Abstract
Freshwater mussels and crayfish are commonly used as biomonitors of trace metals. In the present study, the concentrations of ten metals were determined in mussels (Unio elongatulus eucirrus) and crayfish (Astacus leptodactylus) collected from the Keban Dam Reservoir in Turkey. The significant spatial differences in concentrations of studied metals except As in mussels were not found. However, Co, Cr, Cu, and Zn concentrations in mussels and As, Co, Cu, Fe, Pb, and Zn concentrations in crayfish showed significant seasonal differences. As, Cd, and Mn levels in mussels were about nine times higher than those in crayfish. The concentrations of Cd, Cr, Cu, Pb, Zn, and inorganic As in crayfish and mussels were lower than maximum permissible levels. When compared with other biomonitoring studies using mussels and crayfish, high concentrations of As, Cd, Co, Cr, and Ni in mussels and Cr and Ni in crayfish were observed due to lithogenic sources and anthropogenic activities in the basin. Bioconcentration factor values of Fe, Mn, Cd, and Zn in mussels and Zn, Cu, Fe, and Co in crayfish were > 1000, which indicates that both U. e. eucirrus and A. leptodactylus have potential to bioaccumulate these metals. Therefore, attention should be paid to mussels and crayfish from ecological and human health perspective, because they are potential vectors of metals to higher trophic levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals derived from natural and anthropogenic sources are among the most dangerous contaminants in aquatic environments. Metals pose serious threat to the aquatic organisms and to human health due to their toxicological effects, high persistence, bioaccumulation, and biomagnification in the food chain [1,2,3]. Some metals such as, cobalt, copper, iron, and zinc are essential metals in all living organisms, whereas cadmium, arsenic, lead, and mercury are non-essential metals that do not have beneficial effects on human health and are toxic even at low concentrations. Moreover, essential metals can also produce toxic effects at high concentrations [4, 5].
Contamination of aquatic environments by trace metals can be confirmed in sediment, water, and aquatic biota. Direct analysis of water and sediment cannot afford the powerful evidence on the integrated influence and possible toxicity of metal pollution on organisms and ecosystem [6]. Therefore, organisms may be used to monitor the level of heavy metal contamination, since they concentrate metals continuously, often several orders of magnitude above ambient water concentrations. In addition, because biomonitor organisms are capable of accumulating heavy metals in their body and have been exposed during their entire lifetime, they may reflect the contamination history of a particular location [7, 8].
Freshwater mussels and crayfish are commonly used as biomonitors of heavy metals because they accumulate high concentrations of metals in their tissues [7,8,9,10,11]. Crayfish are in almost constant contact with sediments of aquatic ecosystems and would easily pick up metals from contaminated sediments and from feeding [8, 9]. Mussels feed on plankton filtered from water and could ingest heavy metals, which concentrate in soft tissues. Furthermore, mussels and crayfish are the main sources of food for benthivorous fish. Therefore, they are potential vectors of metals to higher trophic levels in the food web [10, 11].
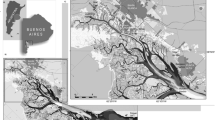
Keban Dam Reservoir (KDR) on the Euphrates River (Turkey) is commonly used for aquaculture production, recreation, fishing, and irrigation. However, it receives both domestic and industrial wastewaters. In addition, there are many fish farms on the reservoir. Agricultural practices are also one of potential sources of contamination due to the use of pesticides and chemical fertilizers in the basin. Streams carry various contaminants from the upstream region to the reservoir [2, 5]. Although there are some reports on metal concentrations in mussels and crayfish collected from the KDR [12, 13], these studies were limited to specific metals and to specific areas of the reservoir. Therefore, in this study, the levels of ten metals (As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) were investigated in soft tissue of mussels (Unio elongatulus eucirrus) collected from 11 sampling sites and muscle tissue of crayfish (Astacus leptodactylus) collected from 5 sites in the KDR.
Materials and Methods
Study Area
With a surface area of 675 km2 and a volume of 30.6 km3, KDR is Turkey’s second largest reservoir. The KDR formed on the Euphrates River is located between latitudes 35° 20′ and 38° 37′ N, and longitudes 38° 15′ and 39° 52′ E. The Keban Dam, built for hydroelectric power generation in 1974, is the first and most upstream of several large-scale dams to be built on the Euphrates River [2, 5, 14].
