Abstract
To determine the negative effects of cadmium (Cd) exposure and the protective role of selenium (Se) on Cd-spiked neutrophils of chicken, forty-eight 28-day-old Isa Brown male chickens were divided randomly into four groups. Group I (control group) was fed with the basic diet containing 0.2 mg/kg Se. Group II (Se-treated group) was fed with the basic diet supplemented with Na2SeO3, and the total Se content was 2 mg/kg. Group III (Se/Cd-treated group) was fed with the basic diet supplemented with Na2SeO3; the total Se content was 2 mg/kg and supplemented with 150 mg/kg CdCl2. Group IV (Cd-treated group) was fed with the basic diet supplemented with 150 mg/kg CdCl2. Analyses of inflammatory factors, cytokines, and heat shock protein (Hsp) messenger RNA (mRNA) expression were detected by real-time PCR (RT-PCR). Additionally, we evaluated the phagocytic rate of neutrophils in peripheral blood. First, we observed that Cd significantly induced the mRNA expression levels of inflammatory factors NF-κB, iNOS, COX-2, and TNF-α, while Se/Cd treatment reduced their mRNA expression, although these expression levels remained higher than that of the control group. In addition, the mRNA expression levels of cytokines (IL-2, IL-4, and IL-10) for the Se-treated group exhibited significant differences between the Se/Cd-treated group and the Cd-treated group. Furthermore, the mRNA expression levels of Hsps demonstrated that the Se/Cd-treated group and the Cd-treated group were significantly higher (P < 0.05) than the control group and the Se-treated group. These results demonstrated that Se presented partial protection on Cd-spiked neutrophils of chicken with Hsps being involved in the process of the Cd-spiked toxic effects in chicken peripheral blood neutrophils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is both highly toxic and omnipresent in natural environments and is frequently used in many industrial applications, including the production of batteries, metal plating, pigments, and plastics, and through these applications, it can be released into the environment and result in damage to physiological functions and the economy. Different aspects of Cd toxicity have been reported in animals, and the accumulation within the body may produce toxic effects in the kidney, liver, lung [1–4], and reproductive organs [5]. Our previous studies have shown Cd-spiked morphological changes and oxidative stress, ion disorder, and apoptosis, suggesting that the toxic effects of Cd are on the chicken splenic lymphocytes [6]. Cd-spiked accumulation was accompanied by a decrease in variables (glutathione, l-glutathione, superoxide dismutase, and glutathione peroxidase) that lead to injury of the kidney [7]. In addition, Cd is an environmental risk factor for destruction of the immune system. Many studies have shown that exposure to Cd can cause immune system injury [6, 8]. Previous study had indicated that Cd exposure seemed to result in a decreased maturation and mobilization of T and B lymphocytes, thereby affecting the immune system [9]. In some works, high Cd levels in Mus musculus liver and plasma increased the transcription of hepatic genes involved in oxidative stress and immune response and apoptosis of neutrophils [10].
Selenium (Se) is an important nutritional trace element. In animals, the main function of Se includes antioxidant actions [11–14] and immunity enhancement [15]. Zhang et al. reported that chickens fed with a Se-deficient diet exhibited lesions in immune organs and lower serum IL-1β, IL-2, and TNF content [16]. Also, some reports have clarified that Se is of great importance for human health, protecting cells from the harmful effects of free radicals through significantly increasing the activities of antioxidant enzymes. Zhang et al. indicated that oxidative stress inhibited the development of immune organs and finally impaired the immune function of chickens [16]. Beside these certain roles, some studies have shown that Se was considered one of the most efficient elements guarding against Cd-spiked injury [17, 18]. According to our group preliminary study, Se attenuated the Cd-spiked inflammatory reaction in lymphocytes and enhanced the immune function, which was partly by way of a downregulation of cytokine mRNA expression via the suppression of NF-κB activation in chicken splenic lymphocytes [19]. It has been shown that supplementation of low-dose Se resulted in the decrease of Cd deposition in analyzed organs including livers and kidneys of hens [20]. El-Boshy et al. reported that Se had a potential to countermeasure the immunosuppressive as well as hepatic and renal oxidative damage induced by Cd in rats by regulating the expression of inflammatory factors [21].
