Abstract
Systematic review (SR) of high-quality studies provides superior evidence, but an SR has not been conducted to evaluate the association between Keshan disease (KD) and selenium deficiency because SR was not available when KD was highly prevalent in the 1950s to 1970s. The objective of this study was to update our understanding of the etiology of KD and provide evidence for policies and strategies in KD surveillance, prevention, and control. We identified related studies by searching the CNKI, Wanfang, CQVIP, SinoMed, CMCI, PubMed, Embase, and EBSCO databases from January 1935 to April 2017. Community trials that met the inclusion criteria were included. Risk ratios (RR) with corresponding 95% confidence intervals (CI) were pooled to compare incidences between the two groups. A total of 17 articles (including 41 studies) were included. In total, the studies included 1,983,238 subjects, 683,075 of which were in experimental groups and 1,300,163 of which were in control groups. The protection rates were over 80% in 35 studies, and the overall effect (risk ratio) was 0.14 [95% CI (0.12, 0.16), P < 0.05]. Potential publication bias was observed in the funnel plots, but the results of Egger’s and Begg’s tests showed that there was no evidence of publication bias. Giving selenium supplements to the residents of KD endemic areas significantly reduced the incidence of KD. Selenium deficiency is therefore a cause of KD by the criterion of causation in modern epidemiology. Selenium should be included in the KD surveillance program. The description of “unknown cause” in the definition of KD may be inappropriate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Context
Keshan disease (KD) is an endemic cardiomyopathy that occurs only in China. Its name was derived from the first reported epidemic of the disease, which occurred in 1935 in Keshan county in the Heilongjiang province [1,2,3]. KD has been found in a wide belt zone from the northeast to the southwest in 2596 townships in 327 counties in 16 provinces in China. As the prevalence rate of KD has been at a low level for the past 10 years, the goal of KD prevention and control in the National Endemic Disease Prevention and Control Plan (2011–2015) in China was “basically eliminating Keshan disease in 90% or more counties of Keshan disease endemic areas to reach the criteria of Keshan disease elimination” [4]. However, the etiology of KD is not fully known, and the disease still exists to some extent today [5], which suggests that the etiologic factor of KD may still exist and that residents of KD endemic areas may potentially develop KD. Therefore, it is important to update our understanding of the etiology of KD. Among the etiology hypotheses for KD, selenium deficiency is the most recognized and convincing hypothesis based on the quantity and quality of evidence [6]. The findings of the observational epidemiological studies indicated that the soil and grains in endemic areas of KD were generally selenium deficient [7,8,9,10]; the levels of selenium in the head-hairs, finger nails, and blood of residents living in endemic areas were low [11, 12], and levels of GSH-Px, a seleno-protein, in residents living in endemic areas were low [13, 14]. KD has already been classified as dietary selenium deficiency in International Classification of Disease (ICD) [15]; in other words, the World Health Organization recognizes that selenium deficiency is a cause of KD. The findings of experimental epidemiological studies of selenium supplementation to residents of endemic areas demonstrated a significant reduction in KD incidence [16,17,18].
Well-conducted systematic reviews (SRs) are regarded as the best-quality evidence [19,20,21]. SRs have become increasingly popular in medicine since the 1970s [22,23,24,25]. In China, therefore, articles of a similar design type were first published in 1990 and have shown a growing trend since 2001 [26]. In modern epidemiology, the cause of a disease is defined as a factor that decreases the incidence of the disease [27]. In China, epidemiology did not develop at the same pace as it did in industrialized countries in the 1950s to1970s, which is the time during which KD was highly prevalent. SR and the concept of causation in modern epidemiology were therefore not widely used in epidemiological studies of KD in this period. On the other hand, this method is suitable for diseases with low incidences, such as KD.
In this paper, we report a SR and meta-analysis of published articles to assess the association between KD and selenium deficiency based on the definition of causation in modern epidemiology in order to update our understanding of the etiology of KD and provide quality evidence for KD surveillance, prevention, and control.
