Abstract
According to undiscovered toxicity and safety of magnesium oxide nanoparticles (MgO NPs) in isolated pancreatic islet cells, this study was designed to examine the effects of its various concentrations on a time-course basis on the oxidative stress, viability, and function of isolated islets of rat’s pancreas. Pancreatic islets were isolated and exposed to different MgO NP (<100 nm) concentrations within three different time points. After that, oxidative stress biomarkers were investigated and the best exposure time was selected. Then, safety of MgO NPs was investigated by flow cytometry and fluorescent staining, and levels of insulin secretion and caspase activity were measured. The results illustrated a considerable decrease in oxidative stress markers such as reactive oxygen species (ROS) and lipid peroxidation (LPO) levels of pancreatic islets which were treated by MgO NPs for 24 h. Also, in that time of exposure, cell apoptosis investigation by flow cytometry and insulin test showed that MgO NPs, in a concentration of 100 μg/ml, decreased the rate of apoptotic cells via inhibiting caspase-9 activity and made a significant increase in the level of insulin secretion. Data of function and apoptosis biomarkers correlated with each other. It is concluded that the use of MgO NPs in concentration of as low as 100 μg/ml can induce antiapoptotic, antioxidative, and antidiabetic effects in rat pancreatic islets, which support its possible benefit in islet transplantation procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scientists are always exploring novel materials for biological applications, especially in the field of medicine as new therapeutic strategies. One of these new compounds that many researches are being done on it is nanoparticles (NPs). Any sub-classification of ultrafine particle which has lengths (in two or three dimensions) greater than 1 nm and smaller than 100 nm is called NPs. Metallic NPs have recently attracted scientists because of their great potential in nanotechnology. Nowadays, NPs have been used in biomedical, engineering, and science as well [1]. In vivo and in vitro studies have recently focused on NPs’ medical effects, as toxic or safe agents. One study has shown that zinc oxide (ZnO) NPs not only are safe in low concentrations but also can cause a huge increment in rat pancreatic islets’ viability and insulin secretion [2]. Also, two other studies have shown that cerium oxide NPs [3] and yttrium oxide NPs [4] can cause a significant enhancement in cells’ viability and improve function of the islets.
Magnesium oxide (MgO) NP is one of the nanomaterials which its effects on live cells have fairly remained undiscovered. Studies have different approaches toward MgO NPs. One study has claimed that exposure to MgO NPs reduces the total antioxidant defense mechanisms by inducing oxidative stress [5]. On the other side, there are a lot of studies claiming that MgO NPs can play a positive role in cells and animals. It seems that MgO NPs have an analgesic effect and also can play an anti-inflammatory role through both central and peripheral mechanisms in mice [6]. MgO NPs also have a noticeable antibacterial effect. A huge inhibition of bacterial growth was seen using Vibrio cholerae that causes intestinal infection [7]. In the other study, water-dispersible MgO NPs at a dose of 150 μg/ml did not show significant changes in glutathione S-transferase and catalase gene expression in human cancer cell lines [8]. Also, the positive effect of MgO NPs on various cell lines, such as human intestinal cell line (INT407) and human cervical cancer cell line (SiHa), has been proved. Using a viability test, it was shown that there was no significant cytotoxicity at the range of 0–350 μg/ml in INT407. Also, for SiHa, the range with no cytotoxicity effect was lower than 300 μg/ml [7].
Diabetes is a major systemic disease with a high-growing prevalence in the world [9]. Islet transplantation would be called as a successful remedy to reduce risk of hyperglycemia without insulin injection. The most severe problems threatening islet transplantation success is loss of healthy cells during this process. It is believed that the two major causes of transplantation failure are enzymatic-mechanical damage during the experience and hypoxia. A physiological concentration of reactive oxygen species (ROS) products protects cells and keeps them live. But, as it is clear, when the number of ROS increases, they can damage different parts of a healthy cell, such as membrane lipids, proteins, and nuclear nucleic acids [10]. Many different studies have been done for decreasing bad effects caused by ROS on cells. On a study working with Nigella sativa, it was shown that this plant has a positive effect on decreasing oxidative stress and protecting beta cells and can be used for treating diabetes [11]. On the other experience, the positive effects of Iranian indigenous plant extracts have been proved. These compounds not only made a great increment in cells’ survivorship but also increased their functionality and insulin levels by decreasing the level of ROS [3].
