Abstract
The aim of this study was to evaluate the protective effects of SeY (selenium-rich yeast) against Al (aluminum)-induced inflammation and ionic imbalances. Male Kunming mice were treated with Al (10 mg/kg) and/or SeY (0.1 mg/kg) by oral gavage for 28 days. The degree of inflammation was assessed by mRNA expression of inflammatory biomarkers. Ionic disorders were assessed by determining the Na+, K+, and Ca2+ content, as well as the alteration in ATP-modifying enzymes (ATPases), including Na+K+-ATPase, Ca2+-ATPase, Mg2+-ATPase, Ca2+Mg2+-ATPase, and the mRNA levels of ATPase’s subunits in kidney. It was observed here that SeY exhibited a significant protective effect on the kidney against the Al-induced upregulation of pro-inflammatory and downregulation of anti-inflammatory cytokines. Furthermore, a significant effect of Al on the Na+, K+, Ca2+, and Mg2+ levels in kidney was observed, and Al was observed to decrease the activities of Na+K+-ATPase, Mg2+-ATPase, and Ca2+Mg2+-ATPase. The mRNA expression of the Na+K+-ATPase subunits and Ca2+-ATPase subunits was regulated significantly by Al. Notably, SeY modulated the Al-induced alterations of ion concentrations, ATPase activity, and mRNA expression of their subunits. These results suggest that SeY prevents renal toxicity caused by Al via regulation of inflammatory responses, ATPase activities, and transcription of their subunits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is the third most abundant element and is known to have negative effects that exceed the beneficial effects. In trace amounts, Al presents a toxic risk potential [1]. Al enters into the human body through drinking water, food, the use of utensils, deodorants, and as components of drugs [2]. Aluminum has gained substantial interest due to its increased bioavailability and negative effects on human health [3]. The toxic consequences of Al exposure have been linked to the dysregulation of other essential metals and ions as well as through the deposition of insoluble Al precipitates in vulnerable tissues [4]. The toxic effects of Al to mammals (both human and experimental animals) have been widely reported, including increased lipid peroxidation (LPO), oxidative stress, defective calcium homeostasis, and altered inflammatory responses [5, 6]. Although our understanding of the mechanisms toxicological responses in critical organs to Al has improved vastly, the mechanisms have not been completely elucidated. The impacts of Al on the kidneys, particularly with respect to disturbances in ionic homeostasis, are less clear.
Ionic pumps are essential for the maintenance of normal cellular functioning. Proper ion concentrations are required for transmembrane ionic balance, membrane potential, pH balance, and the maintenance of the volume of the cell to ensure proper functioning of systems throughout the entire body. ATPases are a group of membrane-bound enzymes responsible for the transport of cations across cellular membranes, the maintenance of intracellular functions, and are widely used as markers for ion regulatory changes [7, 8].
Aluminum toxicity has been attributed to its ability to alter the cellular concentrations of some ions, and the balance of others. It has been reported that Al inhibits the release of Ca2+ from the sarcoplasmic reticulum in myocytes [9]. It has been reported that increasing the concentration of Al resulted in decreased Ca2+Mg2+ ATPase activities in frog muscle [10]. Furthermore, the activity of Na+K+-ATPase has been demonstrated to be regulated by Al in Atlantic salmon [11]. Proper ionic balance plays a critical role in kidney function, and the renal toxicity of Al has been clearly established [12]. Previous studies have also demonstrated that in an inflammatory environment, Na+ and Ca2+ concentrations were abnormal [8]. However, the relationship between kidney ionic disorders and Al-induced renal toxicity remains unclear.
There are a number of potential mechanisms through which selenium (Se) supplementation of feed may protect against oxidative stress and inflammation, acting as the first line of defense against oxidant [13,14,15,16]. Se was responsive to stress, involved in cell immunity, a specific target for heavy metal [17,18,19]. Furthermore, Se is likely effective in limiting morbidity from chronic diseases, including common forms of renal disease [20, 21]. These effects are not only dependent upon the amount of Se, but the speciation of Se as well [22]. Selenium-enriched yeast (SeY) is a potential source of high-quality organic Se for animals. Of the total Se found in SeY products, 97–99% are organic Se. Studies have indicated that the bioavailability of the Se contained in SeY is approximately 1.4–2-fold higher than that of inorganic forms of Se [22]. Experimental poultry models suggest that Se plays an important role in the modulation of tissue damage resulting from inflammation and can also regulate the activity of Na+K+ and Ca2+Mg2+-ATPases [23, 24]. However, little is known about the protective effects of SeY on renal function through the maintenance of ionic equilibria or the reduction in inflammation during exposure to Al resulting in renal toxicity.