Evidence Acquisition
Data Sources
SR and meta-analysis were conducted according to the Preferred Reporting Items for SR and Meta-Analysis (PRIAMA) guidelines [28,29,30]. We searched for potentially relevant studies that were published from January 1935 to April 2017. The following search terms were used without restrictions: “selenium or Se” and “Keshan disease or KD” in the databases of CNKI (China National Knowledge Infrastructure), Wanfang (Wanfang Data Knowledge Service Platform), CQVIP (Chinese Science and Technique Journals Database), SinoMed (Service System for Chinese Biomedical Literature), CMCI (Chinese Medical Citation Index), PubMed (Public Medline), Embase (Excerpta Medical Database), and EBSCO (Elton B. Stephens Company). In addition, we complemented the search using the method of citation pearl growing.
Study Selection
Studies meeting the following criteria were included in the SR: human study articles; community trials; an experimental group and control group in each individual study; selenium supplementation as the intervention; and reported number of participants and morbidity number.
The exclusion criteria were as follows: repeated published studies in which data were included in this analysis and incomplete data.
Data Extraction and Quality Assessment
We extracted the following information from each retrieved article: article title, name of the first author, journal and publication year, sample sizes of the experimental and control groups, and case numbers, and a chi-square test was used to compare the incidence between the two groups. In addition, the protection rate (PR) was not calculated in the original studies because of the lagging development of epidemiology in this country, so we calculated this metric with the extracted data according to the following formula:
where p1 and p2 are the incidence of the control group and experimental group, respectively. The methodological quality of the studies was evaluated using the following criteria: defining the source of information (the risk of bias is low if the reference type was a survey, whereas the risk of bias is high if the reference type was a review); rationality of the research design and choice of control group; description of confounding assessments and/or controls; and completeness of data collection. The level of bias assessment was divided into three categories: high risk of bias, low risk of bias, and unclear risk of bias. Both reviewers independently evaluated the articles, and any disagreement was resolved by consensus.
Statistical Analysis
All analyses were performed using Review Manager Software (version 5.3) and STATA Software (version 12.2). Standard statistical tables and graphs were used to describe the characteristics of the surveyed subjects.
To visually assess the risk ratio (RR) and corresponding 95% confidence intervals (CI) across studies, we generated forest plots sorted by publication year. We assessed the heterogeneity of the risk ratio across studies using forest plots and the inconsistency index (I2). When heterogeneity was not obvious (I2 ≤ 50%), we chose fixed effects models to obtain pooled effect estimates across studies. In the presence of heterogeneity (I2 > 50%), random effects models were used (rather than fixed effects models) to obtain pooled effect estimates across studies. In addition, sensitivity analysis was performed to assess the stability of this meta-analysis. Finally, we used qualitative visual inspections of funnel plots and quantitative Egger’s or Begg’s tests to assess publication bias. All reported P values were two-sided, and P < 0.05was considered significant.
Evidence Synthesis
Literature Search
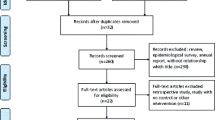
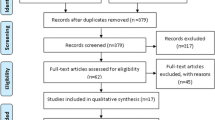
The literature search strategy identified 2230 unique citations, 164 of which were selected manually (Fig. 1). After an initial screen of titles and abstracts, we identified 250 distinct studies as potentially relevant studies for further investigation. Of these articles, 202 did not have a community trial study design, 27 studies had incomplete data, two studies did not have control groups, and two studies were duplicate publications. Thus, 17 articles (including 41 studies) ultimately met the inclusion criteria and were included in this SR and meta-analysis [16,17,18, 31,32,33,34,35,36,37,38,39,40,41,42,43,44].