Collection of Mussel and Crayfish Samples
In the present study, one mussel species (U. e. eucirrus), one crayfish species (A. leptodactylus), and surface water samples were collected from the sampling sites on the KDR in November 2014 (autumn), and February (winter), May (spring) and August 2015 (summer). Mussels were collected from 11 sampling sites (A1–A11) in November 2014 and February 2015. Due to raised water level in the reservoir, mussels were collected from only one site (A7) in May 2015 and from only six sites (A1, A2, A5, A6, A8, and A9) in August 2015. Due to their habitat preferences, A. leptodactylus individuals live in Pertek, Çemişgezek, Ağın, and Keban regions of the reservoir (Fig. 1). Therefore, crayfish individuals were collected from five sites (A5–A9) due to their absence at the other sites (Fig. 1). Crayfish individuals were caught by fyke nets (mesh size 36 mm, total length 178 cm), while mussels were collected by hand at sampling sites in the KDR. All mussel and crayfish individuals were washed with reservoir water in order to remove mud and other fouling substances. In this study, three to eight individuals per species were taken from each site at each sampling time. During the study period, a total of 115 mussels and 104 crayfish were collected. Surface water samples were also taken using 1-L polyethylene bottles from 11 sampling sites (A1–A11). All mussel, crayfish, and water samples were transported in a cooling box to the laboratory immediately after collection. In the laboratory, lengths of mussel and crayfish individuals were recorded. Total length of crayfish individuals ranged from 104 to 112 mm (mean 109 mm), while shell length of mussels ranged from 56 to 77 mm (mean 63.5 mm). Before dissection, all the samples were rinsed with deionized water. Abdominal muscle tissues of crayfish and soft tissues of mussels were removed in the laboratory on the day of sampling. A composite sample (consisting of three to eight individuals) for both mussels and crayfish from each site at each sampling time was prepared and homogenized, and about 10-g (wet weight) test portions were stored in zip-lock bags at − 20 °C until analysis. About 100 mL of water samples were filtered through 0.45-μm nitrocellulose filters (Millipore), acidified with suprapur nitric acid (Merck) for preservation, and stored in pre-cleaned polyethylene bottles at 4 °C until analysis [5]. In this study, for heavy metal analyses, 29 mussel samples, 20 crayfish samples, and 44 water samples were used.
Metal Analysis and Quality Control
In the present study, ten metals, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn, were analyzed in crayfish and mussel samples. One gram of homogenized sample was digested with 8 mL HNO3 and 2 mL H2O2 using a four-step digestion program in a microwave digestion system (MARSXpress, CEM, USA). After cooling to room temperature, the digested solutions were diluted to 50 mL with deionized water. A graphite furnace atomic absorption spectrometer (GF-AAS; Thermo Scientific iCE 3000, USA) was used to measure As, Cd, and Pb levels in the extracts due to lower detection limits, while levels of other elements in the extracts were measured by a flame atomic absorption spectrometer (Thermo Scientific iCE 3000, USA) [2, 5]. Concentrations of ten metals in water samples were measured by GF-AAS [15]. Triplicate analyses were performed on each sample and the average values were used in data analysis. The accuracy of the procedure was checked by determining metal concentrations in a certified reference material (TORT-3, lobster hepatopancreas; NRC, Canada). The recoveries were between 94.4 and 105.7% (Table 1). In addition, spiked water samples were used to check the reliability of GF-AAS measurements. The recoveries of spiked samples ranged from 91.3% (Fe) to 108.6% (Cr).
Maximum Permissible Levels
Trace metal levels determined in mussel and crayfish samples were compared with the maximum permissible levels of trace metals in bivalve molluscs, crustaceans, and fish set by international food standards, such as FSANZ (Food Standards Australia and New Zealand) [16], FAO (Food and Agriculture Organization) [17], WHO/FAO (Codex Alimentarius Commission) [18], EC (European Commission) [19], and MHPRC (Chinese Health Ministry) [20].