Therefore, the aims of the study were to determine the protective efficacy of SeY on Al-induced renal inflammation and to assess whether ion-transporting ATPases were involved in these effects.
Materials and Methods
Compliance with Ethical Standards
All animal studies reported here were approved by the Institutional Animal Care and Use Committee of the Foshan University.
Animals and Experimental Design
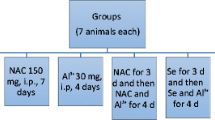
A total of 40 four-week-old male Kunming mice (18–23 g) were allowed a 7-day period for acclimation to their housing prior to initiation of the study. The animals were housed at constant temperature of 22 ± 2 °C, with a relative humidity of 50 ± 15%, and a 12-h light/dark cycle. Beginning at day 0, body weights were measured daily, and the consumption of drinking water and feed (provided ad libitum) were measured daily. Mice were individually housed in polycarbonate cages. SeY and Al were purchased from Angel Yeast Co., Ltd. and Aladdin Chemical Co., Ltd. Prior to initiation of the experiment, baseline levels of Se and Al were measured. The results showed the contents of Se and Al were 0.102 and 0 mg/kg, respectively. Previously, the study used 10 mg/kg/day as toxic dosages [25]. Therefore, to study the effect of Al on mice, we treated animals by administering Al at a dose of 10 mg/kg/day. The dosage of SeY is also from reference [26].
Mice were divided into four groups as follows:
-
Group 1: (C) autoclaved water twice daily for 28 days (n = 8).
-
Group 2: (SeY) autoclaved water with 0.1 mg/kg SeY for 28 days (n = 10).
-
Group 3: (Al) autoclaved water with 10 mg/kg of Al for 28 days (n = 10).
-
Group 4: (SeY + Al) autoclaved water with 10 mg/kg of Al and 0.1 mg/kg SeY for 28 days (n = 12).
The mice were monitored daily for clinical signs and total body weight gain. At the end of the experiment, mice were fasted before the day of sacrifice, and the kidneys were carefully dissected out, hold in − 80 °C after weighing.
Renal Ionic Concentration
On necropsy day, the kidneys were excised on an ice-cold plate and were then immediately washed in physiological saline solution. Concentrations of Na+, K+, Ca2+, and Mg2+ in tissues were measured with detection kits.
Renal ATPase Activity Assays
The activities of the Na+K+-ATPase, Ca2+-ATPase, Mg2+-ATPase, and Ca2+Mg2+-ATPase were measured using the appropriate assay kits according to the manufacturer’s instructions. The activities of the Na+K+-ATPase, the Ca2+-ATPase, and the Mg2+-ATPase were measured by quantifying the inorganic phosphorus (Pi) production from the conversion of ATP into ADP. We then expressed enzymatic activity as units per milligram of protein.
qRT-PCR Analysis
Total RNA was extracted from vein tissues and cell samples, and the complementary DNA was synthesized using a RevertAid first strand cDNA synthesis kit. Gene expression levels were measured by qRT-PCR using a Light Cycler® 480 System and the Fast Universal SYBR Green Master Mix. Primer Analysis Software was used to design target-specific oligonucleotide primers. The primers were commercially synthesized by the Beijing Genomics Institute Co., Ltd., China. Primers used in the experiments are shown in Tab. S1. The relative abundances of mRNA for each gene were calculated using the 2−ΔΔCt method, accounting for gene-specific efficiency, and was normalized to the mean expression of GAPDH.
Statistical Analysis
GraphPad Prism 7.0 (GraphPad Software Inc., USA), Microsoft Office Excel 2010, and SPSS 20 were used to test the effects of the dietary SeY and Al levels on measures. Multiple mean comparisons were performed using one-way ANOVA. Data are presented as the means ± S.D. and values were considered statistically significant if P < 0.05.
Results
Effect of SeY and/or Al on the Body and Kidney Weights
The body and kidney weights of the Al-treated animals were reduced but were observed to have increased in the SeY-treated group, compared to the C group (P < 0.05). When mice were treated with SeY + Al, the body and kidney weights were observed to be similar to those of control animals (Table 1).