Study Characteristics
The details of the included studies are shown in Table 1. Of the 17 articles, 12 (70.6%) articles (including 32 studies) were reported in the 1970s, and the studies were conducted during the peak period in which KD was highly prevalent, so the data from the studies were representative. The protection rates were between 80 and 100% in the 35 studies, and the details are shown in Table 1.
The quality of studies included in the meta-analysis was assessed by applying the above criteria. The assessments of the authors on the risk of bias of the included studies are displayed in the left panel of Fig. 2. The percentages of assessments of the authors on the risk of bias of the included studies are shown in the right panel. The data were mostly derived from retrospective studies and did not describe how confounding was assessed and/or controlled.
Summary of each quality item for each included study and graph of each quality item presented as percentages across all included studies. A, source of information; B, rationality of the research design; C, rationality for choosing a control group; D, description of confounding assessments and/or controls; E, completeness of data collection. Risk of bias summary for each included study and risk of bias graph
Meta-analysis and Sensitivity Analysis
Figure 3 is the forest plot, and it presents little statistical evidence of heterogeneity (P < 0.05, I2 = 41.8%); thus, we choose fixed effects models to obtain pooled effect estimates across studies. In total, the 41 studies included 1,983,238 subjects, 683,075 of which were in experimental groups and 1,300,163 of which were in control groups. Of the 41 studies, 16 studies (39%) had 95% confidence intervals of the risk ratio that crossed the invalid line, but their overall point estimates were < 1.0. The overall effect was 0.14 (95% CI 0.12 to 0.16). In addition, we performed sensitivity analysis by excluding individual studies sequentially. Almost all of the results were similar to the overall effect of the meta-analysis, suggesting that this meta-analysis was stable (Fig. 4).
Publication Bias
The funnel plot showed an asymmetrical distribution of the outcomes of the included studies, indicating that there was likely significant publication bias (Fig. 5). However, we used quantitative Egger’s and Begg’s tests to assess publication bias, and the result confirmed that there was no evidence of publication bias (P for Egger’s test: 0.285; P for Begg’s test: 0.379).
Discussion
KD is an endemic cardiomyopathy occurring only in China. KD has been found in a wide belt zone from the northeast to the southwest in 2596 townships in 327 counties in 16 provinces in China. Now, the prevalence rate of KD is at a low level, so the goal of KD prevention and control is “basically eliminating Keshan disease in 90% or more counties of Keshan disease endemic areas to reach the criteria of Keshan disease elimination.” Elimination was defined as “zero disease in a defined geographic area as a result of deliberate efforts” [45]. People have been concentrating on eliminating many diseases with a known etiology, including polio, measles, and malaria. Not only were these diseases eliminated but also their causes [46,47,48]. However, the etiology of KD is not fully known, but the major hypothesis of its etiology was selenium deficiency. It is necessary to clarify whether selenium deficiency is a cause of KD. Therefore, we conducted this SR and meta-analysis because this method has not been applied to the association between selenium deficiency and KD.
In this study, the protection rates presented in Table 1 quantitatively demonstrate that supplementation of selenium effectively prevented the occurrence of KD. In addition, the details of the forest plot indicate that the risk of occurrence of KD in the control group was eight times of the risk in the experimental group. This quantitative result revealed that reduction of the incidence of KD was causally associated with selenium supplementation. Although the funnel plot, which presented asymmetry, suggested that publication bias might exist, the results of Egger’s and Begg’s tests indicate that there is no publication bias in this study. As demonstrated above, selenium deficiency is a cause of KD. It should be noted that the cause of a non-communicated disease is normally not the only or full cause of the disease. Therefore, we state that selenium deficiency is a cause of KD, which does not mean that selenium deficiency is the only and full cause of KD.