Neutrophils were isolated from bone marrow stem cells, which are the most abundant leukocyte, and account for approximately 50–70% of the total of peripheral blood leukocytes [22]. Neutrophils play an important role in nonspecific cellular immunity of organisms as the first line of defense against infectious diseases, and this role has been extensively studied in many immune system diseases of chicken [23]. During immune dysfunction, activated neutrophils have a great capacity to release a wide variety of cytokines and chemokines, which can regulate almost every element of the immune system and abnormal migration [24]. However, there is no clear explanation about the effect of neutrophil phagocytosis against heavy metal poison on the immune system. Snella et al. reported that manganese caused the migration of peritoneal neutrophils from guinea pigs at a significantly higher rate than control animals [25]. It has also been reported that Cd had adverse effects on lymphocytes and induced higher expression of the metallothionein gene on lymphocytes [26]. Meanwhile, increasing information has demonstrated the protective effect of Se against toxicity of Cd. Liu et al. demonstrated that the mRNA expression levels of iNOS, COX-2, and TNF-α in the Se/Cd-treated group were significantly reduced compared to the Cd-treated group in chicken splenic lymphocytes [19]. In addition, Xie et al. indicated that through altered levels of lipid peroxidation and catalase (CAT) activity, Se was protective against Cd toxicity in the least killifish Heterandria formosa [27].
Although the negative effects of Cd exposure in mechanistic studies are increasing, limited data about the effects of Se and Cd in neutrophils of chicken have been reported. For discovering the toxic effects of Cd on neutrophils and the antagonistic effect of Se to Cd, we detected the phagocytic rate of neutrophils, inflammatory factors, cytokines, and heat shock protein (Hsp) mRNA expression levels in chicken peripheral blood.
Materials and Methods
Chemicals
Neutrophil isolation extraction kit was purchased from Tianjin Haoyang Biological Products Co., Ltd. TRIzol reagent was purchased from Invitrogen, America. Oligo dT primers and Superscript II reverse transcriptase were provided by Promega, China. The mRNA reverse transcription kit was provided by Thermo Fisher Scientific Inc. Wright’s dye was purchased by Beijing Reagan Biotechnology Co., Ltd. Staphylococcus was provided by the Northeast Agricultural University pharmacology laboratory.
Preparation of Animals
Forty-eight 28-day-old Isa Brown male chickens were divided randomly into four groups (n = 10 per group). The remaining two chickens in each group were standby for any unexpected condition. Birds were maintained in the Laboratory Animal Center, College of Veterinary Medicine, Northeast Agricultural University, China. The animal room was maintained at 18–26 °C, and the birds were kept under a 16-h light/8-h dark cycle and given free access to standard food and water for 12 weeks. According to the LC50 of Cd (218.44 mg/kg diet CdCl2), the exposure doses of Cd (150 mg/kg diet CdCl2) were used in this study. In regard to Se doses, we added and supplied 2 mg/kg Se (supernutritional Se but not Se toxicity) with Na2SeO3 supplement. Group I (control group) was fed with the basic diet containing 0.2 mg/kg Se. Group II (Se-treated group) was fed with the basic diet supplemented with Na2SeO3; the total Se content was 2 mg/kg. Group III (Se/Cd-treated group) was fed with the basic diet supplemented with Na2SeO3; the total Se content was 2 mg/kg and supplemented with 150 mg/kg CdCl2. Group IV (Cd-treated group) was fed with the basic diet supplemented with 150 mg/kg CdCl2. All of the procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University.
In the trial period, no mortality was recorded in all groups. Peripheral blood was quickly taken on the 84th day of the experiment, and then, neutrophils were separated using kits according to the manufacturer’s instructions (TBD, China) and stored at −80 °C, in order to isolate the RNA. The other parts of the peripheral blood were used to make the blood smear for detecting the phagocytic rate.
Phagocytic Rate Testing
Two milliliters of heparin anticoagulant was added to 1 mL of staphylococcus culture and incubated at 37 °C for 10 min after mixing. Twenty microliters of the mixture was placed on a glass slide to prepare blood smears, with seven to eight drops of Wright’s dye added to the slide followed by waiting for 3 min; after that, equal amounts of PBS were mixed for 10 min and rinsed with water. The neutrophil phagocytosis rate was detected by microscopic examination observation.