Bioconcentration Factor
The bioconcentration factor (BCF) is expressed as the ratio of metal concentration in an aquatic organism to metal concentration in the surrounding water. The following equation was used to calculate the BCF [21]:
where Cbiota is the metal concentration in mussel or crayfish (μg kg−1 wet weight) and Cwater is the metal concentration in water (μg L−1).
Statistical Analysis
One-way ANOVA was used to determine whether there were significant differences in trace metal levels among sampling sites and between sampling seasons (p < 0.05). One-way ANOVA was done using SPSS 11.5 for Windows.
Results and Discussion
The descriptive statistics of trace metals studied in mussel and crayfish samples collected from the Keban Dam Reservoir are summarized in Table 2.
Metal Concentrations in Mussels
In the present study, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn concentrations (mean ± SD, wet weight) in mussel samples were 1077 ± 385 μg kg−1, 79 ± 24 μg kg−1, 0.70 ± 0.30 mg kg−1, 1.05 ± 0.40 mg kg−1, 0.74 ± 0.45 mg kg−1, 104.5 ± 48.4 mg kg−1, 17.4 ± 14.0 mg kg−1, 1.46 ± 0.52 mg kg−1, 104.4 ± 38.6 μg kg−1, and 10.1 ± 3.1 mg kg−1, respectively (Table 2). The concentrations of heavy metals in mussels followed the order of Fe > Mn > Zn > Ni > As > Cr > Cu > Co > Pb > Cd. The mean concentrations of all metals except As in mussels did not display statistically significant differences among the 11 sampling sites (p > 0.05) (Table 3). The highest concentrations of As and other nine metals were found at sites A11 and A7, respectively, where many rainbow trout cage farms are present. Among the studied metals, the mean concentrations of Co, Cr, Cu, and Zn in mussels showed significant seasonal differences (p < 0.05) (Table 3). The highest concentrations of Co, Cr, Cu, and Zn were found in spring, while the highest concentrations of other metals were recorded in winter. Various environmental and biological factors can cause seasonal fluctuations in concentrations of trace metals in mussels.
In this study, the average concentration of Cd, Cr, Cu, Pb, Zn, and inorganic As (assuming inorganic As is 3% of total As [22, 23]) in mussels was lower than the maximum permissible levels (MPLs) established by EC, FSANZ, MHPRC, FAO, and WHO/FAO (Table 4).
In the present study, the mean metal concentrations in U. e. eucirrus were compared with those of previous studies using freshwater mussels belonging to Unionidae family (Table 5). The mean value of As in U. e. eucirrus was higher than those in mussels from Turkey [24] and Vietnam [7]. However, it was lower than that in mussels from Italy [25]. Cadmium concentration determined in our study was lower than those found in mussels from Italy [25] and Hungary [26], while it was above those found in mussels from Vietnam [7], Poland [27], Iran [28], and Turkey [24]. Co, Cr, and Ni concentrations in the present study were higher than those in mussels from Italy [25], Vietnam [7], Poland [27], and Hungary [26]. The mean value of Cu was comparable to those reported for mussels from Vietnam [7] and Poland [27], while it was below those found in mussels from Hungary [26], Italy [25], and Turkey [24]. Fe and Mn values were lower than those in mussels from Vietnam [7] and Italy [25], while they were higher than those in mussels from Poland [27]. The mean value of Pb in the present study was comparable to that reported for mussels from Vietnam [7], while it was below those found in mussels from Hungary [26], Italy [25], and Turkey [24], and it was higher than those in mussels from Poland [27] and Iran [28]. The mean concentration of Zn was above that in mussels from Turkey [24], while it was below in those found in mussels from Vietnam [7], Hungary [26], and Italy [25], and it was comparable to that reported for mussels from Poland [27]. In addition, the mean value of Cd determined in U. e. eucirrus in our study was lower when compared with a previous study carried out in Keban Dam Reservoir [12], whereas the mean concentrations of Cu and Pb were higher than those of the previous study (Table 5).
