidative stress, even leading to death (at high concentrations) [4,5,6]. In fish, hematological parameters are sensitive and reliable indicators of the physiological stress associated with heavy metal exposure, as fish blood directly interfaces with the external environment [7]. Serum parameters have also been used as pathophysiological indicators of the structural and functional status of fish exposed to toxins [6]. Several studies have suggested that heavy metals damage the blood system, causing hematocytopenia, reducing serum protein levels, and changing ion polarity [8, 9]. Pb may also suppress the immune system by inhibiting non-special immune protein activity and immune-related gene expression [4, 10].
It has recently been shown that dietary supplements protect against heavy metal toxicity [11]. Probiotics are living microorganisms that are beneficial to the host [12]. Of the various available dietary supplements (e.g., essential metals, vitamins, edible plants, phytochemicals, and probiotics) [13,14,15,16], probiotics may potentially reduce Pb toxicity [17]. For instance, it has been suggested that Lactobacillus plantarum alleviates heavy metal toxicity (e.g., Pb and Cd) [16, 18]. However, Lee et al. (2016) suggested that Bacillus subtilis might be a more effective probiotic than L. plantarum for aquacultural applications. B. subtilis, a genus of bacillus, is gram-positive and widely used as a probiotic in aquaculture because it is able to tolerate a wide range of temperatures and low pH [19].
Previously, we found that in C. gibelio exposed to Pb, B. subtilis reduced Pb accumulation in the organs, affected hematological parameters, and influenced antioxidant activity [4, 10, 20]. However, few studies have investigated the effects of dietary Pb and B. subtilis on C. gibelio serum parameters and immune responses. To address this knowledge gap, we herein analyzed the effects of Pb exposure and B. subtilis administration on the serum index, non-special immune protein activity, and immune-related gene expression in C. gibelio. We found that B. subtilis alleviated Pb toxicity in C. gibelio.
Materials and Methods
Fish and Bacteria
C. gibelio (62.51 ± 0.42 g) were obtained from a specialized aquatic fry farm (Jilin province, China). Fish were maintained in 80-L tanks and fed regularly with artificial feed for 2 weeks before the experiment was started. During the acclimation period, fish were fed a Pb-free diet twice daily while constantly maintaining experimental conditions at all times (dissolved oxygen, 5.91 ± 0.21 mg/L; pH, 7.4 ± 0.2; ammonia, less than 0.5 mg/L; nitrites, less than 0.05 mg/L; and temperature, 23 ± 2 °C).
B. subtilis strain was isolated from C. gibelio intestine; the method described by Veras et al. [21]. The bacterial strain was cultivated in Luria-Bertani (LB) medium and grown under aerobic condition at 30 °C with shaking (at 180 rpm) for 12 h.
Diet Preparation
Commercial feed (crude protein 37.7%, crude lipid 7.4%, and ash 10.8%) obtained from Jinyanhong Aquarium Products Co., Hangzhou, China, was used as the basal diet. The experimental diet was formulated by supplementing the basal diet with B. subtilis (at a final dose of 109 cfu/g diet) and/or lead acetate (Pb) (120 mg/kg and 240 mg/kg). The commercial feed was made of powder, and the powder was sifted through 120-μm mesh. B. subtilis and/or Pb at specified concentrations were mixed thoroughly in cooled conditions and then pelleted with a hand pelletizer. The concentration of B. subtilis in the feed was determined by spread plate technique (nutrient agar incubated at 30 °C for 24 h). The control diet was prepared by adding the same volume of sterile saline to the basal diet. The prepared diets were stored at 4 °C before use. The actual dietary Pb concentration is showed in Table 1 using atomic absorption spectrometer AA-6300 (Shimadzu, Japan).