Importance of the Study
The importance of an etiological study is to provide quality evidence for the prevention and control of disease, or in other words, to translate the findings of an etiological study into the practice of prevention and control. In this case, the findings of this study have two aspects of importance: one is that the description of “unknown cause” in the definition of KD does not make sense, and the other is that selenium should be included in national KD surveillance. There are several issues with the current definition of KD. First, “unknown cause” in the definition of any disease does not make sense for the purpose of prevention and control. Second, selenium deficiency has been obviously demonstrated as a cause of KD. Third, the definition is conflicting and confusing. It is conflicting because it neglects the fact that selenium supplementation has effectively reduced the incidence of KD. It is confusing because selenium supplementation has been the primary recommended preventative intervention since the 1970s. Regarding the inclusion of selenium in the national KD surveillance, disease surveillance should normally cover four aspects. The first aspect is incidence or prevalence of the disease. The second aspect is etiology or risks of the disease. The third aspect is implemented interventions for the prevention and control of the disease. The fourth aspect is assessment of the effectiveness of the interventions taken [49]. Regardless of whether selenium deficiency is a cause of KD or not, it is at least a risk factor of KD, so it should be indicated in the etiology or risk of KD and the effectiveness of KD prevention in order to provide evidence for KD elimination and assessment.
Strengths and Limitations
This study has several strengths. First, this work is the first large-scale SR and meta-analysis of community trials for the prevention of KD in which selenium was supplement to residents of endemic areas of KD; it included a literature search of publications from the past 50 years and included 1,983,238 individuals with 683,075 subjects in experimental groups. Second, we updated our understanding of selenium deficiency as a cause of KD by using the criterion of causation in modern epidemiology and provided the most comprehensive synthesis of evidence on this issue so far. Third, we suggested that the findings of this study should be translated into practice for KD prevention and control by including measurements of selenium levels in residents of KD endemic areas in the KD surveillance program and revising the definition of KD.
However, the limitation of this study is that most of the data were derived from studies conducted as early as the 1970s to 1990s.
Conclusion
In summary, supplementation of selenium effectively prevented people from getting KD, and selenium deficiency is a cause of KD. The measurement of selenium levels in residents of KD endemic areas should be included in the KD surveillance program. Defining KD as endemic cardiomyopathy is inappropriate. The description of “unknown cause” should not be included in its definition. KD should be listed in the contemporary definitions and classifications of cardiomyopathies.
References
The Ministry of Health of the People’s Republic of China (2011) The criteria for diagnosis of Keshan disease (WS/T 210–2011). National Standards of the People’s Republic of China. Beijing: Standards Press of China
The Ministry of Health of the People’s Republic of China. (1997) Diagnosis standard for Keshan disease(GB 17021–1997). National Standards of the People’s Republic of China. Beijing: Standards Press of China
Xu GL (1996) Research progress of prevention of Keshan disease by supplementation of selenium and the relationship between selenium deficiency and Keshan disease: commemorating the 60th anniversary of the discover of Keshan disease. Endemic Diseases Bulletin 11(2):1–6
The Central People’s Government of China (2016). General Office of the State Council. The National Endemic Disease Prevention and control plan (2011–2015) in China. http://www.gov.cn/zwgk/2012-01/29/content_2053487.htmChina (in Chinese)
