Abstract
An experiment was conducted to investigate the effects of dietary nanoselenium supplementation at 0, 0.6 and 1.2 mg/kg of diet on growth performance, serum biochemical parameters, immune response, antioxidant capacity, and jejunal morphology of 29-d-old male broilers subjected to heat stress at 37 ± 1°C for 14 d. Broilers were fed for 42 d on the experimental diets. The results showed that nanoselenium supplementation had no effect on growth performance, but it supplementation at the rate of 1.2 mg/kg diet decreased the serum concentration of cholesterol prior to the heat exposure. Further, dietary nanoselenium supplementation linearly increased the high-density lipoprotein cholesterol concentration, while linearly decreased those of low-density lipoprotein cholesterol and aspartate aminotransferase in the serum before applying heat stress. Compared with thermoneutral temperature, heat stress reduced body mass gain, feed intake, percentages of carcass, breast, leg, abdominal fat, bursa of Fabricius, thymus, antibody response against sheep red blood cells, serum concentration of protein, erythrocyte activities of glutathione peroxidase and superoxide dismutase, jejunal villus height, and villus height to crypt depth ratio, while increased feed conversion ratio, percentages of liver, gizzard, pancreas, gallbladder, heart, and the concentrations of aspartate aminotransferase and malondialdehyde. Dietary supplementation of nanoselenium linearly reduced the abdominal fat and liver percentages, while linearly increased the activity of glutathione peroxidase and villus height in heat-stressed broilers. Furthermore, the lower level of nanoselenium decreased the percentages of gizzard and heart in broilers under heat stress. The diet supplemented with 1.2 mg/kg nanoselenium improved feed conversion ratio and increased antibody response against sheep red blood cells, activity of superoxide dismutase, and villus height to crypt depth ratio, but decreased the serum concentrations of cholesterol, low-density lipoprotein cholesterol, and malondialdehyde in heat-stressed broilers. The results suggest that supplemental nanoselenium improved growth performance, internal organs health, immune response, and jejunal morphology by alleviating the oxidative stress induced by heat stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High ambient temperature, which is known as heat stress (HS), is one of the main undesirable factors in poultry production that negatively affects production performance, immunity, and meat quality of broilers, resulting in significant economic losses [1–3]. Currently, micromineral supplementation is used to alleviate or eliminate the detrimental effects of heat stress [2, 4]. Selenium is an integral constituent of thirty identified selenoproteins; including glutathione peroxidases (GPxs) include cytosolic GPx (GPx1), gastrointestinal GPx (GPx2), extracellular GPx (GPx3), phospholipid hydroperoxide GPx (GPx4), three iodothyronine deiodinases (5'DI, 5'DII, 5'DIII), three thioredoxin reductases (TR1, TR2 and TR3), and Selenoprotein-P [5]. Selenium is an essential micromineral for poultry production and low Se status leads to poor growth performance, nutritional muscular dystrophy, exudative diathesis, immunodeficiencies, decreased concentrations of the active form of thyroid hormone, reduced antioxidant status in plasma and various organs, and lipid peroxidation [6–11]. Nutrients consumption and metabolism are influenced by heat stress [1, 12]. Heat stress reduces the feed consumption and, consequently, the intake of micronutrients such as vitamins A, E, and C and Se. Exposing to heat stress also caused increases in the mineral excretion, while it decreased the concentrations of antioxidant vitamins (e.g., Vitamins E, C, and A) and minerals (e.g., Se, Zn, and Cr) in the serum and liver of poultries [13]. Both heat stress and Se deficiency can disturb the balance between production of free radicals and antioxidant defense system result in oxidative stress. The induced-oxidative stress causes damage to a wide variety of biomolecules including lipids, proteins, and DNA, resulting in tissue damages and organ failure [7, 14, 15]. Moreover, synthesizing new selenoproteins such as GPxs may be increased under heat stress conditions, which may exacerbate a marginal Se deficiency or may increase Se requirement [16]. The recommended dietary level of Se for broilers is 0.15 mg/kg [17]. It seems that this level is inadequate for optimum productivity and health of broilers reared under heat stress. Furthermore, chemical form of Se affects its bioavailability and distribution in the body [16]. Today′s sodium selenite is commonly used as a supplement in the poultry diet. Different Se forms including inorganic, such as sodium selenite, organic, such as selenomethionine and Se-enriched yeast, and nanoselenium (Nano-Se) has been compared to choose the best source of Se for maximizing the poultry health and production in thermoneutral or oxidative (heat stress) rearing condition [8, 18–20]. The effects of sodium selenite and Nano-Se on the growth performance, serum GPx activity, Se concentrations in the serum, liver, and breast muscle, and Se retention in the whole body and liver tissue were compared by Hu et al. [21]. Their results showed that both Se sources comparably increased the survival ratio, average daily gain, feed efficiency, and serum GPx activity. However, the concentration of Se in the serum and tissues, the transfer of Se from the intestinal lumen to the body, and the Se retention were markedly higher when broilers fed Nano-Se. Supplementation of various sources of Se (sodium selenite, Se-enriched yeast, and Nano-Se) did not affect the performance parameters in the non-stressed or oxidative-stressed broilers; however, Se improved the antioxidant capacity in broilers under oxidative stress, with a more pronounced impact caused by Nano-Se [20]. However, Wang reported that both sodium selenite and Nano-Se improved the growth performance and increased the tissue Se content and serum and hepatic GPx activity, and there was no noticeable difference between Se sources [18]. Although several studies have evaluated the effects of dietary Se supplementation on broilers kept under high ambient temperature conditions, the results are inconsistent. da Silva et al. reported no significant effects of Se sources and levels on the performance, spleen and bursa indices, hematological profile, and antibody response to infectious bursal disease virus of broilers subjected to HS conditions [22]. However, dietary supplementation with selenomethionine (Se-Met) improved feed efficiency and cell-mediated and humoral immunity in heat-stressed broilers [23]. Another study revealed that Se-Met supplementation had no significant effects on the performance and relative masses of lymphoid organs, while remarkably improved the antibody responses to sheep red blood cells (SRBCs) and blood lipid profile in broilers subjected to heat stress [2]. To our knowledge, very little information about the effects of dietary Nano-Se supplementation on the heat-stressed broilers is available in the literature. Therefore, the aim of this study was to evaluate the effects of Nano-Se supplementation on performance, carcass characteristics, serum biochemical parameters, immune response, antioxidant status, and jejunal morphology in broilers subjected to heat stress.