Extraction of Peripheral Blood Neutrophils
Blood was mixed 1:1 with PBS until uniformity was reached, and an equal quantity was added on the surface of neutrophils for liquid separation. This showed four layers of cells from top to bottom in the centrifuge tube, and we collected the second layer and the third layer. They were put in a test tube with equal quantity of PBS and centrifuged for 10 min at 1000 rpm after being thoroughly incorporated, and the supernatant was abandoned; then, an equal quantity of lymphocyte separation medium was added slowly after being mixed with PBS at 1:1 and centrifuged for 20 min at 2000 rpm, and the supernatant was abandoned; the added 10 mL PBS was resuspended and centrifuged for 15 min at 2000 rpm, and the supernatant was abandoned. To a part of them, 1 mL TRIzol was added and resuspended and another part of 1 mL PBS was added and resuspended and then stored at −80 °C for RT-PCR.
Real-Time Quantitative PCR
Total RNA was dissociated from the neutrophils using the TRIzol reagent according to the manufacturer’s protocol. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm (Gene Quant 1300/100, General Electric Company, USA). First-strand complementary DNA (cDNA) was synthesized from 5 μg of total RNA using oligo dT primers and Superscript II Reverse Transcriptase according to the manufacturer’s instructions (Roche, Switzerland). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C before using.
All of the primers (Table 1) were designed by the Premier software (PREMIER Biosoft International, USA) for RT-PCR. Detected via RT-PCR and gene expression levels were performed on a Light Cycler® 480 System (Roche, Basel, Switzerland) using Fast Universal SYBR Green Master Mix (Roche, Basel, Switzerland). The reactions were performed in a 20-μL reaction mixture containing 10 μL of 2× SYBR Green I PCR Master Mix (Roche, Switzerland), 2 μL of either diluted cDNA, 0.4 μL of each primer (10 μM), 0.4 μL of 50× ROX reference dye II, and 6.8 μL of PCR-grade water. The PCR procedure for all of the primers consisted of heating the reaction mixture at 95 °C for 30 s followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and product purity. The relative mRNA abundance was calculated according to the method of ΔΔCT, accounting for gene-specific efficiencies, and was normalized to the mean mRNA expressions of β-actin.
Statistical Analyses
Statistical analyses of all data were performed using the GraphPad Prism (version 5.0, GraphPad Software Inc., San Diego, CA, USA). When a significant value (P < 0.05) was obtained by one-way analysis of variance, further analysis was performed. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey’s honest significant difference test for post hoc multiple comparisons. Quantitative data are shown as the mean ± SD. Differences of P < 0.05 were considered to be significant.
Results
Neutrophil Phagocytosis and Phagocytic Rate in the Chicken Peripheral Blood
To determine the dietary effect on the phagocytic function of neutrophils in different groups, we compared the rate of chicken neutrophil phagocytosis. According to the Fig. 1a–d, under oil immersion lens, we observed the peripheral blood smears, with the field of view showing neutrophils with intracellular bacteria. Phagocytic rates of different groups on chicken neutrophils are shown in Fig. 1e. Obviously, in the Se group, neutrophils had a greater number of phagocytic bacteria compared to the control group, which referred to fact that neutrophil phagocytosis was optimal. In addition, in the Cd-treated group, neutrophil phagocytosis was the worst and the number of bacteria phagocytized by the neutrophils was the least. Moreover, the number of neutrophils engulfing bacteria of the Se/Cd-treated group was larger than that of the Cd-treated group, which referred to the protective role of Se on the Cd-spiked group.