In the current study, we aimed to measure the effect of various concentrations of MgO NPs on a time-course basis on the oxidative stress, viability, and function of isolated islets of rat’s pancreas in order to achieve an optimum dose of that for using in increments of pancreatic islets’ viability during islet transplantation.
Material Methods
Chemicals
A solution of MgO NPs (<100 nm) was obtained from Nano Zino (Tehran, Iran). Rat-specific insulin ELISA kit was purchased from Mercodia (Sweden), and all other chemicals which were used in this study, including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), thiobarbituric acid (TBA), bovine serum albumin (BSA), RPMI 1640 medium, HEPES sodium salt, 2,4,6-tripyridyl-s-triazine (TPTZ), DNA-binding dyes acridine orange (AO) and ethidium bromide (EB), and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), were obtained from Sigma-Aldrich (GmbH Munich, Germany).
Characteristic Analysis of MgO NPs
For using MgO NPs, it is needed to make sure whether the particle has the proper size and morphology or not, so after sonication of NP suspension for 2 h, the particle size and morphology of MgO NPs were measured using transmission electron microscopy (TEM) and, also, the ultraviolet visible (UV-Vis) spectra of MgO NPs were recorded. The absorption of MgO NPs was investigated in the wavelength of 200–700 nm at room temperature.
Animals
All experimental procedures were approved according to an institutional review board (IRB) with a code number of 94-01-45-28957. Before the experiment, male adult Wistar rats (age 2–3 months, weight 200–250 g) were acclimatized for 1 week in laboratory conditions. The housing temperature was 25 ± 1 °C with a 12-h dark/light cycle and 50 % humidity.
Pancreatic Islets Preparation
After an acceptable period of time that animals spent in the lab environment, they were anesthetized by intraperitoneal injection of a ketamine-xylazine combination in a ratio of 10:1 (100 mg/kg ketamine/10 mg/kg xylazine). After laparotomy, a fresh Krebs buffer was injected into the pancreatic duct and made the pancreas baggy and easy to distinguish for isolation. In the following, the isolated pancreas was propped in the Krebs buffer in the ice bath and homogenized by cutting them off into small pieces. Then, after washing the chopped parts with the Krebs buffer, they were centrifuged at 3000g for 60 s at 4 °C. Collagenase enzyme was used to provide completely separated islets. Ten-minute shaking in a 37 °C bain marie was done. After that, to fix the digestion, BSA was added and then they were washed twice. In the next step, islets were picked up by a stereomicroscope in which every group had a similar size of islets. Finally, hale islets were cultured in RPMI 1640 for 24 h at 37 °C for overcoming all of the negative effects of these stressful procedures and restoring in refreshed and nourished media [2].
Study Design and Islets Treatment
After isolating the pancreatic islets, three concentrations of MgO NPs (10, 100, and 1000 μg/ml) were made in a RPMI medium and then were exposed to each of ten islets for 24, 48, and 72 h at 37 °C. After treatment, oxidative stress biomarkers were investigated and, according to these data, the best time exposure was selected. Then, the safety of MgO NPs and their effects on the function of pancreatic islets were studied.
Assay of Oxidative Stress Biomarkers
Measuring Cytosolic ROS
DCFH-DA was used to measure production of ROS. After homogenizing each group of islets using an extraction buffer, they were centrifuged at 2375g for 15 min. Then, a 50-μl supernatant of the islet extractions was added to the mixture of 10 μl DCFH and 162 μl assay buffer. These solutions were incubated at 37 °C for 15 min. At the end, with a microplate reader, absorbance of samples was read every 10 min up to 60 min. The remaining islets were collected for measurement of protein concentration, which was used to normalize the concentrations as described previously [3].