Materials and Methods
Birds, diets, and experimental design
All procedures were approved by Birjand University Animal Care and Use Committee. One hundred sixty male Ross 308 broiler chicks, one-day-old, were obtained from a commercial hatchery, weighed, and randomly assigned to four groups, each of which had four replicates of 10 birds per replicate. The broilers were fed a starter diet until 10 d of age, a grower diet from 11 to 24 d of age, followed by a finisher diet from 25 to 42 d of age. All diets were formulated to meet the nutrient requirements as recommended by Ross 308 broiler rearing guidelines (Aviagen, 2007), and the ingredients and nutrient composition are shown in Table 1. Two groups were served as the control and fed with the basal diets. The third and fourth groups were randomly received the basal diets supplemented with 0.6 and 1.2 mg/kg Nano-Se from 1 to 42 d of age. Feed in mash form and fresh water were provided ad libitum and broilers were maintained on a 22-h L: 2-h D lightening program through the experimental period. All birds were held in a thermoneutral environment until the 28th day of the experiment and temperature was decreased based on the normal management practice from 32°C to 21°C. Only one of the control groups was reared in a thermoneutral room at 21°C with 55% humidity for 24 h d-1 throughout the experimental period, while the remaining groups were kept at another heat controlled room at 21 °C for 18 h d-1 and 37± 1°C for 6 h d-1 (1030-1630) with 55% relative humidity from 29 to 42 d of age.
Growth performance measurements
Feed intake (FI) and body mass for each replicate pen were recorded at 10, 24, and 42 d of age after fasting for 4 h. Body mass gain (BMG) and feed conversion ratio (FCR) were calculated for each period and the overall experimental period.
Carcass characteristics measurements
At 42 d of age, two birds were selected at random from each replicate and weighted individually after a 4 h fasting. The birds were slaughtered by cervical dislocation and eviscerated carcass, breast, leg, abdominal fat, liver, pancreas, gallbladder, heart, gizzard, bursa of Fabricius, spleen, and thymus were weighed individually. The lengths of duodenum, jejunum, and ileum were also recorded. The weight of organs was expressed as a percentage of live body mass.
Blood Metabolites
At 28 and 42 d of age, two birds from each replicate were randomly selected and blood samples were collected from wing veins after a 4 h feed deprivation. The sera and plasma were harvested by centrifugation of blood at 3,000 g for 10 min, and stored at −20 °C in Eppendorf test tubes until assessed. The plasma activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) activities, and the concentrations of total protein, glucose, triglyceride, cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) concentrations in plasma were analyzed by standard kits (Pars Azmoon Co; Tehran, Iran) according to the manufacturer’s instructions.
Antibody Measurements
To quantitatively analyses of humoral immunity, SRBCs, a T-dependent antigen, were used. Two chicks from each replicate were injected intravenously with 1 mL of 10% SRBCs at 22 and 35 days of age. At the end of the experiment, the blood of these broilers was collected to determine serum antibody titers against SRBCs. The antibody responses were measured using a microhemagglutinaion technique [24].
Antioxidant Parameters
The plasma concentrations of malondialdehyde (MDA) were measured as thiobarbituric acid-reactive substances by the method of Yoshioka et al. [25]. Erythrocyte hemolysates were prepared as described previously [3]. The GPx and superoxide dismutase (SOD) activities were assayed in erythrocyte hemolysates using available commercial kits (Ransel and Ransod test kits, Randox Laboratories Ltd, UK) following the manuals.
Jejunal morphology
At the end of the experiment, Jejunal tissue samples were taken from the midpoint of jejunum, fixed in 10% buffered formalin, dehydrated manually, embedded in paraffin wax, cut to 3 μm thick, and stained with hematoxyline and eosin. Histological parameters were determined using an image analyzer (Image-Pro Plus version 4.5, 0.27). Morphological indices analyzed included: villus height (from the tip of the villi to the junction of villus and crypt), crypt depth (defined as the depth of the invagination between adjacent villi), villus width, and the villus height to crypt depth (V/C) ratio [26].
Statistical Analyses
Before statistical analysis, the Shapiro-Wilk test, stem-and-leaf plots, and normal probability plots were used to test the normal distribution of the data. The significance of the difference between the means of the non-stressed and heat-stressed, unsupplemented birds was tested with t-test if the parametric conditions existed, otherwise, the nonparametric Mann–Whitney test was used to detect the effect of HS. Data from heat-stressed groups were analyzed using the GLM procedure of SPSS (SPSS Inc., Chicago, 1993). Linear and quadratic effects were also analyzed. Statistical differences among the means were determined using Tukey multiple-range test. Statement of significance was based on P ≤ 0.05.
Results
Growth performance was not significantly affected by dietary treatments prior to the onset of heat stress (Table 2). Heat stress impaired the FI, BMG, and FCR of broilers. However, the diet supplemented with 1.2 mg/kg alleviated the adverse effects of HS on the FCR of heat stressed-broilers (Table 4).