Experiment Design
Two hundred seventy healthy fish were randomly distributed into six groups with three replications each (15 fish per replicate). Each group was kept in 80-L plastic tanks. Each group was exposed to dietary Pb and/or B. subtilis. The groups were divided as follows: CK group (control), CB group (B. subtilis, 109 cfu/g), LP group (120 mg/kg Pb), LPB group (120 mg/kg Pb plus B. subtilis, 109 cfu/g), HP group (240 mg/kg Pb), and HPB group (240 mg/kg Pb plus B. subtilis, 109 cfu/g). The fish were fed twice daily (9:00 and 15:00) for 30 days at a rate of 3% bodyweight/day. Lead acetate (CH3COO)2 Pb·3H2O was purchased from Sinopharm Chemical Reagent Company (Shanghai, China). All experiments and handling of the animals were conducted according to the research protocols approved by the Institutional Animal Care and Use Committee, Jilin Agricultural University.
Serum, Intestine, and Spleen Samples
At the end of the feeding trial, fish were fasted were 24 h. All fish were anesthetized and then euthanized using 300 mg/L methane-sulfonate-222 (MS-222). Blood samples were drawn from the caudal veins of all fish with sterile saline and stored at 4 °C for 24 h. The guts and spleens were removed, frozen in liquid nitrogen, and stored at − 80 °C.
Serum Assay
Plasma was separated from each serum sample by centrifugation at 4000g for 5 min at 4 °C. Levels of the biochemical components of the plasma (calcium, magnesium, glucose, cholesterol, total protein, glutamic-pyruvic transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), immunoglobulin M (IgM), and lysozyme (LZM)) were measured using clinical kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Determination of Secretory-IgA (sIgA) in the Gut
The concentration of sIgA in each intestinal mucosa sample was measured using a kit (Shanghai Langdon Biotechnology Co. Ltd.), following the method described by Wang et al. [22].
Total RNA Extraction and cDNA Synthesis
The frozen spleen samples were homogenized in Trizol reagent (Takara, Dalian, China), and total RNA was extracted from each sample following the manufacturer’s instruction. Each dried RNA pellet was dissolved in RNase/DNase free water. Aliquot were stored at − 80 °C. RNA quality was determined using 1% agarose gel electrophoresis, and RNA quantity was measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). One microgram of total RNA was incubated with DNase I (amplification grade; Fermentas) to remove any genomic DNA, and then reverse transcribed into cDNA using a reverse transcriptase M-MLV kit (Takara, Dalian, China). cDNA was stored at − 20 °C.
Relative Expression of Immune-Related Genes
Gene expression was analyzed in the spleen sample after 30 days of treatment. Real-time quantitative reverse transcriptase PCR (RT-qPCR) was performed using 2 × SYBR premix Ex Taq (Takara, Dalian, China). The primers used to amplify the immune genes were designed using Oligo 7.0 (primer sequences are given in Table 2). All RT-qPCR reactions were performed on an ABI 7500 Fast Real-time PCR system (Applied Biosystems) a minimum of three times. PCR reaction mixtures contained 10 μL of 1 × SYBR premix Ex Taq, 200 nM (1 μL) of each primer, 5 μL of 20× diluted cDNA, and nuclease free water to make a final volume of 25 μL. The reaction conditions were 95 °C for 5 min, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 30 s. The qRT-PCR data were converted into Ct values. Gene expression levels were calculated using the 2−ΔΔCt method and normalized to β-actin expression.
Statistical Analysis
Statistical analyses were conducted using SPSS 20.0 (SPSS, Chicago, IL, USA). Data were presented as mean ± standard deviation (S.D.) for each group. The entire experiment was repeated three times. Significant differences among groups were identified using one-way analyses of variance (ANOVAs), followed by Tukey’s multiple comparison tests. We considered P < 0.05 statistically significant.