Metal Concentrations in Crayfish
In the present study, As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn concentrations (mean ± SD, wet weight) in crayfish samples were 98.3 ± 48.2 μg kg−1, 8.1 ± 4.7 μg kg−1, 0.94 ± 0.44 mg kg−1, 0.95 ± 0.35 mg kg−1, 6.9 ± 1.9 mg kg−1, 15.0 ± 14.2 mg kg−1, 1.7 ± 0.9 mg kg−1, 1.41 ± 1.08 mg kg−1, 46.5 ± 31.1 μg kg−1, and 19.7 ± 6.4 mg kg−1, respectively (Table 2). The concentrations of trace metals in crayfish followed the order of Zn > Fe > Cu > Mn > Ni > Cr > Co > As > Pb > Cd. The mean concentrations of trace metals in crayfish did not display significant differences among the five sampling sites (p > 0.05) (Table 3). The highest concentrations of Cu, Fe, Mn, Ni, and Pb were recorded at site A9, which receives wastewater from leather factory, while the highest concentrations of Cd, Co, and Cr were recorded at site A5, where many ferry crossings are available. The highest Zn concentration was recorded at site A7, where many rainbow trout cage farms are present, while the highest As concentration was recorded at site A6. Among the studied metals, the mean concentrations of As, Co, Cu, Fe, Pb, and Zn in crayfish showed significant seasonal differences (p < 0.05) (Table 3). In our study, the highest concentrations of As, Cd, and Pb were found in autumn, the highest concentrations of Cu, Fe, Mn, Ni and Zn were obtained in spring, and the highest concentrations of Co and Cr were recorded in summer. The seasonal variations of concentrations of these metals in crayfish may be due to the natural fluctuations of both environmental and biological factors.
The average concentrations of Cd, Cr, Cu, Pb, Zn, and inorganic As (assuming inorganic As is 3% of total As [22, 23]) in crayfish were below the maximum permissible levels established by FSANZ, FAO, MHPRC, WHO/FAO, and EC (Table 4).
In this study, the mean metal concentrations in A. leptodactylus were compared with those of previous studies using crayfish species belonging to Astacus genus performed in different freshwater sites of Turkey, Iran, Czech Republic, Lithuania, and Sweden (Table 6). In addition, the mean metal concentrations found in our study were compared with global literature on metal levels in muscles of different freshwater crayfish species summarized by Kouba et al. [29] (Table 6). The mean values of Cd found in our study were lower than those in crayfish from Terkos [30] and Yenicaga [33] lakes in Turkey, Iran [31], and Czech Republic [32], while Cr value was higher than those in crayfish from Yenicaga [33] and Kovada [34] lakes in Turkey, Iran [31], Czech Republic [32], Lithuania [35], and Sweden [36]. The mean value of Cu was higher than those in crayfish from Kovada Lake in Turkey [34], Sweden [36], and Lithuania [35], while it was lower than those in crayfish from Lake Terkos in Turkey [30], Iran [31], and Czech Republic [32]. Fe and Mn values were below the values reported for crayfish from Lake Terkos in Turkey [30], while they were above the values in crayfish from Lake Kovada in Turkey [34]. Nickel value was lower than that reported from Lake Kovada in Turkey [34]. However, it was higher than those reported from Czech Republic [32], Sweden [36], and Lithuania [35]. Lead concentration in our study was lower than those reported from Czech Republic [32] and Yenicaga Lake in Turkey [33], while it was higher than those reported from Iran [31], Sweden [36], and Kovada [34] and Terkos [30] lakes in Turkey. Zinc value was lower than those in crayfish from Iran [31] and Czech Republic [32], while it was higher than those reported from Kovada Lake in Turkey [34], Sweden [36], and Lithuania [35] (Table 6). There were only few studies reporting As concentrations in freshwater crayfish. Arsenic concentration (mean = 98.3 μg kg−1 wet weight) obtained in our study was lower than those in crayfish (Procambarus clarkii) collected from China (mean = 253 μg kg−1 wet weight [37]), Italy (median = 364 μg kg−1 wet weight [38]), and Spain (range = 320–1700 μg/kg wet weight [39]). In addition, As value in our study was below that in crayfish (Astacus astacus) from Sweden [36]. In the present study, the mean concentrations of Cd, Cr, Pb, and Zn were between global concentration ranges reported by Kouba et al. [29], while Ni concentration was higher than global concentration ranges for Ni (Table 6). In addition, the average values of As, Cd, Cu, Pb, and Zn determined in A. leptodactylus in our study were lower than those of a previous study carried out in Keban Dam Reservoir [12] (Table 6).