Liu H, Yu F, Shao W, Ding D., Yu Z., Chen F., Geng D., Tan X., Lammi M.J., Guo X. (2017) Associations between selenium content in hair and Kashin-Beck disease/Keshan disease in children in northwestern China: a prospective cohort study. Biol Trace Elem Res https://doi.org/10.1007/s12011-017-1169-x
Zhou HH, Wang T (2015) Advance research on etiology of Keshan disease. Chinese J Endemiology 34(6):466–468
Cheng BR, Ju SJ, Yin ZH, et al. Selenium deficiency in the environment and Keshan disease (1987) In: Office of Endemic Disease Prevention and Control of the Central Committee of the Communist Party of China. Keshan Disease in China and the Research on Its Prevention and Control. Beijing: China Environmental Science Press:265–269 (in Chinese)
Tan JA, Zhu WY, Li RB. Medicinal and Geographical Characterization of Keshan Disease (1987) In: Office of Endemic Disease Prevention and Control of the central Committee of the Communist Party of China, ed. Keshan disease in China and the research on its prevention and control. Beijing: China environmental science press:254–264 (in Chinese)
Compiling Committee of The Atlas of Endemic Diseases and Their Environments in the People's Republic of China (1989). The atlas of endemic diseases and their environments in the People's Republic of China. Beijing: Science Press
Tan JA, Zhu WY, Li RB et al (1991) Association between Keshan disease and environmental selenium. Chinese Endemiology 10(5):269–274
Keshan Disease Research Group of the Chinese Academy of Medical Sciences (1974) Studies on the relationship of selenium and the incidence of Keshan disease. In: The National Collaboratory Group of Etiological Research for Keshan Disease National Symposium about Etiology of Keshan Disease in 1973:181–200
Yang GQ, Wang GY, Yin TA et al (1982) Relationship between Keshan disease distribution and selenium nutrition condition in China. Acta Nutrimenta Sinica 4(3):191–200
Keshan Disease Research Group of the Chinese Academy of Medical Sciences (1977) Determination of glutathione peroxidase in blood. In: the Chinese Academy of Medical Sciences, ed. research data on Keshan disease. Beijing:61–66 (in Chinese)
Xia YM, Zhu LZ (1987) Determination of glutathione peroxidase activity in blood and tissues. J Hygiene Res 16(4):29–33
World Health Organization (2015). International Statistical Classification of Disease and Related Health Problems 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en/
Keshan Disease Research Group of the Chinese Academy of Medical Sciences (1972) Effect observation on prevention of Keshan disease by using sodium selenite. Bulletin Medical Res 2:18–22
Keshan Disease Research Group of the Chinese Academy of Medical Sciences (1972) Continual observation on prevention of Keshan disease by using sodium selenite. Bulletin Medical Res 6:70–74
Anti-epidemic Station of Jilin city (1973) A summary of effect observation on prevention of Keshan disease by using sodium selenite. Bulletin Endemic Diseases (5):15–18 (in Chinese)
Manchikanti L (2008) Evidence-based medicine, systematic reviews, and guidelines in interventional pain management, part I: introduction and general considerations. Pain Physician 11(2):161–186
McKenzie JE, Salanti G, Lewis SC et al (2013) Meta-analysis and the Cochrane collaboration: 20 years of the Cochrane statistical methods group. Syst Rev 2:80
Li YH, Ghosh D (2014) Meta-analysis based on weighted ordered P-values for genomic data with heterogeneity. BMC Bioinformatics 15:226
Moher D, Tetzlaff J, Tricco AC et al (2007) Epidemiology and reporting characteristics of systematic reviews. PLoS Med 4(3):e78
Huang AJ, Zhan SY (2009) Systematic review and meta-analysis. Chinese J Drug Application Monitoring 6(4):257–259
Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L (2011) An international registry of systematic-review protocols. Lancet 377(9760):108–109
Panic N, Leoncini E, Belvis GD et al (2013) Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 8(12):e83138
Wei ML, Liu M, Su W et al (2007) The current status on systematic review/meta-analysis in Chinese publications from 1990-2007. West China Medical J 22(4):697–698
Lilienfeld AM (1957) Epidemiological methods and inferences in studies of non-infectious diseases. Public Health Rep 72(1):51–60
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1–9