Protein Assay
For normalizing the activity of enzymes and other markers such as total antioxidant power (TAP), total protein concentration per sample was determined. In this case, standard protein concentrations between 0 and 10 μg/ml BSA in buffer were prepared. Then, 5 μl of the sample supernatant was diluted with 795 μl of distilled water and 200 μl of Bradford reagent was added to standard and each sample. The absorbance was measured by the spectrophotometer at 595 nm after 5 min [12].
Determination of Lipid Peroxidation Level
In lipid peroxidation (LPO) assay, TBA-reactive substances (TBARSs) were measured. The reaction between TBA and lipid peroxides in the samples leads to a measurable pink color. Briefly, the samples were diluted by buffered saline (1:5) and 800 μl of trichloroacetic acid (TCA, 28 % w/v) was added to 400 μl of this mixture and centrifuged at 3000×g for 30 min. Then, 600 μl of the supernatant was added to 150 μl of TBA (1 % w/v). The mixture was incubated for 15 min in a boiling water bath, 4 ml n-butanol was added, the solution was centrifuged and cooled, and absorption of the supernatant was read by the ELISA reader at 532 nm as set up in our lab previously [13]. The activity was shown as micromolars.
Total Antioxidant Power Assay
TAP was determined by measuring the ability to reduce Fe3+ to Fe2+. The complex between Fe2+ and TPTZ gives a blue color with absorbance at 593 nm. The following procedure leads to the preparation of the ferric reducing antioxidant power (FRAP) react: mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3·6H2O in the ratio of 10:1:1 just before testing. The standard was 0.1–1.5 mM FeSO4·7H2O in methanol. After preparing the FRAP solution, 50 μl of the homogenized islets was added to 1.5 ml reagent and the samples were kept in 37 °C for 30 min. At the end, concentrations were calculated by using calibration curve [14]. Data were shown as nanomoles per microgram protein.
Islets’ Viability Assay
MTT Assay
This assay is based on the reduction of MTT, a yellow tetrazole to purple formazan, by mitochondrial respiration in viable cells. After removing the medium, the pretreated islets were washed twice by Krebs–HEPES buffer and incubated with 50 μl of MTT (0.5 mg/ml) at 37 °C. This yellow solution was reduced and became purple in viable cells. After 4 h, DMSO was added and shaken for 30 min and then the absorbance of the samples was measured at 570 nm using a microplate reader as set up in our lab previously [15].
Microscopic Assay by AO and EB Double Staining
DNA-binding dyes AO and EB were used for the morphological detection of apoptotic and necrotic cells. AO is taken up by both viable and non-viable cells, and it emits green fluorescence if intercalated into DNA. EB is taken up only by non-viable cells, and it emits red fluorescence by intercalation into DNA. After treating the islets with MgO NPs for 24 h, the cells were washed and then stained with a mixture of AO (100 μg/ml) and EB (100 μg/ml) at room temperature for 5 min. The stained cells were observed with a fluorescence microscope (Olympus, Japan) at ×40 magnifications [16]. Intensity of AO and EB has been measured by ImageJ analysis software.
Assay of Apoptosis vs Necrosis by Flow Cytometry Assay
After 24 h, for separating beta cells from exocrine tissue and preparing single cells from pancreatic islets, trypsin was used and, after that, BSA was added to block the digestion process. Then, cell suspension was washed two times by PBS and, for investigating apoptosis vs necrosis, dual staining was done by using ApoFlowEx® FITC kit. Cells with the approximate density of 3 × 105 cells/100 μl were incubated with 5 μl of annexin V–fluorescein isothiocyanate (FITC) and 5 μl of propidium iodide (PI) in room temperature for 15 min. At the end, the samples were analyzed with a flow cytometer (Apogee Flow Systems, UK) [2].