Heat stress decreased the relative masses of carcass, breast, leg, abdominal fat, but increased those of liver, pancreas, gallbladder, gizzard, and heart. Supplementation with Nano-Se reduced the percentages of liver and abdominal fat in birds kept at HS conditions. Moreover, supplementation of Nano-Se at the rate of 0.6 mg/kg reduced the relative masses of gizzard and heart in heat-stressed broilers (Table 5).
Supplementation with Nano-Se linearly decreased the plasma concentrations of LDL-C and AST, but linearly increased that of HDL-C before heat exposure. Moreover, the cholesterol concentration was lower in broilers fed diets supplemented with 0.6 mg/kg Nano-Se than that in the control ones (Table 3). Heat stress decreased the plasma total protein concentration, but increased the AST activity in broiler chickens. Other biochemical parameters were not significantly influenced by HS. Supplementation of Nano-Se at the rate of 1.2 mg/kg reduced the concentrations of cholesterol and LDL-C in broilers subjected to HS. Supplemental Nano-Se tended to increase the protein concentration in birds kept under HS conditions (P<0.06) (Table 6).
The relative masses of bursa of Fabricius and thymus and the antibody production against SRBCs were significantly reduced when broilers were exposed to HS. Dietary Nano-Se supplementation increased the thymus relative mass, also its supplementation at 1.2 mg/kg diet enhanced antibody production against SRBCs in heat-stressed broilers (Table 7).
Heat stress decreased the activities of GPx and SOD, while increased the plasma concentration of MDA in broiler chickens. Dietary Nano-Se supplementation increased the GPx activity in broilers under HS. In addition, Nano-Se supplementation at 1.2 mg/kg diet increased the SOD activity, but decreased the MDA level in heat-stressed broilers (Table 7).
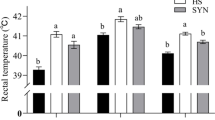
The villus height and the ratio of V/C were significantly decreased due to HS. Diets supplemented with Nano-Se linearly increased the villus height, also it addition at 1.2 mg/kg diet increased the ratio of V/C in broilers subjected to HS conditions (Table 8).
Discussion
It was clear from the obtained results that supplementing 0.6 or 1.2 mg/kg of Nano-Se had no beneficial effect on the growth performance of broilers during the starter and grower phases; and no adverse effect of Se on the growth was observed during these periods. These results confirm the findings of Cai et al. [27] and Liu et al. [28], who reported no significant effect of Nano-Se on performance of broilers reared under thermoneutral conditions. However, Zhou and Wang found the positive effect of Nano-Se supplementation on growth performance of broilers kept under normal conditions and concluded that supplementation with 0.3 ppm Nano-Se was effective in improving the growth performance of broilers and in increasing the Se retention in tissues [29]. The differences among studies might be associated with the length of experimental period or the background of Se in the feedstuffs. The adverse effect of HS on growth performance of broilers has been well documented [3]. The present results showed that heat-stressed broilers had lower FI and BMG, but higher FCR than those kept in thermoneutral condition. Prior studies also demonstrated that high ambient temperatures negatively affected the circulating levels of active form of thyroid hormone and insulin [4], nutrient digestibility [14], post absorption of nutrient metabolism [1], and immune response [2, 3] in broiler chickens. The obtained results suggest that the heat regime applied in the present study resulted in a Se deficiency. According to the present results, supplementation with 1.2 mg/kg Se-Nano improved the FCR in heat-stressed broilers at 42 d. In addition, the overall experimental results showed that the broilers supplemented with 1.2 mg/kg Nano-Se had better BMG and FCR than those supplemented with 0.6 mg/kg Nano-Se or control counterparts, suggesting that Nano-Se supplementation at the rate of 1.2 mg/kg could improve the growth performance of heat-stressed broilers. This improvement might be related to the positive effects of supplemental Se on the concentrations of anabolic hormones, nutrient digestibility, and immunocompetence in heat-exposed broilers. These results confirmed the results of Niu et al. [23], who reported that the effects of various levels of Se-Met on body mass and FI were not significantly different in broilers reared under either heat stress or thermoneutral conditions, whereas FCR was improved by higher dietary Se level when broilers were exposed to heat stress conditions. Compared with the control, both sodium selenite and Se-enriched yeast improved the FI and FCR in heat-stressed broilers [19]. However, different Se levels of the organic [2] and inorganic [22] form had no significant effect on the growth performance of broilers under heat stress conditions. The differences were possibly due to the Se status of animal, the size and chemical form of Se, quantity of Se in feedstuffs, supplemental level, dietary factors such as methionine and vitamin E, and the length and severity of HS [23, 27, 30].
