Abstract
Some trace elements may participate in the pathogenesis of attention-deficit hyperactivity disorder (ADHD). This study aimed to investigate the trace element status of zinc (Zn), copper (Cu), iron (Fe), magnesium (Mg), and lead (Pb) in children with ADHD, and to compare them with normal controls. Associations between examined elements and SNAP-IV rating scores of ADHD symptoms were also assessed. Four hundred nineteen children with ADHD (8.8 ± 2.1 years) and 395 matched normal controls (8.9 ± 1.7 years) were recruited in the study. The concentrations of Zn, Fe, Cu, Mg, and Pb in the whole blood were measured by atomic absorption spectrometry. Lower zinc levels (P < 0.001) and the number out of normal ranges (P = 0.015) were found in children with ADHD when compared with the normal control group. The difference remained when adjusting the factor of BMI z-score. No significant between-group differences were found in levels of other elements. Zinc levels were negatively correlated with parent-rated scores of inattentive subscale of SNAP-IV (r = − 0.40) as well as with total score of SNAP-IV (r = − 0.24). Other significant associations were not observed. The present results indicated that there were alterations in blood levels of zinc, which was associated with the symptom scores of ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a neurobehavioral disorder, characterized by inattention, hyperactivity, and impulsivity [1]. The estimated prevalence of ADHD diagnosis in children ranges from 5 to 10% [2, 3]. ADHD is the most common childhood mental disorder [4], resulting in substantial functional impairments, including cognition and academic accomplishment as well as self-esteem and social relationships. Psychopharmacological treatments can benefit ADHD-related impairments, which have been confirmed by lots of studies [5,6,7]. Although originally considered as a disorder limited in childhood, about one half to two thirds of children with ADHD have the disorder persisting into adulthood [8]. This draws more attention focusing on the intervention strategy and underlying etiology of this disorder. Several studies suggest ADHD may be caused by both genetic and environmental factors and their interactions. However, researchers have not found the exact mechanism of ADHD pathogenesis up to now.
Trace elements include zinc (Zn), copper (Cu), iron (Fe), magnesium (Mg), etc., which play essential roles in normal brain growth, neurotransmitter synthesis [9], catabolism, cellular metabolic process [10], metabolism relevant to neurotransmitters, and dopamine metabolism [11, 12]. Therefore, altered levels of these elements and their imbalance may lead to dysfunction of neurotransmitters, including dopaminergic system. The imbalance of dopaminergic and noradrenergic systems is the possible mechanism of ADHD suggested by several lines of researches [2]. Thus, some studies investigated the association between metabolism of trace elements and ADHD.
The most common elements studied in children with ADHD are zinc and iron. Zinc is a cofactor for enzymes involving in the metabolism of neurotransmitters (including dopamine), melatonin, and prostaglandins [13, 14]. The symptoms of zinc deficiency include inattention, which mimics the manifestations of ADHD. Several case-controlled studies indicated lower zinc levels in children diagnosed with ADHD compared to controls [15,16,17,18,19,20,21,22,23,24]. These results have not been replicated in samples throughout the world, although most meta-analysis suggested an association between low zinc levels and a diagnosis of ADHD [14, 25, 26]. Clinically, although with conflicting results [27], several researches studying the effects of zinc supplements on the ADHD symptoms showed zinc is superior to placebo in reducing both hyperactive and impulsive symptoms [28]. And, zinc could improve the effects of amphetamine in ADHD treatment [29]. These results might be considered as evidences of zinc playing an important role in pathogenesis of ADHD, at least for a subgroup of children.
Iron is another extensively studied element in children with ADHD, which is a cofactor for tyrosine hydroxylase, the rate-limiting enzyme of monoamine synthesis and thus is critical for dopamine and norepinephrine production. Bener et al. [30] found that children with ADHD had low serum iron after adjusting for age, gender, and other variables. Doom et al. [31] found that iron deficiency increased the ADHD symptomology. In addition, some evidences supported the effects of iron supplement on improving ADHD symptoms [32]. These documents demonstrated the low serum iron was associated with ADHD. But there were other studies with controversial results of the iron deficiency in ADHD [33].