Effect of SeY and/or Al on the mRNA Expression of Mediators of Inflammation
To determine whether Al and/or SeY affect the inflammation processes, mRNA expression of ten mediators of inflammation was assessed. As presented in Fig. 1, the relative abundances of the mRNA of inflammation mediators (IL-1β, IL-6, TNF-α, TNF-R1, TNF-R2, COX-2, NF-κB, HO-1, and Nrf2) were significantly increased (P < 0.05) in the Al group compared to the C group. Expression of Keap1 decreased 18.6% (P < 0.05) in the Al group compared to the C group. Interestingly, dietary supplementation with SeY alone did not result in a measurable effect. In contrast, in animals receiving both SeY + Al, the addition of SeY resulted in marked improvements to the inflammatory responses caused by Al, restoring the mRNA expression of the mediators of inflammation to normal levels.
Effect of SeY and/or Al on Na+, K+ Concentrations, Na+K+-ATPase Activity, and mRNA Expression of ATPase Subunits
As presented in Fig. 2, the concentration of Na+ was significantly increased in the Al group, which was restored to normal levels in the SeY + Al group. Similarly, K+ concentrations decreased in the Al group and were restored to normal levels in animals receiving both SeY + Al (P < 0.05). The concentrations of Na+ and K+ were not significantly altered in animals receiving Se alone. The activity of Na+K+-ATPase was significantly increased in both the Al and SeY + Al groups (P < 0.05). The mRNA levels of Na+K+-ATPase subunits (1a1 and 1b1) were significantly increased in the Al group compared with the C group. However, in the SeY + Al group, a moderation in the activity was observed, albeit at a higher level than in the C group (P < 0.05). These results indicate that the transcription of Na+K+-ATPase subunits may be regulated by the SeY pretreatment, thus attenuating the Al-induced effects on Na+K+-ATPase.
Effect of SeY and/or Al on Ca2+ Concentrations, Ca2+-ATPase Activity, and mRNA Expression of ATPase’s Subunits
As presented in Fig. 3, the concentrations of Ca2+ ions were significantly increased in the Al group. Pretreatment with SeY was able to return the Ca2+levels to within normal ranges, compared with the C group. In the present study, alterations in the activity of renal Ca2+-ATPase following SeY and/or Al exposure were observed. A statistically significant decrease in the activity of Ca2+-ATPase was observed after animals were fed a diet supplemented with Al (P < 0.05).
The expression of Ca2+-ATPase subunits (2a3, 2b1, and 2b2) mRNA was significantly reduced in Al-exposed mice, and the transcriptions of 2c1 were significantly increased in the Al group (P < 0.05). When the mice were provided a diet supplemented with SeY, the expression of Ca2+-ATPase (2a3, 2b1, 2b2, and 2c1) subunit mRNAs remained at basal levels.
Effect of SeY and/or Al on Mg 2+ Ion Concentrations and the Activity of Mg2+-ATPase and Ca2+Mg2+-ATPase
As presented in Fig. 4, the concentrations of Mg2+ ions, Mg2+ATPase, and Ca2+Mg2+ATPase activities were significantly decreased in the Al group, whereas the same parameters were maintained at normal levels in the SeY + Al group. These results suggest that the addition of dietary SeY is able to maintain the activities of Ca2+Mg2+-ATPase and Mg2+-ATPase which could be altered by the toxic effects of Al.
Discussion
In the present study, exposure to Al disturbed the mRNA expression of mediators of inflammation, including IL-1β, IL-6, TNF-α, TNF-R1, TNF-R2, COX-2, NF-κB, HO-1, Keap1, and Nrf2. In addition, Al exposure also interfered with the activities of Na+, K+, Ca2+, and Mg2+ATPase; altered the concentrations of Na+, K+, Ca2+, and Mg2+; and altered the expression of mRNA ion-ATPase subunits. Dietary supplementation with SeY returned all these biochemical parameters to baseline levels after exposure to Al. These results indicated that Al exposure results in renal dysfunction, promotes inflammation, and alters ion metabolism. In contrast, dietary supplementation with SeY relieved these pathologies. However, the mechanism through which SeY affords the chemopreventive characteristics against Al exposure remains elusive.