Measuring Activities of Caspase-3 and Caspase-9
Caspase-3 and Caspase-9 activities were measured by colorimetric assays based on the identity of specific amino acid sequences by these caspases. The tetrapeptide substrates were labeled with the chromophore r-nitroaniline (ρNA). ρNA is released from the substrate upon cleavage by caspase and produces a yellow color that is monitored by an ELISA reader at 405 nm. The amount of caspase activity presented in the sample is proportional to the amount of yellow color produced upon cleavage. Briefly, the pretreated pancreatic islets were lysed in the supplied lysis buffer and were incubated on ice for 10 min. The whole cell lysates were incubated in caspase buffer (100 mM HEPES (pH 7.4), 20 % glycerol, 0.5 mM EDTA, 5 mM dithiothreitol) containing 100 mM of caspase-3 and caspase-9-specific substrate (Ac-DEVD-ρNA and Ac-LEHD-ρNA, respectively) for 4 h at 37 °C. Then, absorbance was measured at 405 nm. The caspase-3 and caspase-9 activities of the treatment groups were shown as the percentage of the controls which is assumed to be 100 % [4].
Functional Tests
Insulin Secretion Assay
After treatment of islets with 10, 100, and 1000 μg/ml of MgO NPs and a 24-h incubation, they were translocated in a 1 ml Krebs medium to vials. After centrifuging (3000g for 1 min) and removing the supernatants, the islets were incubated by 2.8 mM glucose for 30 min. After that, the vials were derived into two groups: one for adding 2.8 mM glucose (basal dose) and the other one for 16.7 mM glucose (stimulant dose). After 1 h, the vials were centrifuged and the supernatants were collected for measuring the insulin secretion amount using an insulin kit, according to the manufacturer’s protocol, and were reported in micrograms per milligram of protein per hour [2].
Statistical Analysis
Three independent experiments were carried out in duplicate. Data are performed as mean ± standard error. Tukey’s multi-comparison tests were done for statistical analysis and calculation correlation. The P value of <0.05 was considered significant. StatsDirect software version 3.0.171 was used.
Results
Characteristic Analysis of MgO NPs
In Fig. 1, micrograph indicates that the approximate size of particles after a 2-h dispersion was 100 nm. Also, as it is shown in Fig. 2, the maximum absorption of NP suspension in a range of 200 to 700 nm was 246 nm. It shows that MgO NPs are able to absorb ultraviolet light.
ROS Level
As it is represented in Fig. 3, after a 24-h exposure, a consequential change was observed in ROS production. At a dose of 10 μg/ml, a huge reduction was seen (P < 0.001) and the reduction was to 73.47 % compared to the control group. This result was also repeated for a concentration of 100 μg/ml (P < 0.001) with a slight change in percentage, which was reduced to 75.77 %. On the other side, the reduction in ROS production was reduced to 80 % when treated with the concentration of 1000 μg/ml (P < 0.01). After a 48-h disposal of doses 100 and 1000 μg/ml, no meaningful change in ROS productions was perceived; on the other hand, at the dose of 10, there was a significant alteration (P < 0.01), which shows the reduction of ROS production to 84.84 % of the control group. As indicated, in the treatment of incubated islets after 72 h, no significant change in ROS was detected.
Effect of different concentrations of MgO nanoparticles (NPs) on level of reactive oxygen species (ROS) in rat pancreatic islets after 24, 48, and 72 h of exposure. Data are represented as mean ± standard error with a number of replicates, n = 3. ***P < 0.001; **P < 0.01, statistically significant decrease when compared with the control group
LPO Level
Administration of MgO NPs after 24 h at the dose of 1000 μg/ml had no considerable effect on islets; however, the other concentrations showed a satisfactory reduction. At a concentration of 100 μg/ml, a fulfilling decline in LPO was detected in comparison to the control group (P < 0.01) and also a considerable reduction was detected in the dose of 10 μg/ml (P < 0.05) which is shown in Fig. 4. The exposure of MgO NPs after 48 and 72 h showed no significant change.