The other two elements, Cu and Mg, are possibly related with the ADHD pathogenesis. Cu plays as an essential constituent in many enzymes (i.e., dopamine β-hydroxylase, monoamine oxidase, thyrosinase, and Cu/zinc superoxide dismutase) for their functions. Previous studies reported lower [34, 35] levels of Cu in subjects with ADHD than in normal controls. For Mg, the study by Nogovitsina et al. [36] identified decreased plasma magnesium levels in children with ADHD. However, studies revealed no correlations between both Cu and Mg levels with scores of ADHD symptoms on rating scales [37, 38].
Lead (Pb), a heavy metal naturally found in the environment, is a well-known neurotoxicant, especially harmful to child neurodevelopment. Many studies have shown the harmful effects of higher blood lead levels (BLL) (> 100 μg/L); however, a growing body of evidence shows adverse effects of lower BLLs (e.g., ≤ 50 μg/L), which suggests no threshold of developmental neurotoxicity [39,40,41]. Many studies indicate that low-level lead exposure may contribute to ADHD diagnosis [40,41,42,43,44]. The association between lead exposure and ADHD needs to be examined in different populations.
Overall, the topic continues to interest parents and physicians who prefer an alternative to psychopharmacological treatment or seek a complementary therapy, although there was no definite conclusion of the association between trace elements and ADHD. However, previous investigations had many limitations, which prevented us to draw powerful conclusions including small sample size, lack of controls, not excluding confounding factors (such as nutrient status, BMI), absence of the association between scores of ADHD symptoms and elements levels. Taken these into consideration, we performed a case-controlled study aiming (1) to investigate if the elements were deficient in Chinese children with ADHD comparing to normal controls and (2) to explore if the elements levels were related to ADHD symptomatology.
Methods
Study Design
This case-controlled study was designed to assess if the status of the trace elements were changed in children with ADHD, which was conducted in ADHD and healthy Han Chinese children aged between 6 and 16 years.
Study Procedure
The data were collected from outpatients who were suspected to be affected by ADHD on their first visit to clinic between July 1, 2014, and September 30, 2015, in the Children’s Hospital, Zhejiang University School of Medicine. Participants underwent a comprehensive assessment to define if they had a diagnosis of ADHD. At the same time, trace elements were detected. The study procedures were explained and the written informed consents were obtained from the children and their parents. The study protocol was approved by the Institutional Review Board of the Children’s Hospital, Zhejiang University School of Medicine.
Participants and Diagnostic Assessment
The studied children experienced a comprehensive assessment process by a trained multidisciplinary ADHD diagnostic team, including Vanderbilt ADHD Diagnostic Parent and Teacher Rating Scales, Conners’ Parent and Teacher Rating Scales (Chinese version), SNAP-IV and Raven’s Progressive Matrices, and physical examination, EEG, ECG and liver function. The children, parents, and teachers were interviewed by one psychiatrist to verify the ADHD diagnosis.
Children included in this study met the following criteria: (a) diagnosed with ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), under the following three presentations: predominantly inattentive presentation, predominantly hyperactive-impulsive presentation, and combined presentation; (b) between the ages of 6 and 16 years; (c) having no history of treatment for ADHD; and (d) being Han Chinese.
Individuals were excluded if they had (a) a psychiatric diagnosis with significant symptoms (psychosis, autism, depression, anxiety, mood disorder) and mental retardation; (b) sensorimotor handicaps (paralysis, deafness, blindness); (c) taken drugs such as methylphenidate in any form, clonidine, or atomoxetine for controlling ADHD symptoms; or (d) serious somatic disorders, such as cardiovascular diseases, hyperthyroidism, serious gastrointestinal stenosis, dysphagia, exogenous steroid hormones, and sexual development disorders.
The normal controls were recruited from a local primary school. A total of 395 children enrolled into this group. The control participants had been examined to exclude ADHD condition and other severe medical disorders. The characteristics of the samples are shown in Table 1.
Trace Element Detection
Trace metal analyses were performed with a multichannel atomic absorption spectrophotometer -MB5 (AAS; Beijing Purkinje General Instrument Co., Ltd., Beijing, China) and lead with a graphite furnace atomic absorption spectrometry-MG2 (Beijing Purkinje General Instrument Co., Ltd., Beijing, China) [45]. The detection limits of these instruments for zinc, copper, iron, magnesium, and lead are 0.01, 0.01, 0.01, 0.01 μmol/L and 0.5 μg/L, respectively.