The antioxidant defense system in the cell is based on the three main lines, including antioxidant enzymes, such as SOD and GPx, chain-breaking antioxidants, for example, vitamins A, E, and C, and various enzymatic systems such as lipolytic and proteolytic enzymes and chaperones including heat shock proteins (HSPs). Superoxide dismutase, catalyzes the dismutation of superoxide radical into hydrogen peroxide [31]. GPx isoenzymes this reaction and reduce hydrogen peroxide to water using reduced glutathione as co-substrate. The tissue-specific expression and activity of SOD and GPx can be affected by various factors, including genetic, nutritional, and stress-associated factors [31]. In this study, heat stress decreased the SOD and GPx activities and increased MDA level. Increasing evidence shows that heat stress impairs antioxidant defence system in poultry. For example, Hosseini-Vashan et al. reported that the plasma activities of GPx and SOD were decreased, whereas the concentration of MDA was increased by heat stress [3]. The obtained results also confirm those of Liu et al. [16]. Available evidence shows that heat stress affects the antioxidant defence system in different ways. As mentioned previously, the consumption of antioxidant micronutrients such as Se is reduced under heat stress conditions. Moreover, heat stress can negatively affect the gastrointestinal health and integrity [32, 33], which can diminish the nutrients absorption. It has been proven that the serum and liver concentrations of antioxidant vitamins (e.g., Vitamn A, C, and E) and minerals (e.g., Se, Zn, and Cr) were lower in HS conditions than those in the thermoneutral temperature [13]. In addition, under stress conditions the free radical production increases, which can inactivate the crucial antioxidant enzymes, causing an autocatalytical irreversible oxidation [34]. Therefore, synthesizing new antioxidant enzymes is a most important response to stress conditions. The appropriate response will be achieved when cofactors such as Se for GPx and Cu, Zn, and Mn for SOD are available [13]. The obtained results also demonstrated that dietary Nano-Se supplementation increased the GPx activity, also it addition at 1.2 mg/kg increased the activity of SOD, while reduced the MDA level in broilers under heat stress. It has been demonstrated that the enzymatic activity of GPxs, especially GPx 1-4, is depends on the Se intake [5]. Moreover, current evidences suggest that Se status influences the enzymatic activity of SOD [31]. A huge body of data shows that dietary Se supplementation increases antioxidant status in broiler chickens. For example, the Se sources, including sodium selenite, selenium-enriched yeast, and Nano-Se, increased the glutathione content and GPx activity, but reduced the MDA concentration in the serum of broilers under oxidative stress [20]. These authors concluded that the Nano-Se was more effective than sodium selenite and Se-yeast in improving antioxidant capacity in oxidative-stressed broilers. Moreover, both Nano-Se and sodium selenite increased the erythrocyte activities of catalase, GPx, and SOD in layer birds [8].
The percentages of carcass, breast, and leg were lower in heat-stressed broilers than those at thermoneutrality. This is partially related to an inadequate nutrient intake [1]. Heat stress also affects postabsorptive nutrient metabolism [1, 12]. Evidence demonstrated that both capacity of protein synthesis and protein breakdown were reduced in chronically heat-exposed broilers, however, this effect was more pronounced in the protein synthesis and resulted in a lower protein deposition [12]. Both heat stress and Se deficiency can disturb the steady state concentrations of free radicals and may lead to skeletal muscle damage. Under conditions of adequate vitamin E intake, Se deficiency impaired growth performance, downregulated the liver GPx activity and reduced the ratio of oxidized glutathione to total glutathione, but increased the indicators of muscle damage (AST, creatine kinase, and creatine kinase M and B) in plasma [8]. Moreover, a Se deficient diet downregulated the muscle selenoproteins in broilers leading to oxidative stress. The induced oxidative stress activated the apoptosis cascades, which induced nutritional muscular dystrophy [10]. In this study, supplemental Se did not significantly affect the percentages of carcass, breast, and leg.
The available plasma lipid substrate originating from either the diet or lipogenesis in the liver regulates the lipid storage in the adipose tissue [35]. Therefore, the abdominal fat deposition reduces when the absorption of dietary fat and the fatty acid synthesis reduce or the fatty acid β-oxidation increases. Literature demonstrated that the peripheral lipolysis can decrease at a high ambient temperature, however, the lower absorption of dietary fat as well as the lower capacities of de novo lipogenesis also reported at this situation [1]. In line with previous studies [36, 37], HS resulted in a significant reduction in abdominal fat content, which increases the cutaneous heat loss capacity of broiler chickens [1]. However, Habibian et al. showed clearly that HS conditions markedly increased the abdominal fat deposition in broiler chickens compared with thermoneutral conditions [2]. The obtained results also revealed that dietary Nano-Se supplementation further reduced the abdominal fat percentage in heat-stressed broilers. There are contradictory evidences regarding Se effects on fat metabolism and accumulation. Similarly, the finding of Habibian et al. demonstrated that Se-Met supplementation reduced the abdominal fat percentage in heat-exposed broilers [2]. It has been shown that the Se administration markedly reduced the abdominal fat mass and adipocyte size in obese rats through the differential regulation of the gene expression for fatty acid β-oxidation in fat tissue and liver [38]. Further, the nontoxic selenate concentrations reduced the lipid accumulation in mouse preadipocytes by activation of transforming growth factor beta-β1 signaling pathway [39]. On the other hand, Rahimi et al. found that neither heat stress nor Se sources had significant effect on the percentage of abdominal fat [19]. The response to supplemental Se depends on the diet composition, in particular energy and protein levels, animal-related factors such as breed and age of birds , the heat stress models used in the different studies, and the method used to determine the fat index [40].
Based on the finding of the present study, HS exposure resulted in a cardiac hypertrophy and Nano-Se supplementation at the rate of 0.6 mg/kg reduced the heart percentage in heat-stressed broilers, indicating the cardioprotective effect of Nano-Se. An inverse association between Se status and cardiovascular disease has been reported [41]. It has been found that the Se deficiency markedly reduced the activities of GPx and thioredoxin reductase, while increased the levels of protein carbonyls, an indicator of oxidative injury, in cardiac tissue and resulted in a worsening of cardiac functional parameters and a cardiac hypertrophy [15]. Their results also showed that dietary Se intake reduced the disease severity and mortality in the spontaneously hypertensive rats through increasing the cardiac antioxidant capacity. The currently suggested mechanisms by which Se exerts beneficial effects on cardiac health including increased antioxidant status, reduced myocardial cell apoptosis, reduced the nuclear translocation of nuclear factor kappa-B (NFkB), and reduced the dephosphorylation of connexin-43 [41].
Pancreas is more susceptible to oxidative injury compared with other organs because of its lower antioxidant capacity and the excessive free radical generation within its cells [42]. In this study, a significant increase in the percentage of pancreas was observed due to HS conditions, which may be associated with the oxidative stress-mediated pancreatic dysfunction [42]. The other explanation could be the reductions in the activities of digestive enzymes due to heat stress [43] causing a pancreatomegaly to mount a proper nutrient digestibility. It has been found that deficiency of Se led to pancreatic degeneration in the chicks, which resulted in a reduction in the activity of lipase, chymotrypsin, and trypsin, poor growth, and poor feathering [44]. In this study, Nano-Se supplementation did not alleviate the adverse effect of HS on the pancreas.