Effect of different concentrations of MgO nanoparticles (NPs) on level of lipid peroxidation (LPO) in rat pancreatic islets after 24, 48, and 72 h of exposure. Data are represented as mean ± standard error with a number of replicates, n = 3. *P < 0.05; **P < 0.01, statistically significant decrease when compared with the control group
TAP Level
With regard to Fig. 5, after 24 h, all the doses showed a noticeable increase in TAP level. The dose of 100 μg/ml had a great effect on the percentage of TAP (P < 0.001) which increased the TAP from 53.625 to 93.125 nmol/μg protein. On the other hand, a dose of 10 μg/ml showed exactly the same effect as a dose of 1000 μg/ml (P < 0.01). As demonstrated, after 48 h, not only was there a satisfactory change in TAP level at the dose of 100 μg/ml (P < 0.001) but also the concentrations of 1000 and 10 μg/ml showed a noticeable change (P < 0.01 and P < 0.05, respectively). In the last period of time (72 h), the concentration of 10 μg/ml increased the level of TAP up to 68.875 nmol/μg protein (P < 0.01), the dose of 100 μg/ml made a punctual increase in TAP (P < 0.001), and at the last dose, the enhancement was up to 66.37 nmol/μg protein (P < 0.05).
Effect of different concentrations of MgO nanoparticles (NPs) on level of total antioxidant power (TAP) in rat pancreatic islets after 24, 48, and 72 h of exposure. Data are represented as mean ± standard error with a number of replicates, n = 3. *P < 0.05; **P < 0.01; ***P < 0.001, statistically significant increase when compared with the control group
As it is obvious, the 24-h exposure time had the most positive effect on islets, so that another experiment was done after the 24-h exposure.
Viability
As seen in Fig. 6, MgO NPs at the dose of 100 μg/ml caused a noteworthy change in the metabolic activity of treated islets. This dose increased viability up to 166.04 % compared to the control group (P < 0.001).
AO/EB Staining
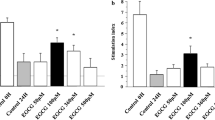
The control group shows necrosis and apoptosis in the islets by absorbing EB, while majority of cells (83.02 %) in islets which were exposed to MgO NPs exhibited green fluorescence (AO), and the rate of dead cells (16.97 %) is much less than that of the control group (Fig. 7).
Pancreatic cells, shown by fluorescence microscopy (magnification ×40), were stained with acridine orange (AO) plus ethidium bromide (EB) after exposing to 100 μl/ml MgO nanoparticles (NPs) compared to the control group. Live cells show green fluorescence, and dead cells show red fluorescence. The intensity of fluorescent stains is also shown in the graph
Apoptosis Evaluation by Flow Cytometry
Flow cytometry assay was done in order to determine the rate of apoptotic cells during the procedure of isleting. In apoptotic phase, phosphatidylserine transferred from inner parts to outer parts of plasma membrane, and then, annexin V–FITC can conjugate with that. In this plot, the upper right field shows the late stage of apoptosis, and the results show a significant reduction in the group exposed to MgO NPs (16 % down to 6.57 %). On the other hand, the lower left field is related to viable cells, and as it is shown, they have not started any apoptotic reactions. In this study, the percent of viable cells by using MgO NPs is 83.3 %. This result shows an increment in the number of live cells, compared to the control group (Fig. 8).
Flow cytometry evaluation of rat pancreatic cells exposed to MgO NPs on the percent of live, early apoptotic, late apoptotic, and necrotic cells. In the above plots, FITC− and PI− in the lower left field show live cells, FITC+ and PI− in the lower right field indicate early apoptotic cells, FITC+ and PI+ in the upper right field represent late apoptotic cells, and FITC− and PI+ in the upper left field express necrotic cells. Percent of cells in the stage of early apoptosis and live cell is also shown in the chart. *P < 0.05; ***P < 0.001, statistically significant changes when compared with the control group
Caspase-3 and Caspase-9 Activities
Figure 9 represents the enzyme activities of caspase-3 and caspase-9. MgO NPs have no meaningful effect on the level of caspase-3 activity, but it is shown that 100 μg/ml of MgO NPs causes a significant reduction in the level of caspase-9 activity compared to the control group (P < 0.01).