The kidneys are responsible for the elimination of drugs and metabolites from the bloodstream. It has been demonstrated that organ toxicity induced by chemical agents is one consequence of the accumulation of certain metabolites in the kidneys. It has been reported that the renal damage caused by Al occurs through mechanisms such as oxidative stress and ATPase metabolism dysregulation [27, 28]. Studies in human Jurkat and lung carcinoma cells have shown that Se supplementation of macrophages decreases the expression of COX-2 via the inactivation of NF-κB. Furthermore, previous studies have also demonstrated the anti-inflammatory effects of Se [29, 30]. In the present study, the changes in biochemical indicators including markers of inflammation, ion concentrations, ATPase activities, and the mRNA expression of ATPase subunits were observed in Al-exposed and SeY-treated mice.
According to previous studies, Al can induce inflammatory responses in multiple tissues and cell lines [6, 31, 32]. Additionally, accumulation of Al was detected in kidneys [33], suggesting that the organ is likely involved in the metabolism of Al. In the present study, our results demonstrated a pronounced inflammatory response in mice exposed to Al. The inflammation observed in Al-exposed mice was mitigated when the mice diets were concurrently supplemented with SeY. It has been reported that exposure to Al causes an inflammatory reaction by regulating inflammatory factors [34]. In this study, the data showed that Al caused the dysregulation of pro-inflammatory (TNF-α, IL-6, COX-2, and IL-1β) mediators. Moreover, we demonstrated that SeY had a protective role against Al-induced inflammation in mouse kidneys. These observations recorded here were similar to the previously published research [34, 35]. Therefore, it can be hypothesized that Al-induced kidney injury occurs though the activation of pro-inflammatory pathways.
Proper ionic balance is crucial for a number of physiological processes and is vital to ensure correct functioning of the entire body, especially the kidney. Therefore, the kidneys have been identified as target organs for Al-induced ionic disorders. Functioning of Na+-K+-ATPase, Mg2+-ATPase, and Ca2+-ATPase has been determined to be powerful markers of cellular impairment during toxic exposure [36]. In the kidneys, ATPases are widely distributed, but predominantly located in the basement membrane of renal tubular epithelial cells, and aid in the transport of sodium, potassium, calcium, amino acids, and other substances. ATPase is the main source of renal tubular reabsorption and material exchange, but also to maintain the stability of the internal environment. In a previous report by Seven et al., the authors demonstrated that Na+K+-ATPase activity was decreased in guinea pig kidneys when exposed to LPS [37]. In another study, vitamin E treatment prevented the diabetes-induced increase in Ca2+-ATPase activity [38]. In the present study, significant ionic imbalances were observed in the kidneys of mice. The activities of the Na+-K+-ATPase, Mg2+-ATPase, and Ca2+-ATPase were significantly decreased following exposure to Al. Furthermore, the concentrations of Na+, K+, Ca2+, and Mg2+ were also altered following Al exposure. The decrease of Na+K+-ATPase activity resulted in an increase of Na+ levels, which served to further raise the exchange of Na+ and Ca2+. This in turn resulted in increased intracellular Ca2+ levels. As a result, this imbalance causes tan overload of Ca2+, leaving Ca2+ATPase unable to keep up with the cellular Ca2+ transport needs, causing the perceived decrease of Ca2+-ATPase activity. Conversely, the decreased Ca2+-ATPase activity results in the accumulation of Ca2+. As activator of Na+K+-ATPase, the decrease of Mg2+ similarly corresponded with the measured decrease of Na+K+-ATPase. This resultant cellular electrophysiological deficiency induces ion imbalance, leading to cellular dysfunction and tissue damage. With the treatment of SeY, the activities of ATPases appeared, to varying degrees, to return to normal. This observation suggests that SeY can protect cells from the imbalance of ATPase metabolism caused by Al exposure. Moreover, mRNA expression of ion-ATPase subunits (1a1, 1b1, 2a3, 2b1, 2b2, and 2c1) significantly changed following Al exposure. These data suggest that Al-mediated renal toxicity in mice occurred through the imbalance of ATPase activities and subunit transcription, as well as through imbalance in ion levels. Interestingly, the ATPase activities, ion contents, and ion subunits returned to normal following SeY treatment, which implies the protective efficacy of SeY. In a recently published report by Silva et al., the authors demonstrated that the reduction of Na+K+-ATPase activity is correlated with increasing dosage of Al [39]. In another study, it was reported that the activities of Na+K+-ATPase, Mg2+-ATPase, and Ca2+-ATPase all decreased in rats exposed to AlCl3 [40]. Our results reported here are consistent with previously published research, suggesting that dietary intake of SeY may have protective effects against renal toxicity caused by Al.