Phagocytosis and phagocytic rate of different groups on chicken neutrophils. a Phagocytosis status from the control group; b Phagocytosis status from the Se-treated group; c Phagocytosis status from the Se/Cd-treated group; d Phagocytosis status from the Cd-treated group. e Phagocytic rate of different groups on chicken neutrophils. The black arrows in a-d refer to the intracellular bacteria in peripheral neutrophils
The mRNA Expression of NF-κB, iNOS, COX-2, and TNF-α from Peripheral Blood Neutrophils
As shown in Fig. 2, the mRNA expression levels of NF-κB, iNOS, COX-2, and TNF-α were examined in peripheral blood neutrophils of chicken. The mRNA expressions of NF-κB, iNOS, COX-2, and TNF-α for the Se/Cd-treated group and the Cd-treated group were significantly higher than those for the control group and the Se-treated group (P < 0.05), but there was no significant difference for NF-κB between the Se-treated group and the Se/Cd-treated group. Conversely, mRNA expression levels of these inflammatory factors had significant differences between the Se-treated group and the control group apart from NF-κB (P < 0.05). In addition to TNF-α, significant differences existed between the Se/Cd-treated group and the Cd-treated group (P < 0.05).
The mRNA expression of NF-κB, iNOS, COX-2, and TNF-α in peripheral blood neutrophils of different groups. The mRNA levels of NF-κB, iNOS, COX-2, and TNF-α in peripheral blood neutrophils of chicken. Effects of Se on Cd-induced changes in the mRNA levels of NF-κB, iNOS, COX-2, and TNF-α in peripheral blood neutrophils of chicken. The relative mRNA levels from the control group were used as reference values. The different letters indicate significant differences (P < 0.05) between any two groups. Each value represents the mean ± SD (n = 10)
The mRNA Expression of IL-1β, IL-2, IL-4, IL-10, IL-17, and IFN-γ from Peripheral Blood Neutrophils
With regard to cytokines, our results showed that the mRNA levels of IL-2, IL-4, and IL-10 were significantly promoted (P < 0.05) in chicken peripheral blood neutrophils of the Se-treated group compared to the control group (Fig. 3). The mRNA expression levels of these genes for the Se-treated group showed significant differences (P < 0.05) between the Se/Cd-treated group and the Cd-treated group, of which their values for the Se-treated group were significantly higher (P < 0.05) than those for the Se/Cd-treated group apart from IL-1β and IL-17. Moreover, there are no significant differences between the control group and the Cd-treated group for IL-1β, IL-2, and IL-4.
The mRNA expression of IL-1β, IL-2, IL-4, IL-10, IL-17, and IFN-γ in peripheral blood neutrophils of different groups. The mRNA levels of IL-1β, IL-2, Il-4, IL-10, IL-17, and IFN-γ in peripheral blood neutrophils of chicken. The relative mRNA expression levels from the control group were used as the reference values, and the different letters indicate that there were significant differences (P < 0.05) between any two groups. Each value represented the mean ± SD (n = 10)
The mRNA Expression of Hsp 40, Hsp 60, Hsp 70, and Hsp 90 from Peripheral Blood Neutrophils
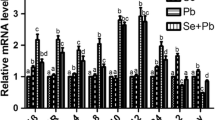
As Fig. 4 illustrates, the mRNA expression levels of Hsps in the Se/Cd-treated group and the Cd-treated group were significantly higher (P < 0.05) than in the control group and the Se-treated group. However, the mRNA expression level of Hsp 90 showed no significant difference between the Se-treated group and the control group. Additionally, we also found that mRNA expression levels of these Hsps in the Cd-treated group were significantly higher (P < 0.05) than in the Se/Cd-treated group except Hsp 60 and Hsp 90. This result indicated that Cd promoted the mRNA expression of Hsp 40, Hsp 60, Hsp 70, and Hsp 90 mRNA, while Se could alleviate the adverse effect of Cd on the Hsp mRNA expression.
The mRNA expression of Hsp 40, Hsp 60, Hsp 70, and Hsp 90 in peripheral blood neutrophils of different groups. The mRNA levels of Hsp 40, Hsp 60, Hsp 70, and Hsp 90 in peripheral blood neutrophils of chicken. The relative mRNA expression levels from the control group were used as the reference values, and the different letters indicate that there were significant differences (P < 0.05) between any two groups. Each value represented the mean ± SD (n = 10)
Discussion
Se is an essential trace element in the body with obvious antagonistic Cd effects. Previous studies have already demonstrated the protective effects of Se against subchronic exposure to dietary Cd, and it causes hepatotoxicity, oxidative stress, and apoptosis in chicken liver and kidney [17, 28]. Liu et al. suggested the great importance of Se for chicken immune tissues because Se supplementation during Cd exposure attenuated Cd-induced immune dysfunction [6].