Exposing to a high ambient temperature increased the relative gizzard mass in this study. However, the addition of 0.6 mg/kg Nano-Se attenuated this negative effect in broilers under HS. Myopathy of the smooth muscle of gizzard is associated with the Selenium or Vitamin E deficiency. It has also been demonstrated that the Se deficiency decreased the digestion and absorption of Vitamin E [44]. Supplementation of Se at the rate of 0.4 ppm to a low Se diet increased the concentration of Se in the gizzard and the plasma activity of GPx, while reduced the plasma AST activity, resulted in a lower incidence of gizzard myopathy in turkeys [6]. Selenoprotein W has antioxidant property and its expression in the gastrointestinal tract of avian is easily affected by dietary Se content. Supplementation of 1-3 ppm Se to a practical diet markedly increased the expression of this selenoprotein in the various gastrointestinal parts, such as gizzard [45].
Following the results, the plasma activities of ALT and LDH and the serum concentration of glucose as well as total protein concentration were unaffected by dietary manipulation before heat stress, which are in line with the results of Selim et al. who found that different sources of Se (inorganic, organic, and Nano) did not influence the plasma ALT activity and the total protein concentration in the non-stressed broilers [46]. In this study, the Nano-Se supplementation linearly decreased the plasma AST activity prior to the thermal challenge. In contrast with these results, Mohapatra et al. found that dietary Nano-Se supplementation increased the AST activity, but decreased the ALT activity in grower birds kept under thermoneutrality [8]. The upregulation of the HSPs (60, 70, and 90) gene expressions by Se deficiency reduced the activities of L-glutathione and GPx, while increased the concentration of MDA in the liver of chickens, which resulted in serious hepatic injuries [47]. The obtained results also showed that HS treatment resulted in higher plasma AST activity, an indicator of damage to liver and to skeletal and cardiac muscles [4], and liver percent. Further, supplemental Se did not alter the AST activity, while reduced the liver percentage in heat-stressed birds. In a more recent study, Amin et al. reported that the acetaminophen oral overdosing caused significant increases in the serum activities of the ALT, AST, and ALP, hepatic lipid peroxidation, hepatic catalase and SOD activities, decresed the level of reduced glutathione and glutathione reductase activity, and increased DNA fragmentation, a hepatic biomarker of cell death, in hepatocytes [48]. Their results also showed that Nano-Se supplementation protected rat liver against acetaminophen toxicity and restored its cellular structure by improving liver function and antioxidant enzymes activities as well as reducing the hepatic DNA fragmentation. In the present study, the serum concentration of total protein was lower in heat-stressed broilers than that in the non-stressed broilers. This effect is explained by the reduced protein consumption and digestibility under heat stress conditions. Contrary to this result, heat exposure did not alter the serum total protein, albumin, and globulin concentrations in broilers [49]. The results of the present study also demonstrated that the Nano-Se administration did not attenuate the adverse effect of HS on the total protein concentration. In this study, the plasma concentration of glucose was not influenced by both heat stress and Nano-Se supplementation. Similarly, Imik et al. stated that serum glucose concentration did not affect by heat stress [49]. However, Habibian et al. reported that heat stress elevated the serum glucose concentration in broilers and supplementation with Se-Met alleviated this adverse effect [2].
The obtained results showed that dietary Se supplementation caused a linear decrease in the serum concentrations of LDL-C, but resulted in a linear increase in that of HDL-C before heat stress conditions. Further, the lower level of Nano-Se reduced the cholesterol concentration in broilers. Exposure to HS did not alter plasma lipid profile, which is in accordance with the results of previous reports [3, 49]. However, addition of Se at 1.2 mg/kg decreased the serum concentrations of cholesterol and LDL-C in heat-stressed broilers. Moreover, the increasing Se level to 1.2 mg/kg significantly decreased the concentration of HDL-C compared with that in the broilers supplemented with 0.6 mg/kg. These results are partly consistent with the findings of Habibian et al. [2], who reported that Se-Met supplementation did not positively affect the serum lipid profile of broilers reared under normal temperature conditions, while decreased the concentrations of triglyceride, total cholesterol, and LDL-C and increased the concentration of HDL-C in broilers kept under a high temperature. It has been proven that the Se deficiency caused increases in 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (HMG-CoA reductase, rate controlling enzyme in the cholesterol biosynthesis) activity and apolipoprotein E concentration [50]. Supplementation of Se in hypercholesterolemic rats upregulates the expression of 5'DI, which in turn regulates the thyroid hormones metabolism leading the downregulation of the apolipoprotein B and HMG-CoA reductase expressions [51]. Ness et al. previously revealed that thyroid hormone administration caused an increase in the activity of cholesterol 7α hydroxylase activity, the enzyme which catalyzes the rate-limiting step in bile acid synthesis [52]. Furthermore, the supplementation of Se up to 1 mg/kg increased the LDL receptor activity and mRNA expression during hypercholesterolemia [53]. Another possible mechanism whereby Se exerts its hypolipidemic effect is by changing excretion of cholesterol and bile acids. The incorporation of 10 μg/kg Se-enriched Japanese radish sprouts in laying hens diet enhanced the fecal excretion of cholesterol and bile acids, whereas decreased the concentrations of triglyceride and cholesterol in the serum and yolk and increased the serum HDL-C concentration [54].