Shamseer L, Moher D, Clarke L et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647
Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Research Laboratory of Keshan Disease in Xi’an Medical University (1973) A primary report on research of pathogenic factor in soil and water of Keshan disease. Bulletin Endemic Diseases (3):11–25
Collaboratory Group of Preventing Keshan Disease by Sodium Selenite (1975) A summary over the past 7 years on prevention of Keshan disease by using sodium selenite. J Hygiene Res 3:190–194
Collaboratory Group of Preventing Keshan Disease by Sodium Selenite (1976) Effect on prevention of Keshan disease by using sodium selenite in 1975. Bulletin Medical Res 5:29–32
Collaboratory Group of Preventing Keshan Disease by Sodium Selenite (1976) Effect on prevention of Keshan disease by using sodium selenite. Bulletin Endemic Diseases 3:15–19
Anti-epidemic Station of Jilin city (1977) Effect observation on prevention of acute Keshan disease by using sodium selenite. Chinese Journal Control of Endemic Disease (2):31–35
Research Laboratory of Keshan Disease in Xi’an Medical University (1978) Effect observation on prevention of acute Keshan disease by using sodium selenite. Shannxi Medical Journal (2):1–6 (in Chinese)
Anti-epidemic Station of Jilin city (1979) Effect observation on prevention of Keshan disease by using sodium selenite - a summary over the past 3 years in Jilin City from 1976 to 1978. Bulletin Endemic Diseases (2):29–33
National Collaboratory Group of Etiological Research for Keshan Disease: Task Force on Selenium (1979) Comprehensive report on prevention of Keshan disease by using sodium selenite. Bulletin Endemic Diseases 2:40–43
Research Laboratory of Keshan Disease in Xi’an Medical University (1979) Report on effect of acute Keshan disease prevention by using sodium selenite. Bulletin Endemic Diseases (2):1–5
Cao HC, Lou ZY, Qu FR et al (1982) Prevention of Keshan disease and influence of abnormal heart by using sodium selenite. Chinese J Endemiology 1(3):150–154
Yu DL, Zhou DZ, Yang PJ (1983) A summary over the past 7 years on prevention of Keshan disease by using sodium selenite. Chinese J Control Endemic Disease 1:41–42,73
Liu CD, Xu YC, Wang DQ (1988) The observation of relationship between trace element selenium and sub-acute Keshan disease onset. Chinese J Control Endemic Disease 4(2):93–94
Li GY, Wang TG (1992) Selenium and acclimation disease. Translations on Endemic Disease 13(5):1–6
Song HB, Xu GL, Yang YX et al (1992) Clinical evaluation on the association between selenium deficiency and Keshan disease. Shaanxi Medical Journal 21(10):589–592
Hopkins DR (2013) Disease eradication. NEJM 368:54–63
Toole MJ (2016) So close: remaining challenges to eradicating polio. BMC Med 14:1–4
Khanal S, Sedai TR, Choudary GR, Giri JN, Bohara R, Pant R, Gautam M, Sharapov UM, Goodson JL, Alexander J, Dabbagh A, Strebel P, Perry RT, Bah S, Abeysinghe N, Thapa A (2016) Progress toward measles elimination - Nepal, 2007–2014. MMWR Morb Mortal Wkly Rep 65:206–210
Newby G, Bennett A, Larson E, Cotter C, Shretta R, Phillips AA, Feachem RGA (2016) The path to eradication: a progress report on the malaria-eliminating countries. Lancet 387:1775–1784
Wang T (2012) The translational epidemiology of Keshan disease surveillance. Foreign Medical Sci(Section Medgeography) 33(3):143–147
Funding
This study was supported by grants from the National Natural Science Foundation of China (81202154, 81372938, and 81773368).
Author information
Authors and Affiliations
Contributions
Huihui Zhou was the principal investigator of this paper. Huihui Zhou and Tong Wang developed the hypothesis and study design and supervised this study. All authors contributed to the study concept and design, analysis, and interpretation of data and drafted or critically revised the manuscript for important intellectual content and/or data acquisition.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhou, H., Wang, T., Li, Q. et al. Prevention of Keshan Disease by Selenium Supplementation: a Systematic Review and Meta-analysis. Biol Trace Elem Res 186, 98–105 (2018). https://doi.org/10.1007/s12011-018-1302-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1302-5