Neutrophils contribute as an essential component to defenses against heavy metal injury through enhancing immune system activity. The numbers of phagocytes and neutrophil phagocytic activity are indicators of nonspecific immune activity. Several metals have been demonstrated to have influence on the phagocytic activity of human neutrophils. With regard to Se and Cd, they can cause a variety of biological effects including alterations of immune responses. To clarify the effects of Se and Cd on the peripheral blood neutrophils of the chicken, we first calculated the phagocytosis of neutrophils under microscope as well as the neutrophil phagocytosis rate of the different groups. Our results revealed that the neutrophil phagocytosis rate of the Se-treated group was the highest and the Cd-treated group was the lowest. Simultaneously, the phagocytosis rate of the Se/Cd-treated group showed slight enhancement compared to the Cd-treated group, which also meant that Se had a potential alleviating role on Cd-spiked peripheral blood neutrophils of chicken. Viñuelas-Zahínos E et al. indicated that the treatment of human neutrophils with Cd did not cause any cellular damage at a concentration of 1 × 10−3 mM for 30 min [29]. The study revealed inconsistent results, which may be, at least in part, due to the different experimental conditions used including different application regimes or varying experimental objects. However, many studies have shown that Cd affected immune cell injuries and Se alleviated this Cd-spiked dysfunction. Liu et al. suggested that Se played a protective role in inflammation induced by Cd via regulating the mRNA expression of inflammatory cytokines in chicken splenic lymphocytes [19]. Similarly, Xu et al. also reported the protective effect of Se against Cd in chicken splenic lymphocytes [8]. Obviously, the results of this study are in accordance with the results of the previously mentioned study. We inferred that the injurious effect of Cd on the phagocytic capacity of neutrophils could be partly changed by Se.
Inflammatory cytokines play a critical role in the inflammatory response induced by various environmental challenges such as exposure to Cd in living organisms, which can also be identified as the predominant mediators of inflammation. This research sought to understand the toxic effect of Cd and the protective role of Se against this toxic effect by determining the mRNA expression levels of inflammatory factors (NF-κB, iNOS, COX-2, and TNF-α) and cytokines (IL-1β, IL-2, IL-4, IL-10, IL-17, and IFN-γ) in the peripheral blood neutrophils of chicken. Our results showed that the mRNA expression levels of inflammatory factors (NF-κB, iNOS, COX-2, and TNF-α) were upregulated in the Cd-treated group and the Se/Cd-treated group compared to the control group. Interestingly, it was higher in the Cd-treated group. Some studies have demonstrated that immune cells’ inflammatory response is closely related to the expression of inflammatory cytokines. TNF-α is the most important cytokine, which activates neutrophils and lymphocytes and promotes the synthesis and release of other cytokines [30]. As a pro-inflammatory factor, the main function of iNOS in inflammatory neutrophils induces the production of NO, and NO is a free radical which was synthesized under inflammatory conditions and takes part in immunoregulation [31]. COX-2, another important inflammatory cytokine, not normally expressed in tissues or organs, is stimulated by physiological or pathological condition factors [32]. NF-κB has the ability to participate in regulation of multiple cytokines during different phases of the early immune response and inflammatory response. It has been reported that Cd stimulation caused the expression of ICAM-1 via NF-κB activation in cerebrovascular endothelial cells [33]. Similar to Liu and Cao et al.’s studies [6, 34], in our experiment, the mRNA expression of the inflammatory factors (NF-κB, iNOS, COX-2, and TNF-α) increased due to Cd exposure, but in the Se/Cd-treated group, the mRNA levels of iNOS, COX-2, and TNF-α were significantly reduced compared to the Cd-treated group. Moreover, Låg M et al. have shown that the mRNA expression levels of IL-1β and TNF-α were reduced after exposure to Cd in lung cells from rats [35]. Furthermore, the Se/Cd-treated group showed a significant reversal of the mRNA expression levels of inflammatory cytokines’ increases in the Cd-treated group; we identified that this is the first evidence of the effect of Se on mRNA expressions of pro-inflammatory cytokines in the neutrophils of chicken.