Growth Measurement and Assessment
Growth data were taken during the first recruitment visit by one assistant. Body weight was measured to the nearest 0.10 kg while the subjects were wearing lightweight clothing, and height was measured to the nearest 0.10 cm without shoes and hats. BMI was calculated by dividing the subject’s weight (in kg) by the square of his or her height (in meters). BMI percentiles and BMI z-score were used to determine the individuals’ growth status, which was evaluated using the National Growth Reference for Chinese Children and Adolescents [26]. Individuals’ BMI percentile was determined according to the age- and gender-specific standardized growth chart. BMI z-score was defined as the difference between a subject’s absolute BMI and the mean BMI for children of the same age and gender, divided by the standard deviation of the BMI for that subgroup. Age in months was calculated from the date of birth to the date of the visit.
Data Analysis
Data were entered into Excel software and analyzed in the SPSS (version 13.0) software package. Before a t test was used to compare the differences of trace elements levels between two groups, the normal distributions of the sample values were tested. Pearson (or Spearman) correlation test was used for correlation analysis. To compare the demographic data between ADHD and control groups, chi-square (for dichotomous variables) or t test (for continuous variables) were performed. A P value of less than 0.05 was regarded as statistically significant for all statistical tests.
Results
A total of 421 children were diagnosed with ADHD during the study period. Two children were excluded from the study because of the incomplete information. The characteristics of the subjects are summarized in Table 1. The mean age was 8.8 years, with a standard deviation of 2.1 (they ranged from 6.3–15.17 years), and most (92.1%, n = 386) were in primary school. The sample consisted mainly of children with combined presentation (50.6%, n = 80) of ADHD. There were more boys (72.8%, n = 305) than girls. There were no statistical differences between the ADHD and normal control groups for the demographic information.
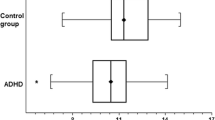
The whole blood levels of Zn, Fe, Cu, Mg, and Pb in the ADHD and normal control groups are shown in Table 2. The level of Zn was significantly lower in the ADHD group than that in the normal control one (P < 0.001). For other biochemical parameters, no statistical differences were observed. After adjusting BMI z-score, the trends still remained. In addition, the information of the number out of normal ranges (NON) in every group was collected. The number out of normal ranges of the element of zinc was much more in ADHD group than that in normal control group (P = 0.015). Further analysis of comparison were performed according to different age groups of childhood (6–11 years old) and adolescent (12–16 years old). Table 3 showed the results. Statistical differences of zinc levels and NON were also found between ADHD group and normal controls aged 6–11 years.
Furthermore, the relationships between levels of Zn, Fe, Cu, Mg, and Pb and variables of gender, age, BMI z-score, and ADHD symptoms in ADHD children were assessed. Statistical analysis showed moderate correlations between zinc level and inattention subscale score (r = − 0.40), and weak correlation between zinc level and total score of SNAP-IV (r = − 0.24). For other elements, no significant relationship was indicated between them and BMI-z score, subscales score and total score of SNAP-IV.
Discussion
Up to date, many research studies have been designed for elucidating if the trace elements were altered in the subjects with ADHD [19, 20, 23, 24, 46]. Some studies indicated the lower level of elements in children with ADHD compared with normal controls [19, 23, 24], although others did not [10, 47]. There were no consistent conclusions so far. But researchers still keep the increasing interest about understanding the participation of trace elements in the pathophysiology of ADHD.
In the present investigation, we found mean zinc level was much lower in the ADHD group than that in the controls. The results were consistent with other literature. Bekaroglu et al. found that serum zinc was greatly lower in children with ADHD than that in healthy ones (60.6 ± 9.9 vs. 105.8 ± 13.2 μg/dl) [15]. Zhou et al. found that blood zinc level was negatively related to the symptoms of ADHD [24]. In a meta-analysis including 2177 Chinese children with ADHD in 17 studies, Sun and colleagues concluded that serum zinc levels may be associated with susceptibility to ADHD in Chinese children [21]. All of these researches and our present one indicated that zinc level was inversely associated with ADHD.