In conclusion, the present study suggests that Al causes a renal inflammatory reaction, regulating the activities of ATPase and the transcription of their subunits. We also demonstrated that SeY protects kidneys against Al-induced tissue toxicity. This study provides novel insights into the inflammatory responses and ionic imbalances caused by Al exposure, and a potential protective role for dietary supplementation with SeY.
References
Muselin F, Dumitrescu E, Cristina R, Doma A, Trif A (2015) Protective effect of melatonin on aluminum accumulation in some organs of rats. Istanbul Univ Vet Fak Dergisi 41:26–30
Nampoothiri M, John J, Kumar N et al (2015) Modulatory role of simvastatin against aluminium chloride-induced behavioural and biochemical changes in rats. Behav Neurol 2015:1–9
Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev 10:1–269
Maya S, Prakash T, Madhu KD, Goli D (2016) Multifaceted effects of aluminium in neurodegenerative diseases: a review. Biomed Pharmacother 83:746–754
Umesalma SAS (2015) Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain—histopathological, and biochemical approach. Journal of Molecular Biomarkers J & Diagnosis 6:1
Ligi D, Santi M, Croce L, Mannello F (2015) Aluminum induces inflammatory and proteolytic alterations in human monocytic cell line. J Inorg Biochem 152:190–198
Lin J, Li HX, Qin L, du ZH, Xia J, Li JL (2016) A novel mechanism underlies atrazine toxicity in quails (Coturnix Coturnix coturnix): triggering ionic disorder via disruption of ATPases. Oncotarget 7:83880–83892
Lin J, Zhao HS, Xiang LR, Xia J, Wang LL, Li XN, Li JL, Zhang Y (2016) Lycopene protects against atrazine-induced hepatic ionic homeostasis disturbance by modulating ion-transporting ATPases. J Nutr Biochem 27:249–256
Bohuts’Ka KI, Iui PK, Nozdrenko DM (2014) The use of aluminum and its compounds for the biomedical purposes. Fiziol Zh 60:91–97
Nozdrenko DM, Abramchuk OM, Soroca VM, Miroshnichenko NS (2015) Aluminum chloride effect on Ca2+, Mg(2+)-ATPase activity and dynamic parameters of skeletal muscle contraction. Ukr Biochem J 87:38–45
Nilsen TO, Ebbesson LOE, Kverneland OG et al (2010) Effects of acidic water and aluminum exposure on gill Na+, K+-ATPase alpha -subunit isoforms, enzyme activity, physiology and return rates in Atlantic salmon (Salmo salar L.) Aquat Toxicol 97:250–259
Liu J, Wang Q, Sun X, Yang X, Zhuang C, Xu F, Cao Z, Li Y (2016) The toxicity of aluminum chloride on kidney of rats. Biol Trace Elem Res 173:339–344
Li X, Xing M, Chen M, Zhao J, Fan R, Zhao X, Cao C, Yang J, Zhang Z, Xu S (2017) Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol Environ Saf 139:447–453
Xing M, Jin X, Wang J, Shi Q, Cai J, Xu S (2017) The antagonistic effect of selenium on lead-induced immune dysfunction via recovery of cytokine and heat shock protein expression in chicken neutrophils. Biol Trace Elem Res 1:1–8
Jin X, Xu Z, Zhao X, Chen M, Xu S (2017) The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 180:259–266
Liu LL, Li CM, Zhang ZW, Zhang JL, Yao HD, Xu SW (2014) Protective effects of selenium on cadmium-induced brain damage in chickens. Biol Trace Elem Res 158:176–185
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta Gen Subj 1830:3112–3120
Yao L, Du Q, Yao H et al (2015) Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 28:255–265
Chen X, Zhu YH, Cheng XY, Zhang ZW, Xu SW (2012) The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17:14565–14572
Iglesias P, Selgas R, Romero S, Díez JJ (2013) Selenium and kidney disease. J Nephrol 26:266–272
Zhu SY, Li XN, Sun XC, Lin J, Li W, Zhang C, Li JL (2017) Biochemical characterization of the selenoproteome in Gallus gallus via bioinformatics analysis: structure-function relationships and interactions of binding molecules. Metallomics 9:124–131
Kubachka K, Hanley T, Mantha M et al (2016) Evaluation of selenium in dietary supplements using elemental speciation. Food Chem 218:313–320