Metal Sources for Mussels and Crayfish
When compared with previous studies (Tables 5 and 6), our results indicated that high concentrations of As, Cd, Co, Cr, and Ni in mussels and Cr and Ni in crayfish were observed. The primary source of these metals in mussels and crayfish can be lithogenic (natural) sources. Kalender and Uçar [40] reported that the KDR region is one of the most important base metal deposits in Turkey. Therefore, mussels and crayfish can be exposed to elevated levels of heavy metals from sediments, because both organisms are in physical contact with aquatic sediments [41, 42]. The second source of them can be anthropogenic activities, such as rainbow trout cage farms, ferry crossings, wastewater from leather factory, and agricultural activities (Fig. 1). In our study, mussels with the highest total mean concentrations of ten elements were collected at sites A7 (197.3 mg kg−1) and A11 (175.0 mg kg−1), where many rainbow trout cage farms are located. Crayfish with the highest total mean concentration was collected at site A9 (55.8 mg kg−1), which receives wastewater from leather factory.
Essential and Non-essential Metals in Mussels and Crayfish
A. leptodactylus has a long lifespan up to 20 years and eats a varied diet. U. e. eucirrus belonging to the Unionidae family is a sedentary filter feeder. It can concentrate particulate-associated trace metals. It is reported that Unionid mussels and A. leptodactylus are good indicators of heavy metal pollution in aquatic environments [7, 43]. In this study, As, Cd, Cr, Fe, Mn, Ni, and Pb concentrations were higher in mussels, while Co, Cu, and Zn concentrations were higher in crayfish. As, Cd, and Mn levels found in mussels were nine times higher in relation to As, Cd, and Mn levels in crayfish. However, Cu level in crayfish was nine times higher in relation to Cu level in mussels. These resulted indicated that U. e. eucirrus can be a good indicator of As, Cd, and Mn contamination, while A. leptodactylus can be a good indicator of Cu contamination in the KDR.
Zn, Cu, Fe, and Mn are essential elements. The essential elements were more abundant than non-essential elements in mussels and crayfish. Among the essential elements, Fe, Mn, and Zn had the highest mean concentrations in mussels, respectively, while Zn, Fe, and Cu had the highest mean concentrations in crayfish, respectively. Essential elements are easily assimilated by mussels and crayfish and are needed in physiological functions. In addition, essential elements can be regulated by mussels and crayfish until a certain threshold level [7, 10]. However, they can also cause harmful effects at high concentrations. Conversely, non-essential elements such as Cd, As, and Pb are not regulated by the living organisms. Among the non-essential elements, As was the most abundant element in mussels and crayfish. Non-essential elements tend to be detoxified by metallothioneins and stored in tissues of mussels and crayfish, becoming harmful elements to organisms [10, 11].
Bioconcentration Factor
A metal is considered to be not bioaccumulative if its bioconcentration factor (BCF) value is less than 1000, bioaccumulative if its BCF value is between 1000 and 5000, and very bioaccumulative if its BCF value is greater than 5000 [44]. The calculated BCF values of trace metals in mussels and crayfish are presented in Table 7. BCF values of Fe and Mn in mussels were higher than 5000, which indicates that these elements are considered very bioaccumulative. BCF values of Cd and Zn in mussels and Co, Cu, Fe, and Zn in crayfish were between 1000 and 5000, which indicates that these elements are considered bioaccumulative. These results revealed that U. e. eucirrus has potential to bioaccumulate Fe, Mn, Cd, and Zn elements, while A. leptodactylus has potential to bioaccumulate Co, Cu, Fe, and Zn elements. However, BCF values of As, Co, Cr, Cu, Ni, and Pb in mussels and As, Cd, Cr, Mn, Ni, and Pb in crayfish were less than 1000, which indicates that mussels and crayfish have no potential to accumulate them (Table 7).