Furthermore, because of the broad age range from 6 to 16 years, further analysis were done according to the subgroup of childhood (6–11 years old) and adolescent (12–16 years old), respectively. For the childhood group, similar results were found as the above results. But no difference was found for the zinc levels between 2 groups for children aged 12–16 years. One noted point is there is a difference in numerical values of zinc levels between ADHD and control groups, but no statistical difference. It is speculated that the small sample size of adolescents group may contribute to this result. Large sample-size study including more adolescents is warranted.
To draw more powerful conclusion, we did the statistical analysis of comparing elemental levels between two groups after controlling the factor of BMI z-score. As was well known, the elemental status was greatly affected by the nutritional condition. One study found that serum trace elements were changed by the factor of BMI z-score [48]. In our study, BMI z-score was higher in the ADHD group than that in the normal controls, although with a statistical value at gray level (P = 0.061). However, the difference for zinc levels still remained when adjusting the confounding factor of BMI z-score. Moreover, the number of out of normal ranges for Zn was another parameter indicating zinc deficiency in ADHD. The NON was much larger in the ADHD group, which could also confirm that lower Zn was closely related to ADHD. It was suggested that low zinc level played an important role in ADHD by both the methods.
In addition, the relation between low zinc level and ADHD was supported by correlation analysis. Our results revealed that Zn was inversely related to total scores and inattention subscale score of ADHD symptoms. This is another proof that zinc plays an important role in the ADHD manifestation. The study [23] by Viktorinova and colleagues indicated that the level of zinc was significantly related to ADHD symptoms. Our results were consistent with a study by Arnold et al. that showed lower serum zinc levels were associated with greater parent and teacher ratings of inattention (r = − 0.45) in children with ADHD [16]. However, the exact mechanism of how zinc might contribute to ADHD is not clear. But there were several potential pathways: (1) zinc is a cofactor for more than 300 enzymes and is involved in the pathway for the body’s production of prostaglandins and neurotransmitters, including norepinephrine [49]; (2) zinc has the assistant effects on regulating melatonin which is an important factor in the pathophysiology of ADHD due to its modulation of dopamine [50, 51]; (3) zinc also can bind to and regulate the dopamine transporter, which is the target of psychostimulants treating ADHD [50]. From this point of view, zinc is recommended in RDA/RDI dosages as part of a balanced vitamin/mineral supplement, which is a safe and costly effective intervention in ADHD treatment [52].
Although other elements have been reported to be associated with ADHD, no statistically differences were found in the present study. For heave metal of lead, the exposure was commonly considered as a high environmental risk factor for the development of ADHD [53]. But the lead levels in two groups were comparable and both in normal ranges, which meant lead exposure was not correlated with ADHD in this group of children. Further large-scale study is warranted to confirm it.
Strength and Limitations
The first strength was the collected information including the nutritional information of BMI z-score, which can exclude the influence of nutrition on the trace element status. The second was the large sample size, which could minimize the selective bias. Moreover, the scores of ADHD symptoms of children were used to do the relationship analysis between ADHD, BMI, and trace elements. These strengths would make this investigation more powerful. However, there were limitations for it. Firstly, this is a cross-sectional survey that makes it difficult to infer a relationship between trace elements and ADHD symptoms. These collected data do not allow us to speculate on potential developmental periods of susceptibility to zinc deficiency. In addition, the duration of low zinc level in ADHD children was unknown. Secondly, data of dietary intake of Zn were not collected. But in our design, BMI z-score was matched between two groups, which could exclude the confounding factors of nutritional status that affected zinc status. Or, it is also a good alternative method to measure the bioelements in hair as the research by Tippairote and colleagues [46]. Thirdly, we only studied the most common four elements routinely measured in clinical practice. These limitations should be taken into consideration when the results interpreted.
Conclusion
Our study shows that blood zinc levels are lower in patients with ADHD than those in normal controls, and zinc levels are inversely related to inattention subscale of ADHD, which suggests that zinc is correlated with ADHD. In this study, we failed to find a correlation between other trace elements and ADHD.