Cao C, Zhao X, Fan R, Zhao J, Luan Y, Zhang Z, Xu S (2016) Dietary selenium increases the antioxidant levels and ATPase activity in the arteries and veins of poultry. Biol Trace Elem Res 172:222–227
Cao C, Fan R, Chen M et al (2017) Inflammatory response occurs in veins of broiler chickens treated with a selenium deficiency diet. Biol Trace Elem Res 9:1–9
Guo CH, Hsu GS, Chuang CJ, Chen PC (2009) Aluminum accumulation induced testicular oxidative stress and altered selenium metabolism in mice. Environ Toxicol Pharmacol 27:176–181
Krohn RM, Lemaire M, Negro Silva LF, Lemarié C, Bolt A, Mann KK, Smits JE (2016) High-selenium lentil diet protects against arsenic-induced atherosclerosis in a mouse model. J Nutr Biochem 27:9–15
Mahieu S, Contini MC, Gonzã lM, Millen N (2009) Melatonin reduces oxidative damage induced by aluminium in rat kidney. Toxicol Lett 190:9–15
Zhao HJ, Ning LI, Liu P (2011) Effect of deferiprone on activities of kidney ATPase and xanthine oxidase of aluminum induced mice. Food Drug 11:1
Vunta H, Davis F, Palempalli UD, Bhat D, Arner RJ, Thompson JT, Peterson DG, Reddy CC, Prabhu KS (2007) The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J Biol Chem 282:17964
Bahmani F, Kia M, Soleimani A, Mohammadi AA, Asemi Z (2016) The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: a randomised, double-blind, placebo-controlled trial. Br J Nutr 116:1–7
Zhu YZ, Liu DW, Liu ZY, Li YF (2013) Impact of aluminum exposure on the immune system: a mini review. Environ Toxicol Pharmacol 35:82–87
Alexandrov PN, Kruck TP, Lukiw WJ (2015) Nanomolar aluminum induces expression of the inflammatory systemic biomarker C-reactive protein (CRP) in human brain microvessel endothelial cells (hBMECs). J Inorg Biochem 152:210–213
Karabulutbulan O, Bayrak BB, Ardapirincci P et al (2015) Role of exogenous melatonin on cell proliferation and oxidant/antioxidant system in aluminum-induced renal toxicity. Biol Trace Elem Res 168:1–9
Campbell A, Bondy SC (2000) Aluminum induced oxidative events and its relation to inflammation: a role for the metal in Alzheimer’s disease. Cell Mol Biol (Noisy-le-Grand, France) 46:721–730
Duntas LH (2009) Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res 41:443–447
Speit G, Merk O (2002) Evaluation of mutagenic effects of formaldehyde in vitro: detection of crosslinks and mutations in mouse lymphoma cells. Mutagenesis 17:183–187
Seven I, Turkozkan N, Cimen B (2005) The effects of nitric oxide synthesis on the Na+, K(+)-ATPase activity in guinea pig kidney exposed to lipopolysaccharides. Mol Cell Biochem 271:107–112
Dogru PB, Daş EN, Ulusu NN, Bali M, Karasu C (2010) Effects of vitamin E on microsomal Ca(2+) -ATPase activity and calcium levels in streptozotocin-induced diabetic rat kidney. Cell Biochem Funct 21:177–182
Silva VS, Gonçalves PP (2014) Effect of lysine acetylsalicylate on aluminium accumulation and (Na+/K+) ATPase activity in rat brain cortex synaptosomes after aluminium ingestion. Toxicol Lett 232:167–174
Sun X, Sun H, Yu K et al (2017) Aluminum chloride causes the dysfunction of testes through inhibiting the ATPase enzyme activities and gonadotropin receptor expression in rats. Biol Trace Elem Res 9:1–9
Funding
This work was supported by Foshan University research star-up project (099/gg07037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal studies reported here were approved by the Institutional Animal Care and Use Committee of the Foshan University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 30 kb)
Rights and permissions
About this article
Cite this article
Cao, C., Luo, J., Li, X. et al. Selenium-Rich Yeast Protects Against Aluminum-Induced Renal Inflammation and Ionic Disturbances. Biol Trace Elem Res 186, 467–473 (2018). https://doi.org/10.1007/s12011-018-1324-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1324-z