Exposure to HS caused significant decreases in the relative masses of bursa and thymus and antibody response to SRBCs in broilers. Increasing evidence suggests that HS conditions compromise the immunity of broilers. The destruction of lymphoid organs, decreased T-helper 2 cytokines production, and increased inflammatory cytokines production were reported under heat stress conditions [55]. Data from Liu et al. demonstrated that exposure of black-boned broilers to HS markedly reduced the enzymatic activities of SOD, GPx, and catalase in the serum and the growth indices of the bursa of Fabricius and spleen, while increased the concentration of MDA [16]. The obtained results also suggested that the supplemental Se increased the index of thymus in heat-stressed broilers, also Nano-Se supplementation at 1.2 mg/kg improved antibody titer against SRBCs. Selenium is essential for normal immune function and its deficiency impairs both cellular and humoral immunity. The deficiency of Se induced oxidative stress and increased the expressions of HSPs in the spleen, thymus, and bursa of Fabricius of broilers, which lead to defects in immune organ morphology and function [11]. Moreover, Xu and Tian found that the acute heat stress increased the levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4, HSP60, HSP70, HSP90, and MDA, while reduced the levels of GPx and SOD in the lymphoid organs [56]. Their result also demonstrated that the supplemental Se restored the adverse effects of acute HS on the TNF- α, IFN-γ, and HSPs expressions and on the activity of antioxidant enzymes in these organs. The present findings are partly consistent with the results of Habibian et al. who found that the relative masses of immune organs and primary and secondary antibody responses to SRBCs were markedly reduced by HS and Se supplementation as Se-Met only alleviated the negative effect of HS on the secondary antibody response to SRBCs [2].
Although supplementation with Nano-Se had no significant effect on the indices of immune organs under normal conditions, however, it supplementation at the doses of 0.3 to 1.0 ppm improved IgM production and at the dose of 0.3 ppm improved IgG production in broilers at 42 d [27]. On the other hand, different sources and levels of Se on the immunologic responses of broilers against Newcastle vaccine and SRBCs were not significant [23]. The differences among studies may be associated with the Se background of birds, stress condition, the level and intensity of heat stress, bioavailability of supplemental Se, breed and age of birds, and type of antigen applied in experiments [1, 24, 27, 57].
Following to results, HS resulted in a shorter villus and a lower V/C ratio, and supplementation with 1.2 mg/kg Nano-Se attenuated these adverse effects in heat-stressed broilers. When broilers are exposed to heat stress, the blood and nutrient flow to gastrointestinal tract reduces, which causes intestinal hypoxia, ATP depletion, intracellular acidosis as well as oxidative and nitrosative stress resulting in altered intestinal function and integrity [32]. Increased intestinal permeability increases the lipopolysaccharide leakage to internal environment leading to multiple organ failure [32]. It has also been reported that HS upregulated the mRNA and protein expression of HSP70, HSP90, and NFkB, but reduced epidermal growth factor in the jejunal mucosa of black-boned chickens [33]. Gastrointestinal damages reduced the Se absorption, which may induce Se deficiency. In addition, Se can impact the gastrointestinal histology through the regulation of the inflammatory cytokine productions and through increasing the antioxidant status. Se deficiency induced the production of harmful free radicals including oxygen and nitrogen free radicals, while reduced the antioxidant capacity in the intestine, which resulted in oxidative damage to chicken intestinal tissues [9]. The current research suggests that Nano-Se supplementation at a rate of 1.2 ppm diminishes the lipid peroxidation and helps broilers maintain the intestinal structure under heat stress circumstances. It has been shown that dietary supplementation with 1-3 ppm sodium selenite for 90 days increased the Se concentration and selenoprotein W expression in the bird gastrointestinal tract including small intestine [45]. In another study, supplementing with 0.4 mg sodium selenite per kg diet enhanced the GPx activity in the blood and liver as well as of the thioredoxin in duodenal mucosa, liver, and kidney of broiler chickens [58].
In conclusion, dietary supplementation with Nano-Se improved growth performance, internal organs health, immune response, and jejunal morphology by decreasing the oxidative stress induced by heat stress. Further research would be necessary to elucidate the potential of Nano-Se as a nutritional supplement to ameliorate the negative effects of heat stress in poultry.
References
Renaudeau D, Collin A, Yahav S, De Basilio V, Gourdine J, Collier R (2012) Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi:10.1017/S1751731111002448
Habibian M, Ghazi S, Moeini MM, Abdolmohammadi A (2014) Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int J Biometeorol 58:741–752. doi:10.1007/s00484-013-0654-y
Hosseini-Vashan S, Golian A, Yaghobfar A (2015) Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int J Biometeorol. doi:10.1007/s00484-015-1112-9
Perai AH, Kermanshahi H, Nassiri Moghaddam H, Zarban A (2015) Effects of chromium and chromium + vitamin C combination on metabolic, oxidative, and fear responses of broilers transported under summer conditions. Int J Biometeorol 59:453–462. doi:10.1007/s00484-014-0860-2