References
Verma R, Balhara YP, Mathur S (2011) Management of attention-deficit hyperactivity disorder. J Pediatr Neurosci 6(1):13–18. https://doi.org/10.4103/1817-1745.84400
Biederman J (2005) Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 57(11):1215–1220. https://doi.org/10.1016/j.biopsych.2004.10.020
Akinbami LJ, Liu X, Pastor PN, Reuben CA (2011) Attention deficit hyperactivity disorder among children aged 5-17 years in the United States, 1998-2009. NCHS Data Brief 70:1–8
Wolraich ML (2006) Attention-deficit hyperactivity disorder. Semin Pediatr Neurol 13(4):279–285. https://doi.org/10.1016/j.spen.2006.09.008
Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE (2012) A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med 10:99. https://doi.org/10.1186/1741-7015-10-99
Reale L, Bartoli B, Cartabia M, Zanetti M, Costantino MA, Canevini MP, Termine C, Bonati M (2017) Comorbidity prevalence and treatment outcome in children and adolescents with ADHD. Eur Child Adolesc Psychiatry 26:1443–1457. https://doi.org/10.1007/s00787-017-1005-z
Maia CR, Cortese S, Caye A, Deakin TK, Polanczyk GV, Polanczyk CA, Rohde LA (2017) Long-term efficacy of methylphenidate immediate-release for the treatment of childhood ADHD. J Atten Disord 21(1):3–13. https://doi.org/10.1177/1087054714559643
Barkley RA, Fischer M, Smallish L, Fletcher K (2002) The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 111(2):279–289
Li Y, Hasenhuetl PS, Schicker K, Sitte HH, Freissmuth M, Sandtner W (2015) Dual action of Zn2+ on the transport cycle of the dopamine transporter. J Biol Chem 290(52):31069–31076. https://doi.org/10.1074/jbc.M115.688275
Konofal E, Lecendreux M, Arnulf I, Mouren MC (2004) Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 158(12):1113–1115. https://doi.org/10.1001/archpedi.158.12.1113
Arnold LE, Pinkham SM, Votolato N (2000) Does zinc moderate essential fatty acid and amphetamine treatment of attention-deficit/hyperactivity disorder? J Child Adolesc Psychopharmacol 10(2):111–117
Pifl C, Wolf A, Rebernik P, Reither H, Berger ML (2009) Zinc regulates the dopamine transporter in a membrane potential and chloride dependent manner. Neuropharmacology 56(2):531–540. https://doi.org/10.1016/j.neuropharm.2008.10.009
Sandstead HH, Penland JG, Alcock NW, Dayal HH, Chen XC, Li JS, Zhao F, Yang JJ (1998) Effects of repletion with zinc and other micronutrients on neuropsychologic performance and growth of Chinese children. Am J Clin Nutr 68(2 Suppl):470s–475s
Arnold LE, DiSilvestro RA (2005) Zinc in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 15(4):619–627. https://doi.org/10.1089/cap.2005.15.619
Bekaroglu M, Aslan Y, Gedik Y, Deger O, Mocan H, Erduran E, Karahan C (1996) Relationships between serum free fatty acids and zinc, and attention deficit hyperactivity disorder: a research note. J Child Psychol Psychiatry 37(2):225–227
Arnold LE, Bozzolo H, Hollway J, Cook A, DiSilvestro RA, Bozzolo DR, Crowl L, Ramadan Y, Williams C (2005) Serum zinc correlates with parent- and teacher- rated inattention in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 15(4):628–636. https://doi.org/10.1089/cap.2005.15.628
Kiddie JY, Weiss MD, Kitts DD, Levy-Milne R, Wasdell MB (2010) Nutritional status of children with attention deficit hyperactivity disorder: a pilot study. Int J Pediatr 2010:767318–767317. https://doi.org/10.1155/2010/767318
Oner O, Oner P, Bozkurt OH, Odabas E, Keser N, Karadag H, Kizilgun M (2010) Effects of zinc and ferritin levels on parent and teacher reported symptom scores in attention deficit hyperactivity disorder. Child Psychiatry Hum Dev 41(4):441–447. https://doi.org/10.1007/s10578-010-0178-1
Mahmoud MM, El-Mazary AA, Maher RM, Saber MM (2011) Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital J Pediatr 37:60. https://doi.org/10.1186/1824-7288-37-60