Results
Serum Index
After 15 days of treatment, serum cholesterol and AST levels were significantly lower in the groups treated with 0 or 120 mg/kg Pb-B. subtilis, as compared to the Pb-only treatment groups (P < 0.05; Fig. 1). Serum glucose levels in the 240 mg/kg Pb-B. subtilis treatment groups were also significantly lower than in the Pb-only groups (P < 0.05), as was serum ALP in the 120 mg/kg Pb-B. subtilis treatment group (P < 0.05). After 30 days, serum glucose and ALT levels were significantly lower in the 120 and 240 mg/kg Pb-B. subtilis treatment groups as compared with the Pb-only treatment groups (P < 0.05), while serum magnesium was significantly higher (P < 0.05). Compared to the Pb-only treatment group, serum calcium was significantly higher in the 240 mg/kg Pb-B. subtilis treatment group after 30 days (P < 0.05), but AST, cholesterol, and ALP levels were significantly lower in the 0, 120, and 240 mg/kg Pb-B. subtilis treatment groups. Serum total protein was significantly lower in the 240 mg/kg Pb-B. subtilis treatment group than in the Pb-only group after 30 days (P < 0.05).
Non-special Immune Protein Activity
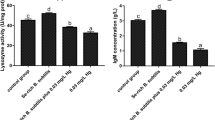
After 15 days of treatment, serum IgM concentration and LZM activity level in the 0 mg/kg Pb-B. subtilis treatment group were significantly higher than those in the Pb-only treatment group (P < 0.05; Fig. 2). After 30 days of treatment, serum IgM concentration and LZM activity were significantly greater in the 0, 120, and 240 mg/kg Pb-B. subtilis treatment groups as compared to the Pb-only treatment group (P < 0.05). After 15 days, the sIgA level in the gut was significantly greater in the 0 mg/kg Pb-B. subtilis treatment group as compared to the Pb-only treatment group (P < 0.05); after 30 days, sIgA levels in the gut were significantly greater in the 0, 120, and 240 mg/kg Pb-B. subtilis treatment groups as compared to the Pb-only treatment group (P < 0.05). Compared with the Pb-only treatment group, serum LDH was significantly lower in the 0 mg/kg Pb-B. subtilis treatment group after 15 days (P < 0.05), and in the 120 and 240 mg/kg Pb-B. subtilis treatment groups after 30 days (P < 0.05).
Immune-Related Gene Expression
Compared with the Pb-only treatment group, IgM expression levels in the kidneys were significantly greater in the 0 and 240 mg/kg Pb-B. subtilis treatment groups (P < 0.05; Fig. 3). Compared with the Pb-only treatment group, the gene expression levels of HSP70, HSP90, IL-1β, IL-6, and TNF-α were significantly greater in the 120 and 240 mg/kg Pb-B. subtilis treatment groups (P < 0.05).
Discussion
Probiotics enhances the immunity and adjusts the serum plasma properties of the target species [23, 24]. B. subtilis is becoming more widely used as a probiotic for farmed aquatic species [19]. The heavy metal Pb has serious toxic effects on the hematology and immunity of aquatic organisms [4, 20]. In this study, we evaluate the effects of Pb exposure and B. subtilis administration on the serum index, non-special immune protein activity, and related-immune gene expression for the first time.
Hematology has been widely used to evaluate the health of animals exposed to various heavy metals, including Pb, Cd, and Sn [9, 25, 26]. Hematological changes have been reported in fish exposed to various stress-inducing substances [27, 28]. Previously, we found that fish blood noticeably accumulated heavy metals [10]. Pb accumulation in the blood can suppress protein activity and damage the plasma balance [29]. Kim et al. (2017) demonstrated that Pb exposure affected calcium and magnesium concentrations in the blood of the rockfish, Sebastes schlegelii [25]. Similar results were observed in the rainbow trout [30]. Rogers et al. (2004) suggested that Pb exposure affected ion regulation by significantly reducing the Ca2+ concentration, and Ca2+ ATPase activity in rainbow trout [31].
Here, although Pb exposure led to considerable decreases in blood calcium and magnesium levels in C. gibelio, B. subtilis supplementation reversed these decreases. Zhang et al. (2016) reported that tributyltin disrupted feeding and energy metabolism in goldfish [32]. Blood glucose and cholesterol levels are general secondary responses to stress in fish; thus, these levels can be considered sensitive indicators of environmental stress [33]. Pb damages the conversion of glucose to glycogen [34].