Effect of different concentrations of MgO nanoparticles (NPs) on level of caspase activities of rat pancreatic islets after 24 h of exposure. Data are represented as mean ± standard error with a number of replicates, n = 3. **P < 0.01, statistically significant changes when compared with the control group
Insulin Secretion
As it is represented in Fig. 10, although there is no remarkable change in insulin secretion at doses of 10 and 1000 μg/ml, there was a substantial change at the dose of 100 μg/ml. This dose not only increased insulin secretion (P < 0.01) in the stimulated phase (16.7 mM glucose) but also made a fundamental increment (P < 0.001) in the basal state (2.8 mM glucose).
Effect of different concentrations of MgO nanoparticles (NPs) on level of insulin secretion in rat pancreatic islets after 24 h of exposure. Islets were incubated for 1 h in the presence of 2.8 mM glucose as a basal concentration and 16.7 mM glucose as a stimulant concentration. Data are represented as mean ± standard error with a number of replicates, n = 3. *P < 0.05; **P < 0.01; ***P < 0.001, statistically significant changes when compared with the control group
Correlation Between Results
Table 1 shows a positive correlation (P < 0.05) between viability and insulin secretion in both stimulant and basal concentrations of glucose and also a fine-negative correlation (P < 0.05) between viability and caspase-9 activity. No correlation between the level of insulin in basal dose of glucose and stimulant dose or caspase activity was seen.
Discussion
Nowadays, one of the attractive strategies to remedy diabetic patients is islet transplantation, and the main challenge of that is to reduce cellular antioxidant defense system and generation of ROS products in islets and attenuation of their functions [17]. In this study, the safety of MgO NPs and their other effects on oxidative stress and function of isolated rats’ pancreatic islets were investigated to obtain a new option for improving the efficiency of islet transplantation and reduction of risk factors during that process.
Previous studies showed that metal particles, due to their size, have different effects. Karlsson et al. found that a nano size of CuO and Fe2O3 has more cytotoxicity on alveolar type II-like epithelial cell line, compared to their micro size [18]. On the other hand, positive effects of ZnO NPs on pancreatic islet cells, by preventing apoptosis and oxidative stress, have been shown [2]. Except size of NPs, shapes and their surface area [19], concentration [20], and also the duration time of exposure [21] are effective in giving them toxic or safety features.
About MgO NPs, there are many researches which show its various roles on cell lines and animal models. A study had discovered that MgO NPs have a toxic effect on rats by reducing antioxidant capacity and superoxidase enzymes, dose dependently [5]. On the other hand, there are some published papers claiming that MgO NPs have positive effects as well [6].
In the first phase of the study, MgO NPs have been characterized. As it was shown in TEM micrograph, the average diameter of MgO NPs was 100 nm. Previous studies revealed that this size is in the range which is suitable for pharmacological studies and has therapeutic effects [22]. Also, as shown in Fig. 2, the UV-Vis spectrum of MgO NPs indicates the range of pure MgO NPs [23].
In the second phase of the study, oxidative stress tests were assayed on rat pancreatic islets. Results showed that MgO NPs had the best positive effect on decreasing ROS level after a 24-h exposure, which is obviously more effective than the other periods of exposure. In the other oxidative stress tests, the LPO level had the most reduction after 24 h either. Surprisingly, in both ROS and LPO assays, the most effective concentration, reducing stress oxidative, was 100 μg/ml.
But, what makes MgO an effective particle in this procedure? It has been claimed in a study that Mg plays a vital role in protecting cells against oxidative stress. It also has been claimed that there is a direct relation between Mg deficiency and increasing oxidative stress, which results in the increment of ROS and LPO level. So, this would be logical, if we claim that cells exposed to MgO NPs can act highly better when facing oxidative stress [24]. Also, it has been reported that a metal form of Mg reduced LPO level in cadmium-induced oxidative stress rats. The authors concluded that Mg by effecting glutathione peroxidase enzyme can decrease LPO level [25]. Another study revealed that a metal form of Mg has neuroprotective effects and reduces LPO after spinal cord injury in rats [26]. For sure, to avoid any toxic effects, exposure of MgO NPs has to be in a specific period of time and a specific concentration. In the other concentrations or in different exposure times, MgO NPs can induce oxidative stress to islets, which is followed by a reduction in total antioxidant capacity, and as it is obvious, all these make ROS and LPO levels increase [5]. Maybe that is why in some studies, they had found toxic effects in MgO NPs.