Villagomez A, Ramtekkar U (2014) Iron, magnesium, vitamin D, and zinc deficiencies in children presenting with symptoms of attention-deficit/hyperactivity disorder. Children (Basel) 1(3):261–279. https://doi.org/10.3390/children1030261
Sun GX, Wang BH, Zhang YF (2015) Relationship between serum zinc levels and attention deficit hyperactivity disorder in children. Zhongguo Dang Dai Er Ke Za Zhi 17(9):980–983
Salehi B, Mohammadbeigi A, Sheykholeslam H, Moshiri E, Dorreh F (2016) Omega-3 and zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J Res Pharm Pract 5(1):22–26. https://doi.org/10.4103/2279-042x.176561
Viktorinova A, Ursinyova M, Trebaticka J, Uhnakova I, Durackova Z, Masanova V (2016) Changed plasma levels of zinc and copper to zinc ratio and their possible associations with parent- and teacher-rated symptoms in children with attention-deficit hyperactivity disorder. Biol Trace Elem Res 169(1):1–7. https://doi.org/10.1007/s12011-015-0395-3
Zhou F, Wu F, Zou S, Chen Y, Feng C, Fan G (2016) Dietary, nutrient patterns and blood essential elements in Chinese children with ADHD. Nutrients 8(6). https://doi.org/10.3390/nu8060352
Kirby K, Floriani V, Bernstein H (2001) Diagnosis and management of attention-deficit/ hyperactivity disorder in children. Curr Opin Pediatr 13(2):190–199
Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (2012) Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatry 51(10):1003–1019.e1020. https://doi.org/10.1016/j.jaac.2012.08.015
Ghanizadeh A, Berk M (2013) Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr 67(1):122–124. https://doi.org/10.1038/ejcn.2012.177
Bilici M, Yildirim F, Kandil S, Bekaroglu M, Yildirmis S, Deger O, Ulgen M, Yildiran A, Aksu H (2004) Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 28(1):181–190. https://doi.org/10.1016/j.pnpbp.2003.09.034
Arnold LE, Disilvestro RA, Bozzolo D, Bozzolo H, Crowl L, Fernandez S, Ramadan Y, Thompson S, Mo X, Abdel-Rasoul M et al (2011) Zinc for attention-deficit/hyperactivity disorder: placebo-controlled double-blind pilot trial alone and combined with amphetamine. J Child Adolesc Psychopharmacol 21(1):1–19. https://doi.org/10.1089/cap.2010.0073
Bener A, Kamal M, Bener H, Bhugra D (2014) Higher prevalence of iron deficiency as strong predictor of attention deficit hyperactivity disorder in children. Ann Med Health Sci Res 4(Suppl 3):S291–S297. https://doi.org/10.4103/2141-9248.141974
Doom JR, Georgieff MK, Gunnar MR (2015) Institutional care and iron deficiency increase ADHD symptomology and lower IQ 2.5-5 years post-adoption. Dev Sci 18(3):484–494. https://doi.org/10.1111/desc.12223
Sarris J, Kean J, Schweitzer I, Lake J (2011) Complementary medicines (herbal and nutritional products) in the treatment of attention deficit hyperactivity disorder (ADHD): a systematic review of the evidence. Complement Ther Med 19(4):216–227. https://doi.org/10.1016/j.ctim.2011.06.007
Hariri M, Azadbakht L (2015) Magnesium, Iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med 6:83. https://doi.org/10.4103/2008-7802.164313
Kozielec T, Starobrat-Hermelin B, Kotkowiak L (1994) Deficiency of certain trace elements in children with hyperactivity. Psychiatr Pol 28(3):345–353
Starobrat-Hermelin B (1998) The effect of deficiency of selected bioelements on hyperactivity in children with certain specified mental disorders. Ann Acad Med Stetin 44:297–314
Nogovitsina OR, Levitina EV (2007) Neurological aspects of the clinical features, pathophysiology, and corrections of impairments in attention deficit hyperactivity disorder. Neurosci Behav Physiol 37(3):199–202. https://doi.org/10.1007/s11055-007-0001-z
Kul M, Kara M, Unal F, Tuzun Z, Akbiyik F. Serum copper and ceruloplasmin levels in children and adolescents with attention deficit hyperactivity disorder. Klin Psikofarmakol B 24(2):7
Irmisch G, Thome J, Reis O, Hassler F, Weirich S (2011) Modified magnesium and lipoproteins in children with attention deficit hyperactivity disorder (ADHD). World J Biol Psychiatry 12(Suppl 1):63–65. https://doi.org/10.3109/15622975.2011.600292