As a critical structural component of various membranes, cholesterol is a sensitive indicator of heavy metal–induced environmental stress. Firat et al. (2011) reported that cholesterol levels in Nile tilapia were significantly increased by dietary Pb exposure, possibly because liver and kidney failure led to the release of cholesterol into the blood [35]. ALT and AST, which are synthesized in the liver, are frequently used as critical indicators of liver impairment. Heydarnejad et al. (2013) reported that the levels of ALT, AST, total protein, and ALP in rainbow trout blood were significantly increased by dietary Cd [9]. Similar results were observed in rockfish [25]. Previously, we found that dietary exposure to Pb in C. gibelio resulted in a substantial accumulation of Pb in the blood and liver. Here, we demonstrated that the serum index of C. gibelio was altered by dietary Pb exposure. Probiotics and secondary metabolites from probiotics benefit the host [36, 37]. Secondary metabolites mainly include microbial exopolysaccharides (EPS) and proteins [36]. Feng et al. (2012) reported that microbial exopolysaccharides from lactic acid bacteria absorb Pb(II) [38]. Here, B. subtilis supplementation effectively reversed and reduced the alterations caused by dietary Pb exposure. This was probably because B. subtilis increased the absorption of Pb by EPS in the blood and liver. Once the concentration of lead in the tissues was reduced, the serum index recovered.
Pb inhibits immune protein activity and alters the expression of immune-related genes [4, 20]. Immune proteins in the serum and gut play important roles in non-special immune responses [26]. Dai et al. (2018) suggested that the levels of IgM and LZM in the blood of Crucian carp were significantly decreased after exposure to waterborne Pb (at 0.05, 0.5, and 1 mg/L) [4]. Wu et al. (2017) reported that Pb exposure injured the crustacean metabolic organ, inhibiting the non-special immune response [5]. Zhang et al. (2016) demonstrated that zebrafish exposed to tributyltin for up to 56 days had lower concentrations of IgM and LZM in the gut [26]. LDH may also play an important role in the response of the immune system to viral infection or heavy metal exposure.
However, dietary supplementation with B. subtilis may enhance the activity of immune proteins in the serum and gut [22, 26]. In the common carp, different combinations of Bacillus supplements enhanced IgM and LZM activity in the serum and sIgA activity in the gut [22]. Consistent with these results, immune proteins in the serum and gut decreased significantly after Pb exposure in C. gibelio, perhaps because Pb disrupted the structures of these proteins. However, B. subtilis supplementation effectively reduced the alterations caused by dietary Pb exposure. This was probably because B. subtilis induced the repair of protein structures by secondary metabolites. Secondary metabolites from probiotics may regulate the host immune response [39]. Several studies have demonstrated that secondary metabolites play a protective role in mammalian models of inflammatory bowel disease [40, 41]. In the common carp, the expression of immune-related genes (including HSP70, IL-1β, TNF-α, and IL-10) was improved, and disease resistance was increased due to the presence of secondary metabolites after B. licheniformis administration [39]. Here, the expression levels of immune-related genes (including HSP70, HSP90, IL-1β, IL-6, and TNF-α) increased significantly after Pb exposure in C. gibelio, while IgM significantly decreased. B. subtilis supplementation also effectively regulated immune-related gene expression after dietary Pb exposure. Thus, secondary metabolites may stimulate immunity. Immune regulation might be one of the main mechanisms by which B. subtilis alleviates Pb toxicity.
Conclusion
Our results suggested that B. subtilis regulates the serum index, increases the activity of immune proteins, and alters immune-related gene expression following Pb exposure in C. gibelio. Thus, B. subtilis might potentially alleviate the toxic effects of Pb exposure in aquaculture species.