Conclusions
In the present study, the concentrations of ten metals in mussels (U. e. eucirrus) collected from 11 sampling sites and crayfish (A. leptodactylus) collected from 5 sites in the Keban Dam Reservoir were determined. The concentrations of all trace metals except As in mussels did not display statistically significant differences among the sampling sites. Significant seasonal variations were observed in metal concentrations in mussels and crayfish. The levels of trace metals in crayfish and mussels were below the MPLs determined by international food standards. As, Cd, Cr, Fe, Mn, Ni, and Pb concentrations were higher in mussels, while Co, Cu, and Zn concentrations were higher in crayfish. Lithogenic sources and anthropogenic activities are important contributors of metal contamination. Because BCF values of Fe, Mn, Cd, and Zn in mussels and Zn, Cu, Fe, and Co in crayfish were > 1000, U. e. eucirrus and A. leptodactylus have potential to bioaccumulate these metals. Therefore, attention should also be paid to the roles of mussels and crayfish as vectors of pollutants, as these organisms can transfer these metals to higher trophic levels.
Change history
22 January 2018
The original version of this article contained mistakes: the units mg/kg-1 and μg/kg-1 found on tables 1, 2, 4, 5 and 6 should be mg kg−1 and μg kg−1, respectively.
References
Djedjibegovic J, Larssen T, Skrbo A, Marjanovic A, Sober M (2012) Contents of cadmium, copper, mercury and lead in fish from the Neretva River (Bosnia and Herzegovina) determined by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem 131(2):469–476. https://doi.org/10.1016/j.foodchem.2011.09.009
Varol M, Sünbül MR (2017) Organochlorine pesticide, antibiotic and heavy metal residues in mussel, crayfish and fish species from a reservoir on the Euphrates River, Turkey. Environ Pollut 230:311–319. https://doi.org/10.1016/j.envpol.2017.06.066
Noel L, Chekri R, Millour S, Merlo M, Leblanc JC, Guerin T (2013) Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 90(6):1900–1910. https://doi.org/10.1016/j.chemosphere.2012.10.015
Wei YH, Zhang JY, Zhang DW, TH T, Luo LG (2014) Metal concentrations in various fish organs of different fish species from Poyang Lake, China. Ecotoxicol Environ Saf 104:182–188. https://doi.org/10.1016/j.ecoenv.2014.03.001
Varol M, Sünbül MR (2017) Comparison of heavy metal levels of farmed and escaped farmed rainbow trout and health risk assessment associated with their consumption. Environ Sci Pollut Res 24(29):23114–23124. https://doi.org/10.1007/s11356-017-9958-5
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606(2):135–150. https://doi.org/10.1016/j.aca.2007.11.018
Wagner A, Boman J (2004) Biomonitoring of trace elements in Vietnamese freshwater mussels. Spectrochim Acta Part B 59(8):1125–1132. https://doi.org/10.1016/j.sab.2003.11.009
Schilderman PAEL, Moonen EJC, Mass LM, Welle I, Kleinjans JCS (1999) Use of crayfish in biomonitoring studies of environmental pollution of the river Meuse. Ecotoxicol Environ Saf 44(3):241–252. https://doi.org/10.1006/eesa.1999.1827
Richert JC, Sneddon J (2007) Determination of inorganics and organics in crawfish. Appl Spectrosc Rev 43(1):51–67. https://doi.org/10.1080/05704920701702950
Suarez-Serrano A, Alcaraz C, Ibanez C, Trobajo R, Barata C (2010) Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicol Environ Saf 73(3):280–286. https://doi.org/10.1016/j.ecoenv.2009.11.001
Kraak MHS, Scholten MCT, Peeters WHM, de Kock WC (1991) Biomonitoring of heavy metals in the western european rivers Rhine and Meuse using the freshwater mussel Dreissena polymorpha. Environ Pollut 74(2):101–114. https://doi.org/10.1016/0269-7491(91)90107-8
Aksu Ö, Yabanli M, Can E, Kutluyer F, Kehayias G, Can ŞS, Kocabaş M, Demir V (2012) Comparison of heavy metals bioaccumulation by Dreissena polymorpha (Pallas, 1771) and Unio elongatulus eucirrus (Bourguignat, 1860) from Keban Dam Lake, Turkey. Fresenius Environ Bull 21:1942–1947