Kim Y, Cho SC, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, Bhang SY (2010) Association between blood lead levels (<5 mug/dL) and inattention-hyperactivity and neurocognitive profiles in school-aged Korean children. Sci Total Environ 408(23):5737–5743. https://doi.org/10.1016/j.scitotenv.2010.07.070
Nigg JT, Knottnerus GM, Martel MM, Nikolas M, Cavanagh K, Karmaus W, Rappley MD (2008) Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry 63(3):325–331. https://doi.org/10.1016/j.biopsych.2007.07.013
Wang HL, Chen XT, Yang B, Ma FL, Wang S, Tang ML, Hao MG, Ruan DY (2008) Case-control study of blood lead levels and attention deficit hyperactivity disorder in Chinese children. Environ Health Perspect 116(10):1401–1406. https://doi.org/10.1289/ehp.11400
Nigg JT, Nikolas M, Mark Knottnerus G, Cavanagh K, Friderici K (2010) Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry 51(1):58–65. https://doi.org/10.1111/j.1469-7610.2009.02135.x
Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS (2009) Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics 124(6):e1054–e1063. https://doi.org/10.1542/peds.2009-0738
Ha M, Kwon HJ, Lim MH, Jee YK, Hong YC, Leem JH, Sakong J, Bae JM, Hong SJ, Roh YM, Jo SJ (2009) Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children's health and environment research (CHEER). Neurotoxicology 30(1):31–36. https://doi.org/10.1016/j.neuro.2008.11.011
Qi L, Song Y, He N (2005) The development of professional medicinal instrument for trace elements inspection of blood - MB5 and MG2 multi-channel AAS. Mod Sci Instrum 21(04):12–16
Tippairote T, Temviriyanukul P, Benjapong W, Trachootham D (2017) Hair zinc and severity of symptoms are increased in children with attention deficit and hyperactivity disorder: a hair multi-element profile study. Biol Trace Elem Res 179(2):185–194. https://doi.org/10.1007/s12011-017-0978-2
Donfrancesco R, Parisi P, Vanacore N, Martines F, Sargentini V, Cortese S (2013) Iron and ADHD: time to move beyond serum ferritin levels. J Atten Disord 17(4):347–357. https://doi.org/10.1177/1087054711430712
Azab SF, Saleh SH, Elsaeed WF, Elshafie MA, Sherief LM, Esh AM (2014) Serum trace elements in obese Egyptian children: a case-control study. Ital J Pediatr 40:20. https://doi.org/10.1186/1824-7288-40-20
Rink L, Gabriel P (2000) Zinc and the immune system. Proc Nutr Soc 59(4):541–552
Lepping P, Huber M (2010) Role of zinc in the pathogenesis of attention-deficit hyperactivity disorder: implications for research and treatment. CNS Drugs 24(9):721–728. https://doi.org/10.2165/11537610-000000000-00000
Chen MD, Lin PY, Sheu WH (1999) Zinc coadministration attenuates melatonin's effect on nitric oxide production in mice. Biol Trace Elem Res 69(3):261–268. https://doi.org/10.1007/bf02783878
Arnold LE, Hurt E, Lofthouse N (2013) Attention-deficit/hyperactivity disorder: dietary and nutritional treatments. Child Adolesc Psychiatr Clin N Am 22(3):381–402. https://doi.org/10.1016/j.chc.2013.03.001
Ji Y, Hong X, Wang G, Chatterjee N, Riley AW, Lee LC, Surkan PJ, Bartell TR, Zuckerman B, Wang X (2018) A prospective birth cohort study on early childhood lead levels and attention deficit hyperactivity disorder: new insight on sex differences. J Pediatr. https://doi.org/10.1016/j.jpeds.2018.03.076
Acknowledgments
We thank the children and their parents to participate our study. We also thank Miss Lili Yang for her copyediting our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Institutional Review Board of the Children’s Hospital, Zhejiang University School of Medicine.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yang, R., Zhang, Y., Gao, W. et al. Blood Levels of Trace Elements in Children with Attention-Deficit Hyperactivity Disorder: Results from a Case-Control Study. Biol Trace Elem Res 187, 376–382 (2019). https://doi.org/10.1007/s12011-018-1408-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1408-9