References
Landrigan PJ, Boffetta P, Apostoli P (2000) The reproductive toxicity and carcinogenicity of lead: a critical review. Am J Ind Med 38(3):231–243
Lim DI, Choi JY, Jung HS, Choi HW, Kim YO (2007) Natural background level analysis of heavy metal concentration in Korean coastal sediments. Ocean Polar Res 29(4):379–389
Cicik B, Ay O, Karayakar F (2004) Effects of lead and cadmium interactions on the metal accumulation in tissue and organs of the Nile tilapia (Oreochromis niloticus). Bull Environ Contam Toxicol 72(1):141–148
Dai J, Zhang L, Du X, Zhang P, Li W, Guo X (2018) Effect of lead on antioxidant ability and immune responses of Crucian carp. Biol Trace Elem Res:1–8. https://doi.org/10.1007/s12011-018-1316-z
Wu YS, Huang SL, Chung HC, Nan FH (2017) Bioaccumulation of lead and non-specific immune responses in white shrimp (Litopenaeus vannamei) to Pb exposure. Fish Shellfish Immunol 62:116–123
Knops M, Altenburger R, Segner H (2001) Alterations of physiological energetics, growth and reproduction of daphnia magna, under toxicant stress. Aquat Toxicol 53(2):79–90
Kim JH, Kang JC (2014) The selenium accumulation and its effect on growth, and haematological parameters in red sea bream, Pagrus major, exposed to waterborne selenium. Ecotoxicol Environ Saf 104(104):96–102
Norouzi M, Mansouri B, Hamidian AH, Zarei I, Mansouri A (2012) Metal concentrations in tissues of two fish species from Qeshm island, Iran. Bull Environ Contam Toxicol 89(5):1004–1008
Heydarnejad MS, Khosravianhemamai M, Nematollahi A (2013) Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Ir Vet J 66(1):11
Yin Y, Yue X, Zhang D, Zhang P, Abdallah A, Yin Y (2018) Study of bioaccumulation, hematological parameters, and antioxidant responses of Carassius auratus gibelio exposed to dietary lead and Bacillus subtilis. Biol Trace Elem Res 3–4:1–8
Kumar P, Prasad Y, Patra AK, Ranjan R, Swarup D, Patra RC (2009) Ascorbic acid, garlic extract and taurine alleviate cadmium-induced oxidative stress in freshwater catfish (Clarias batrachus). Sci Total Environ 407(18):5024–5030
Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S (2018) Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174:1388–1405.e21
Pérez Díaz MF, Acosta M, Mohamed FH, Ferramola ML, Oliveros LB, Gimenez MS (2013) Protective effect of soybeans as protein source in the diet against cadmium-aorta redox and morphological alteration. Toxicol Appl Pharmacol 272(3):806–815
El-Sokkary GH, Awadalla EA, El-Sokkary GH, Awadalla EA (2011) The protective role of vitamin c against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. Open Neuroendocrinol J 411(1):1–8
Luchese C, Brandão R, De OR, Nogueira CW, Santos FW (2007) Efficacy of diphenyl diselenide against cerebral and pulmonary damage induced by cadmium in mice. Toxicol Lett 173(3):181–190
Tian F, Zhai Q, Zhao J, Liu X, Wang G, Zhang H (2012) Lactobacillus plantarum ccfm8661 alleviates lead toxicity in mice. Biol Trace Elem Res 150(1–3):264–271
Zhai Q, Yin R, Yu L, Wang G, Tian F, Yu R (2015) Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control 54:23–30
Zhai Q, Wang G, Zhao J, Liu X, Narbad A, Chen YQ (2014) Protective effects of lactobacillus plantarum ccfm8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl Environ Microbiol 80(13):4063–4071
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A (2016) Comparative evaluation of dietary probiotics Bacillus subtilis, wb60 and Lactobacillus plantarum kctc3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 61:201–210
Yin Y, Zhang P, Yue X, Du X, Li W, Yin Y, Li Y (2018) Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: bioaccumulation, antioxidant responses and immune responses. Ecotoxicol Environ Saf 161:755–765
Veras FF, Correa AP, Welke JE, Brandelli A (2016) Inhibition of mycotoxin-producing fungi by Bacillus strains isolated from fish intestines. Int J Food Microbiol 238:23–32