With regard to cytokines, our results showed that the mRNA levels of IL-1β, IL-2, IL-4, and IL-10 were significantly promoted in the Se-treated group compared to the control group and the mRNA expression levels of the Se-treated group were significantly higher than the Se/Cd-treated group apart from IL-1β and IL-17. We also found several other studies that have demonstrated that cytokines could interact with each other, thereby regulating the immune system. Like IFN-γ, IL-2, and TNF-α, which were secreted from Th1 cells to enhance the clearance of many intracellular pathogens, there has been a study that has proven that these three cytokines can promote the phagocytic activity of neutrophils. IFN-γ is also important to neutrophil activation because it is necessary for TNF-α to synergize with IFN-γ to induce a variety of neutrophil activating genes, including NOS. It was shown that IFN-γ promoted the level of NO by inducing the release of iNOS [36], then causing an inflammatory response. Moreover, Chen et al. have reported the protective effect of Se against Cd and chicken splenic lymphocytes [37]. El-Boshy M E et al. showed that Se significantly increased the mRNA expression level of IFN-γ, while IL-10 was decreased in rats and Se/Cd treatment significantly improved the elevation of serum IL-1β, TNF-α, and IL-10[21]. To summarize our results, Cd had a toxic effect on chicken neutrophils through inducing inflammation, while Se had a protective role against Cd toxicity.
Hsp is a kind of protective protein produced under adverse conditions that has important roles in the immune defenses of organism [38]. To evaluate the effect of Se and Cd on the mRNA expression of Hsps (Hsp 40, Hsp 60, Hsp 70, and Hsp 90) on neutrophils of chicken peripheral blood, we separated neutrophils from cocks’ peripheral blood that received either Se, Cd, or Se/Cd in their diets for 84 days and determined the mRNA expression levels of Hsps. Our results showed that the heavy metal Cd could induce the mRNA expression of Hsps, and this status was slightly reduced by Se but was still higher than the control group. Many studies have shown that the synthesis of Hsps under stress conditions is one of the first physiological events that protect cells from consequent injury [39, 40]. Cao et al. have reported that the mRNA expressions of Hsp 60, Hsp 70, and Hsp 90 were significantly enhanced in livers of duck, which were exposed to molybdenum and/or Cd [34]. It has also been demonstrated that the mRNA expression levels of Hsp 60 and Hsp 90 in Labeo rohita after Cd exposure were significantly upregulated [41]. Brunt JJ et al. have shown that Cd exposure promoted Hsp accumulation in A6 kidney epithelial cells of Xenopus laevis [42]. In addition, we detected that the mRNA expression levels of Hsps in the Se/Cd-treated group were reduced compared to those in the Cd-treated group in our study. Chen et al. illustrated that treatment of chicken splenic lymphocytes with Se in combination with Cd promoted the mRNA expression of Hsps, which was reduced by Cd treatment [37]. Quite notably, these results were consistent with our previously mentioned studies.
In summary, Cd treatment decreased the phagocytic rate of neutrophils in peripheral blood. Cd induced the mRNA expression levels of inflammatory factors (NF-κB, iNOS, COX-2, and TNF-α), while the mRNA expression levels of cytokines (IL-2, IL-4, and IL-10 and IFN-γ) were lower than the control group; the mRNA expression level of IL-10 was higher due to Cd. In addition, Cd treatment enhanced the mRNA expression levels of Hsps. However, Se exhibited antagonistic roles against the Cd-spiked inflammatory factors and Hsp mRNA expression levels in chicken peripheral blood neutrophils. We suggested that Hsps were involved in the process of the Cd-spiked toxic effect in chicken peripheral blood neutrophils.