Aksu O, Adiguzel R, Demir V, Yildirim N, Danabas D, Seker S, Can SS, Ates M (2014) Temporal changes in concentrations of some trace elements in muscle tissue of crayfish, Astacus leptodactylus (Eschscholtz, 1823), from Keban Dam Lake. Bioinorg Chem Appl 120401
Güner B (2015) Aquafishing in Keban Dam Lake. Fırat Univ J Soc Sci 25:1–8
Varol M, Kaya GK, Alp SA, Sünbül MR (in press) Trace metal levels in rainbow trout (Oncorhynchus mykiss) cultured in net cages in a reservoir and evaluation of human health risks from consumption. Biol Trace Elem Res. https://doi.org/10.1007/s12011-017-1156-2
FSANZ (Food Standards Australia and New Zealand) (2013) Australia New Zealand Food Standards Code, Standard 141. Contaminants and natural toxicants. http://www.legislationgovau/Details/F2013C00140. Accessed 24 Feb 2017
FAO (Food and Agriculture Organization) (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular No 464. Food and Agriculture Organization of the United Nations, Rome
WHO/FAO (World Health Organization/Food and Agriculture Organization) (2015) Codex Alimentarius Commission. General Standard for Contaminants and Toxins in Food and Feed, CODEX STAN 193–1995
EC (Commission of the European Communities) (2006) Commission Regulation (EC) No 1881/2006 of 19 December 2006: setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union Legislation 364. http://www.eur-lexeuropaeu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881from=EN. Accessed 19 Dec 2016
MHPRC (Ministry of Health of the People’s Republic of China) (2013) National Food Safety Standard. Maximum Levels of Contaminants in Foods (GB2762-2012)
Tao Y, Yuan Z, Xiaona H, Wei M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake, China. Ecotoxicol Environ Saf 81:55–64. https://doi.org/10.1016/j.ecoenv.2012.04.014
Borak J, Hosgood HD (2007) Seafood arsenic: implications for human risk assessment. Regul Toxicol Pharmacol 47(2):204–212. https://doi.org/10.1016/j.yrtph.2006.09.005
Li Y, Liu H, Zhou H, Ma W, Han Q, Diao X, Xue Q (2015) Concentration distribution and potential health risk of heavy metals in Mactra veneriformis from Bohai Bay, China. Mar Pollut Bull 97(1-2):528–534. https://doi.org/10.1016/j.marpolbul.2015.05.017
Yarsan E, Bilgili A, Türel İ (2000) Heavy metal levels in mussels (Unio stevenianus Krynicki) obtained from Van Lake. Turk J Vet Anim Sci 24:93–96
Ravera O, Cenci R, Beone GM, Dantas M, Lodigiani P (2003) Trace element concentrations in freshwater mussels and macrophytes as related to those in their environment. J Limnol 62(1):61–70. https://doi.org/10.4081/jlimnol.2003.61
Nguyen HL, Leermakers M, Osan J, Török S, Baeyens W (2005) Heavy metals in Lake Balaton: water column, suspended matter, sediment and biota. Sci Total Environ 340(1-3):213–230. https://doi.org/10.1016/j.scitotenv.2004.07.032
Rzymski P, Niedzielski P, Klimaszyk P, Poniedzialek B (2014) Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ Monit Assess 186(5):3199–3212. https://doi.org/10.1007/s10661-013-3610-8
Pourang N, Richardson CA, Mortazavi MS (2010) Heavy metal concentrations in the soft tissues of swan mussel (Anodonta cygnea) and surficial sediments from Anzali wetland, Iran. Environ Monit Assess 163(1-4):195–213. https://doi.org/10.1007/s10661-009-0827-7
Kouba A, Buric M, Kozak P (2010) Bioaccumulation and effects of heavy metals in crayfish: a review. Water Air Soil Pollut 211(1-4):5–16. https://doi.org/10.1007/s11270-009-0273-8
Kurun A, Balkıs N, Erkan M, Balkıs H, Aksu A, Erşan MS (2010) Total metal levels in crayfish Astacus leptodactylus (Eschscholtz, 1823), and surface sediments in Lake Terkos, Turkey. Environ Monit Assess 169(1-4):385–395. https://doi.org/10.1007/s10661-009-1181-5