Wang L, Ge C, Wang J, Dai J, Zhang P, Li Y (2017) Effects of different combinations of bacillus, on immunity and antioxidant activities in common carp. Aquac Int 25(1):1–9
Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA (2018) Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol 36:1–8. https://doi.org/10.1038/nbt.4222
Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS (2017) A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548:407–412
Kim JH, Kang JC (2017) Toxic effects on bioaccumulation and hematological parameters of juvenile rockfish Sebastes schlegelii exposed to dietary lead (Pb) and ascorbic acid. Chemosphere 176:131–140
Zhang CN, Zhang JL, Ren HT, Zhou BH, Wu QJ, Sun P (2017) Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol Environ Saf 138(1–8):1–8
Reckziegel P, Dias VT, Benvegnú DM, Boufleur N, Barcelos RCS, Segat HJ (2017) Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol Rep 3(C):351–356
Rodríguez-Estival J, Barasona JA, Mateo R (2012) Blood Pb and δ-ALAD inhibition in cattle and sheep from a Pb-polluted mining area. Environ Pollut 160(1):118–124
Alsop D, Ng TY, Chowdhury MJ, Wood CM (2016) Interactions of waterborne and dietborne Pb in rainbow trout, Oncorhynchus mykiss: bioaccumulation, physiological responses, and chronic toxicity. Aquat Toxicol 177:343–354
Clark NJ, Shaw BJ, Handy RD (2018) Low hazard of silver nanoparticles and silver nitrate to the haematopoietic system of rainbow trout. Ecotoxicol Environ Saf 152:121–131
Rogers JT, Wood CM (2004) Characterization of branchial lead-calcium interaction in the freshwater rainbow trout Oncorhynchus mykiss. J Exp Biol 207(5):813–825
Zhang J, Sun P, Yang F, Kong T, Zhang R (2016) Tributyltin disrupts feeding and energy metabolism in the goldfish (Carassius auratus). Chemosphere 152:221–228
Carvalho CS, Fernandes MN (2008) Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp Biochem Physiol A Mol Integr Physiol 151(3):437–442
Oner M, Atli G, Canli M (2010) Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ Toxicol Chem 27:360–366
Özgür F, Cogun HY, Yüzereroğlu TA, Gülbin G, Özge F, Ferit K (2011) A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 37(3):657–666
Postler TS, Ghosh S (2017) Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26(1):110–130
Balzaretti S, Taverniti V, Guglielmetti S, Fiore W, Minuzzo M, Ngo HN (2017) A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl Environ Microbiol 83(3):AEM.02702–16
Feng M, Chen X, Li C, Nurgul R, Dong M (2012) Isolation and identification of an exopolysaccharide-producing lactic acid bacterium strain from Chinese Paocai and biosorption of Pb(II) by its exopolysaccharide. J Food Sci 77(6):T111–T117
Chen XM, Ru HM, Niu XT, Wang GQ, Zhang DM (2015) Enhancement of secondary metabolites from Bacillus Licheniformis XY-52 on immune response and expression of some immune-related genes in common carp, Cyprinus carpio. Fish Shellfish Immunol 45(1):124–131
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohloolyy M (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341(6145):569–573
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature 504(7480):446–450
Funding
This work was supported by the National Natural Sciences Foundational of China (no. 30972191) and the 948 Program from Ministry of Agriculture of China (no. 2014Z34).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of Jilin Agricultural University with ID no. 20121008. All authors read this guide carefully. All subjects signed their informed consents before participation.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cai, Y., Yin, Y., Wang, L. et al. Effect on Serum Parameters and Immune Responses of Carassius auratus gibelio Exposed to Dietary Lead and Bacillus subtilis. Biol Trace Elem Res 190, 217–225 (2019). https://doi.org/10.1007/s12011-018-1544-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1544-2