References
Låg M, Refsnes M, Lilleaas EM, Holme JA, Becher R, Schwarze PE (2005) Role of mitogen activated protein kinases and protein kinase C in cadmium-induced apoptosis of primary epithelial lung cells. Toxicology 211(3):253–264
Lin W, Chen D, Wang H, Liu Z (2009) Effects of lead and/or cadmium on the expression of metallothionein in the kidney of rats. Biol Trace Elem Res 129(1):190–199
Wang L, Lin SQ, He YL, Liu G, Wang ZY (2013) Protective effects of quercetin on cadmium-induced cytotoxicity in primary cultures of rat proximal tubular cells. Biomed Environ Sci 26(4):258–267
Zhang Z, Bi M, Liu Q, Yang J, Xu S (2016) Meta-analysis of the correlation between selenium and incidence of hepatocellular carcinoma. Oncotarget: doi: 10.18632/oncotarget.12804
Yang S, Zhang Z, He J, Li J, Zhang J, Xing H, Xu S (2012) Ovarian toxicity induced by dietary cadmium in hen. Biol Trace Elem Res 148(1):53–60
Liu S, Xu FP, Yang ZJ, Li M, Min YH, Li S (2014) Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: mechanisms of oxidative stress and apoptosis. Biol Trace Elem Res 160(3):340–351
Jihen EH, Fatima H, Nouha A, Baati T, Imed M, Abdelhamid K (2010) Cadmium retention increase: a probable key mechanism of the protective effect of zinc on cadmium-induced toxicity in the kidney. Toxicol Lett 196(2):104–109
Xu F, Liu S, Li S (2015) Effects of selenium and cadmium on changes in the gene expression of immune cytokines in chicken splenic lymphocytes. Biol Trace Elem Res 165(2):214–221
Ilbäck NG, Fohlman J, Friman G, Ehrnst A (1994) Immune responses and resistance to viral-induced myocarditis in mice exposed to cadmium. Chemosphere 29(6):1145–1154
Jin Y, Liu L, Zhang S, He R, Wu Y, Chen G, Fu Z (2016) Cadmium exposure to murine macrophages decreases their inflammatory responses and increases their oxidative stress. Chemosphere 144:168–175
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of SE-deficient chicks. J Nutr 143(5):613–619
Yao H, Liu W, Zhao W, Fan R, Zhao X, Khoso PA, Zhang Z, Xu S (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv 4(109):64032–64042
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830(4):3112–3120
Yao H, Fan R, Zhao X, Zhao W, Liu W, Yang J, Sattar H, Zhao J, Zhang Z, Xu S (2016) Selenoprotein W redox-regulated Ca2+ channels correlate with selenium deficiency-induced muscles Ca2+ leak. Oncotarget 7(36):57618–57632
Broome CS, Mcardle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80(1):154–162
Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149(3):352–361
Li JL, Jiang CY, Li S, Xu SW (2013) Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicology & Environmental Safety 96(8):103–109
Li JL, Gao R, Li S, Wang JT, Tang ZX, Xu SW (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23(4):695–705
Liu S, Xu F, Fu J, Li S (2015) Protective roles of selenium on nitric oxide and the gene expression of inflammatory cytokines induced by cadmium in chicken splenic lymphocytes. Biol Trace Elem Res 168(1):1–9
Marettová E, Maretta M, Legáth J, Košutzká E (2012) The retention of cadmium and selenium influence in fowl and chickens of F1 generation. Biol Trace Elem Res 147(1):130–134
El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 29:104–110
Mayadas TN, Cullere X, Lowell CA (2014) The multifaceted functions of neutrophils. Pathology: Mechanisms of Disease 9(9):181–218
Kumar V, Sharma A (2010) Neutrophils: Cinderella of innate immune system. Int Immunopharmacol 10(11):1325–1334
Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V (2016) Human neutrophils in auto-immunity. Semin Immunol 28(2):159–173
Snella MC (1985) Manganese dioxide induces alveolar macrophage chemotaxis for neutrophils in vitro. Toxicology 34(2):153–159
Lu J, Jin T, Nordberg G, Nordberg M (2005) Metallothionein gene expression in peripheral lymphocytes and renal dysfunction in a population environmentally exposed to cadmium. Toxicology & Applied Pharmacology 206(2):150–156