Mehdi Y, Hornick JL, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18:3292–3311. doi:10.3390/molecules18033292
Cantor AH, Moorhead PD, Musser MA (1982) Comparative effects of sodium selenite and selenomethionine upon nutritional muscular dystrophy, selenium-dependent glutathione peroxidase, and tissue selenium concentrations of turkey poults. Poult Sci 61:478–484. doi:10.3382/ps.0610478
Fischer J, Bosse A, Pallauf J (2008) Effect of selenium deficiency on the antioxidative status and muscle damage in growing turkeys. Arch Anim Nutr 62:485–497. doi:10.1080/17450390802453468
Mohapatra P, Swain RK, Mishra SK, Behera T, Swain P, Mishra SS, Behura NC, Sabat SC, Sethy K, Dhama K, Jayasankar P (2014) Effects of dietary nano-selenium on tissue selenium deposition, antioxidant status and immune functions in layer chicks. Int J Pharmacol 10:160–167
Yu J, Yao H, Gao X, Zhang Z, Wang JF, Xu SW (2015) The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol Trace Elem Res 163:144–153. doi:10.1007/s12011-014-0164-8
Huang JQ, Ren FZ, Jiang YY, Xiao C, Lei XG (2015) Selenoproteins protect against avian nutritional muscular dystrophy by metabolizing peroxides and regulating redox/apoptotic signaling. Free Radic Biol Med 83:129–138. doi:10.1016/j.freeradbiomed.2015.01.033
Yang Z, Liu C, Zheng W, Teng X, Li S (2016) The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol Trace Elem Res 169:341–351. doi:10.1007/s12011-015-0407-3
Temim S, Chagneau AM, Peresson R, Tesseraud S (2000) Chronic heat exposure alters protein turnover of three different skeletal muscles in finishing broiler chickens fed 20 or 25% protein diets. J. Nutr 130:813–819
Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS (2009) Role of dietary zinc in heat-stressed poultry: a review. Poult Sci 88:2176–2183. doi:10.3382/ps.2008-00560
Lymbury RS, Marino MJ, Perkins AV (2010) Effect of dietary selenium on the progression of heart failure in the ageing spontaneously hypertensive rat. Mol Nutr Food Res 54:1436–1444. doi:10.1002/mnfr.201000012
Liu LL, He JH, Xie HB, Yang YS, Li JC, Zou Y (2014) Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 93:54–62. doi:10.3382/ps.2013-03423
Lyons MP, Papazyan TT, Surai PF (2007) Selenium in food chain and animal nutrition: Lessons from nature. Asian Australas J Anim Sci 20:1135–1155. doi:10.5713/ajas.2007.1135
NRC (1994) Nutrient requirement for poultry, 9th edn. National Academies Press, Washington, DC, p. 62
Wang Y (2009) Differential effects of sodium selenite and nano-Se on growth performance, tissue Se distribution, and glutathione peroxidase activity of avian broiler. Biol Trace Elem Res 128:184–190. doi:10.1007/s12011-008-8264-y
Rahimi S, Farhadi D, Valipouri AR (2011) Effect of organic and inorganic selenium sources and vitamin E on broiler performance and carcass characteristics in heat stress condition. Vet J 91:25–35
Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N (2015) Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci 178:330–336. doi:10.1016/j.livsci.2015.05.004
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210. doi:10.1016/j.anifeedsci.2012.08.010
da Silva IC, Ribeiro AM, Canal CW, Trevizan L, Macagnan M, Gonçalves TA, Hlavac NR, De Almeida LL, Pereira RA (2010) The impact of organic and inorganic selenium on the immune system of growing broilers submitted to immune stimulation and heat stress. Revista Brasileira de Ciência Avícola 12:247–254. doi:10.1590/S1516-635X2010000400005
Niu Z, Liu F, Yan Q, Li L (2009) Effects of different levels of selenium on growth performance and immunocompetence of broilers under heat stress. Arch Anim Nutr 63:56–65. doi:10.1080/17450390802611610
Nelson N, Lakshmanan N, Lamont S (1995) Sheep red blood cell and Brucella abortus antibody responses in chickens selected for multitrait immunocompetence. Poult Sci 74:1603–1609. doi:10.3382/ps.0741603
Yoshioka T, Kawada K, Shimada T, Mori M (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 135:372–376. doi:10.1016/0002-9378(79)90708-7
Hajati H, Hassanabadi A, Golian AG, Nassiri-Moghaddam H, Nassiri MR (2015) The effect of grape seed extract and vitamin c feed supplements carcass characteristics, gut morphology and ileal microflora in broiler chickens exposed to chronic heat stress. Iran J Appl Anim Sci 5(1):155–165
Cai SJ, Wu CX, Gong LM, Song T, Wu H, Zhang LY (2012) Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci 91:2532–2539. doi:10.3382/ps.2012-02160
Liu S, Tan H, Wei S, Zhao J, Yang L, Li S, Zhong C, Yin Y, Chen Y, Peng Y (2015) Effect of selenium sources on growth performance and tissue selenium retention in yellow broiler chicks. J Appl Anim Res 43:487–490. doi:10.1080/09712119.2014.978780
Zhou X, Wang Y (2011) Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poult Sci 90:680–686. doi:10.3382/ps.2010-00977
Thomson C (1998) Selenium speciation in human body fluids. Analyst 123:827–831. doi:10.1039/A707292I
Surai P (2016) Antioxidant Systems in Poultry Biology: Superoxide Dismutase. J Anim Nutr 1(1):8
Lambert GP (2009) Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 87(E. Suppl):E101–E108. doi:10.2527/jas.2008-1339
Liu L, Fu C, Yan M, Xie H, Li S, Yu Q, He S, He J (2016) Resveratrol modulates intestinal morphology and jejunal mucosa HSP70/90, NF-κB and EGF expression in black-boned chicken exposure to circular heat stress. Food Funct 7:1329–1338. doi:10.1039/C5FO01338K
Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, Zachary MD, Remacle J (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51:283–297. doi:10.1016/0047-6374(90)90078-T