Hosseini SV, Amininasab SM, Tahergorabi R, Sari AE, Bor S (2004) Determination of heavy metals content in water, sediments and muscle of crayfish, Astacus leptodactylus in southern coasts of Caspian Sea (Abbasa river of Nour city). Proceedings of the Fourth International Iran & Russia Conference, Shahrekord, Iran, pp 1463–1467
Kuklina I, Kouba A, Buric A, Horka I, Duris Z, Kozak P (2014) Accumulation of heavy metals in crayfish and fish from selected Czech reservoirs. BioMed Res Int 306103
Tunca E, Atasagun S, Saygı Y (2012) Pre-investigation of some heavy metal accumulation in the water, sediment and crayfish (Astacus leptodactylus) in Yenicaga Lake (Bolu-Turkey). Ecology 21:68–76
Kır İ, Tuncay Y (2010) The investigation of some heavy metals in crayfish (Astacus leptodactylus) inhabiting Kovada Lake. SDU J Sci 5:179–186
Mackeviciene G (2002) Bioaccumulation of heavy metals in noble crayfish (Astacus astacus L.) tissues under aquaculture conditions. Ekologia (Vilnius) 2:79–82
Jorhem L, Engman J, Sundström B, Thim AM (1994) Trace elements in crayfish: regional differences and changes induced by cooking. Arch Environ Contam Toxicol 26(2):137–142
Peng Q, Nunes LM, Greenfield BK, Dang F, Zhong H (2016) Are Chinese consumers at risk due to exposure to metals in crayfish? A bioaccessibility-adjusted probabilistic risk assessment. Environ Int 88:261–268. https://doi.org/10.1016/j.envint.2015.12.035
Bellante A, Maccarone V, Buscaino G, Buffa G, Filiciotto F, Traina A, Del Core M, Mazzola S, Sprovieri M (2015) Trace element concentrations in red swamp crayfish (Procambarus clarkii) and surface sediments in Lake Preola and Gorghi Tondi natural reserve, SW Sicily. Environ Monit Assess 187(7):404. https://doi.org/10.1007/s10661-015-4613-4
Devesa V, Suner MA, Lai VWM, Granchinho SCR, Martinez JM, Velez D, Cullen WR, Montoro R (2002) Determination of arsenic species in a freshwater crustacean Procambarus clarkii. Appl Organomet Chem 16(3):123–132. https://doi.org/10.1002/aoc.269
Kalender L, Uçar SÇ (2013) Assessment of metal contamination in sediments in the tributaries of the Euphrates River, using pollution indices and the determination of the pollution source, Turkey. J Geochem Explor 134:73–84. https://doi.org/10.1016/j.gexplo.2013.08.005
Knowlton ME, Boyle TR, Jones JR (1983) Uptake of lead from aquatic sediment by submersed macrophytes and crayfish. Arch Environ Contam Toxicol 12(5):535–541. https://doi.org/10.1007/BF01056549
Allert AL, DiStefano RJ, Fairchild JF, Schmitt CJ, McKee MJ, Girondo JA, Brumbaugh WG, May TW (2013) Effects of historical lead–zinc mining on riffle-dwelling benthic fish and crayfish in the Big River of southeastern Missouri, USA. Ecotoxicology 22(3):506–521. https://doi.org/10.1007/s10646-013-1043-3
Tunca E, Ucuncu E, Ozkan AD, Ulger ZE, Tekinay T (2013) Tissue distribution and correlation profiles of heavy-metal accumulation in the freshwater crayfish Astacus leptodactylus. Arch Environ Contam Toxicol 64(4):676–691. https://doi.org/10.1007/s00244-012-9863-3
Costanza J, Lynch DG, Boethling RS, Arnot JA (2012) Use of the bioaccumulation factor to screen chemicals for bioaccumulation potential. Environ Toxicol Chem 31(10):2261–2268. https://doi.org/10.1002/etc.1944
Funding
The study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) (Project No: 114Y018).
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: the units mg/kg-1 and μg/kg-1 found on tables 1, 2, 4, 5 and 6 should be mg kg−1 and μg kg−1, respectively.
Rights and permissions
About this article
Cite this article
Varol, M., Sünbül, M.R. Biomonitoring of Trace Metals in the Keban Dam Reservoir (Turkey) Using Mussels (Unio elongatulus eucirrus) and Crayfish (Astacus leptodactylus). Biol Trace Elem Res 185, 216–224 (2018). https://doi.org/10.1007/s12011-017-1238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1238-1