Xie L, Xing W, Chen H, Wu D, Cazan AM, Klerks PL (2016) A low level of dietary selenium has both beneficial and toxic effects and is protective against Cd-toxicity in the least killifish Heterandria formosa. Chemosphere 161:358–364
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. Journal of Trace Elements in Medicine & Biology Organ of the Society for Minerals & Trace Elements 28(1):89–93
Viñuelas-Zahínos E, Luna-Giles F, Torres-García P, Rodríguez AB, Bernalte-García A (2011) Effects of a derivative thiazoline/thiazolidine azine ligand and its cadmium complexes on phagocytic activity by human neutrophils. Inorg Chim Acta 366(1):373–379
Lee HS, Park HW, Song WJ, Jeon EY, Bang B, Shim EJ, Moon HG, Kim YK, Kang HR, Min KU (2015) TNF-α enhance Th2 and Th17 immune responses regulating by IL23 during sensitization in asthma model. Cytokine 79:23–30
Andrés MCD, Takahashi A, Oreffo ROC (2016) Demethylation of an NF-κB enhancer element orchestrates iNOS induction in osteoarthritis and is associated with altered chondrocyte cell cycle. Osteoarthritis & Cartilage 24(11):1951–1960
Herz C, Márton MR, Tran HTT, Gründemann C, Schell J, Lamy E (2016) Benzyl isothiocyanate but not benzyl nitrile from Brassicales plants dually blocks the COX and LOX pathway in primary human immune cells. J Funct Foods 23:135–143
Jeong EM, Moon CH, Kim CS, Lee SH, Baik EJ, Moon CK, Jung YS (2004) Cadmium stimulates the expression of ICAM-1 via NF-kappaB activation in cerebrovascular endothelial cells. Biochemical & Biophysical Research Communications 320(3):887–892
Cao H, Gao F, Xia B, Zhang M, Liao Y, Yang Z, Hu G, Zhang C (2016) Alterations in trace element levels and mRNA expression of Hsps and inflammatory cytokines in livers of duck exposed to molybdenum or/and cadmium. Ecotoxicology & Environmental Safety 125:93–101
Låg M, Rodionov D, Øvrevik J, Bakke O, Schwarze PE, Refsnes M (2010) Cadmium-induced inflammatory responses in cells relevant for lung toxicity: expression and release of cytokines in fibroblasts, epithelial cells and macrophages. Toxicol Lett 193(3):252–260
Evans DM, Ralston SH (1996) Nitric oxide and bone. J Bone Miner Res 11(3):300–305
Chen X, Zhu YH, Cheng XY, Zhang ZW, Xu SW (2012) The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17(12):14565–14572
Ghasemi Y, Dabbagh F, Rasoulamini S, Borhani HA, Morowvat MH (2012) The possible role of HSPs on Behcet’s disease: a bioinformatic approach. Computers in Biology & Medicine 42(11):1079–1085
Nazir A, Saxena DK, Kar CD (2003) Induction of hsp70 in transgenic Drosophila: biomarker of exposure against phthalimide group of chemicals. Biochim Biophys Acta 1621(2):218–225
Snyder MJ, Girvetz E, Mulder EP (2001) Induction of marine mollusc stress proteins by chemical or physical stress. Archives of Environmental Contamination & Toxicology 41(1):22–29
Giri SS, Sen SS, Jun JW, Sukumaran V, Park SC (2016) Immunotoxicological effects of cadmium on Labeo rohita, with emphasis on the expression of HSP genes. Fish & Shellfish Immunology 54:164–171
Brunt JJ, Khan S, Heikkila JJ (2012) Sodium arsenite and cadmium chloride induction of proteasomal inhibition and HSP accumulation in Xenopus laevis A6 kidney epithelial cells. Comparative Biochemistry & Physiology Part C Toxicology & Pharmacology 155(2):307–317
Acknowledgements
The authors are grateful to the members of the Veterinary Physiology and Veterinary Internal Medicine Lab for sample collection and processing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The Institutional Animal Care and Use Committee of Northeast Agricultural University approved all procedures used in this experiment.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31472161).
Additional information
All other authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the journal.
Rights and permissions
About this article
Cite this article
Tan, S., Chi, Q., Liu, T. et al. Alleviation Mechanisms of Selenium on Cadmium-Spiked Neutrophil Injury to Chicken. Biol Trace Elem Res 178, 301–309 (2017). https://doi.org/10.1007/s12011-016-0924-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0924-8