Hermier D (1997) Lipoprotein metabolism and fattening in poultry. J Nutr 127:805S–808S
Sands J, Smith M (1999) Broilers in heat stress conditions: effects of dietary manganese proteinate or chromium picolinate supplementation. J Appl Poult Res 8:280–287. doi:10.1093/japr/8.3.280
Dai S, Gao F, Zhang W, Song S, Xu X, Zhou G (2011) Effects of dietary glutamine and gamma-aminobutyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. Anim Feed Sci Technol 168:51–60. doi:10.1016/j.anifeedsci.2011.03.005
Kim JE, Choi SI, Lee HR, Hwang IS, Lee YJ, An BS, Lee SH, Kim HJ, Kang BC, Hwang DY (2012) Selenium significantly inhibits adipocyte hypertrophy and abdominal fat accumulation in OLETF rats via induction of fatty acid β-oxidation. Biol Trace Elem Res 150:360–370. doi:10.1007/s12011-012-9519-1
Kim CY, Kim GN, Wiacek JL, Chen CY, Kim KH (2012) Selenate inhibits adipogenesis through induction of transforming growth factor-β1 (TGF-β1) signaling. Biochem Biophys Res Commun 426:551–557. doi:10.1016/j.bbrc.2012.08.125
Lu Q, Wen J, Zhang H (2007) Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci 86:1059–1064. doi:10.1093/ps/86.6.1059
Tanguy S, Grauzam S, De Leiris J, Boucher F (2012) Impact of dietary selenium intake on cardiac health: experimental approaches and human studies. Mol Nutr Food Res 56:1106–1121. doi:10.1002/mnfr.201100766
Bashir N, Manoharan V, Miltonprabu S (2016) Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J Nutr Biochem 32:128–141. doi:10.1016/j.jnutbio.2016.03.001
Hai L, Rong D, Zhang ZY (2000) The effect of thermal environment on the digestion of broilers. J. Anim. Physiol. Anim Nutr 83:57–64. doi:10.1046/j.1439-0396.2000.00223.x
Thompson JN, Scott ML (1970) Impaired lipid and vitamin E absorption related to atrophy of the pancreas in selenium-deficient chicks. J Nutr 100:797–809
Li JL, Li HX, Li S, Jiang ZH, Xu SW, Tang ZX (2011) Selenoprotein W gene expression in the gastrointestinal tract of chicken is affected by dietary selenium. Biometals 24:291–299. doi:10.1007/s10534-010-9395-0
Selim NA, Radwan NL, Youssef SF, Eldin TS, Elwafa SA (2015) Effect of Inclusion Inorganic, Organic or Nano Selenium Forms in Broiler Diets On: 2-Physiological, Immunological and Toxicity Statuses of Broiler Chicks. Int J Poult Sci 14:144–155
Liu CP, Fu J, Xu FP, Wang XS, Li S (2015) The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals 28:163–173. doi:10.1007/s10534-014-9812-x
Amin KA, Hashem KS, Alshehri FS, Awad ST, Hassan MS (2016) Antioxidant and Hepatoprotective Efficiency of Selenium Nanoparticles Against Acetaminophen-Induced Hepatic Damage. Biol Trace Elem Res. doi:10.1007/s12011-016-0748-6
Imik H, Kaynar O, Ozkanlar S, Gumus R, Polat H, Ozkanlar Y (2013) Effects of vitamin C and α-lipoid acid dietary supplementations on metabolic adaptation of broilers to heat stress. Rev Méd Vét 164:52–59
Nassir F, Moundras C, Bayle D, Serougne C, Gueux E, Rock E, Rayssiguier Y, Mazur A (1997) Effect of selenium deficiency on hepatic lipid and lipoprotein metabolism in the rat. Br J Nutr 78:493–500. doi:10.1079/BJN19970166
Dhingra S, Bansal MP (2006) Modulation of hypercholesterolemia-induced alterations in apolipoprotein B and HMG-CoA reductase expression by selenium supplementation. Chem Biol Interact 161:49–56. doi:10.1016/j.cbi.2006.02.008
Ness GC, Pendleton LC, Li YC, Chiang JY (1990) Effect of thyroid hormone on hepatic cholesterol 7α hydroxylase, LDL receptor, HMG-CoA reductase, farnesyl pyrophosphate synthetase and apolipoprotein AI mRNA levels in hypophysectomized rats. Biochem Biophys Res Commun 172:1150–1156. doi:10.1016/0006-291X(90)91568-D
Dhingra S, Bansal MP (2006) Hypercholesterolemia and LDL receptor mRNA expression: modulation by selenium supplementation. Biometals 19:493–501. doi:10.1007/s10534-005-5393-z
Hossain MS, Afrose S, Takeda I, Tsujii H (2010) Effect of selenium-enriched Japanese radish sprouts and Rhodobacter capsulatus on the cholesterol and immune response of laying hens. Asian Australas J Anim Sci 23:630–639. doi:10.5713/ajas.2010.90394
Mashaly M, Hendricks G, Kalama M, Gehad A, Abbas A, Patterson P (2004) Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 83:889–894. doi:10.1093/ps/83.6.889
Xu D, Tian Y (2015) Selenium and polysaccharides of Atractylodes macrocephala koidz play different roles in improving the immune response induced by heat stress in chickens. Biol Trace Elem Res 168:235–241. doi:10.1007/s12011-015-0351-2
Liao X, Lu L, Li S, Liu S, Zhang L, Wang G, Li A, Luo X (2012) Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biol Trace Elem Res 150:158–165. doi:10.1007/s12011-012-9517-3
Placha I, Takacova J, Ryzner M, Cobanova K, Laukova A, Strompfova V, Venglovska K, Faix S (2014) Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci 55:105–114. doi:10.1080/00071668.2013.873772
Rama Rao SV, Prakash B, Raju MVLN, Panda AK, Poonam NS, Murthy OK (2013) Effect of supplementing organic selenium on performance, carcass traits, oxidative parameters and immune responses in commercial broiler chickens. Asian Australas J Anim Sci 26:247–252. doi:10.5713/ajas.2012.12299
Acknowledgments
The authors would like to thank the Birjand University for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Safdari-Rostamabad, M., Hosseini-Vashan, S.J., Perai, A.H. et al. Nanoselenium Supplementation of Heat-Stressed Broilers: Effects on Performance, Carcass Characteristics, Blood Metabolites, Immune Response, Antioxidant Status, and Jejunal Morphology. Biol Trace Elem Res 178, 105–116 (2017). https://doi.org/10.1007/s12011-016-0899-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0899-5