Viability, rate of apoptosis, and also the release of insulin were investigated in the third phase of the study. The results of MTT assay showed that MgO NPs in all three concentrations are safe and 100 μg/ml of MgO NPs even increases viability more than the control group (P < 0.001). Interestingly, the perfect correlation between MTT and other tests can be seen and it has a good correlation with other studies. In one of our references, on HUVE cells, it was shown that MgO NPs in a concentration up to 200 μg/ml not only have no toxic effect but also promote proliferation in cells [27]. Fluorescent staining showed that islets exposed to 100 μg/ml MgO NPs absorbed EB less than the control group. So, the rate of necrosis and apoptosis decreased by using this concentration of NPs. Statistical comparisons of viability and caspase-9 activity with the results of insulin secretion show a significant Pearson correlation (P < 0.05) which means that controlling apoptosis pathway and cell death has a key role in improving the function of the islets. Also, the significant negative correlation between MTT and caspase-9 activity suggests that apoptosis of the islets may be due to the intrinsic apoptotic signaling pathway [28].
The above results have been confirmed by flow cytometric evaluation. The process of isolating islets by adding collagenase enzyme and making single beta cells by adding trypsin disturbs beta cells and infuses apoptosis (16 %) in the control group. As we have expected, exposing cells to 100 μg/ml of MgO NPs caused cells’ resistance and decreased rate of apoptosis down to 6.57 % and increased viable cells up to 83.3 %. Annexin V binds to phosphatidylserine which is translocated to the outer side of cell membrane [2]; MgO NPs by increasing the stability of plasma membrane can prevent this binding and decrease apoptosis. Also, previous studies showed that Mg sulfate made a reduction in apoptosis of nerve cells [29] and MgO NPs reduced apoptotic cells in lymphocytes by suppression of caspase activity [23]. Another mechanism in apoptosis cascade is increasing levels of cytochrome C and apoptosis-inducing factor (AIF) by making dysfunction in mitochondria [30]. For the MTT assay of this study, it can be concluded that improvement of mitochondrial function may lead to cytochrome C and AIF reduction.
It has been proved that the increment in ROS level in short time can make an obvious increase in glucose-stimulated insulin secretion (GSIS), but when time passes and after a long period of time, this increment will play a negative role and decrease insulin secretion [31]. As mentioned above, MgO NPs play an important role in reducing oxidative stress, which helps ROS and LPO levels reduce, and as a result of this reduction, insulin secretion increases. Other studies have investigated antidiabetic effects of silver and ZnO NPs on streptozotocin-induced diabetic rats. These NPs increased the activity of glucokinase and raised the expression level of GLUT-2 glucokinase genes and also insulin receptors [32]. MgO NPs may also make changes in the abovementioned genes.
Therefore, MgO NPs in a concentration of 100 μg/ml are able to increase insulin secretion and viability of the cells and also decrease apoptosis, ROS production, and LPO level.
Conclusion
The most severe problem in islet transplantation to reduce risk of hyperglycemia without insulin injection is loss of healthy cells during this process, and oxidative stress is one of these problems. Antioxidant compounds such as metal NPs can overcome this problem.
In this study, positive effects of MgO NPs on rat pancreatic cells were investigated. Interestingly, our results together with the previous studies demonstrate that MgO NPs in approximate 100 nm diameter and in low concentration (100 μg/ml) not only are safe but also attenuate oxidative stress. Furthermore, these NPs are able to reduce apoptosis, which are investigated by flow cytometry and fluorescent staining, via caspase suppressing and making cell membrane resistance. The other effect of them is increasing the level of insulin in basal and stimulant concentrations of glucose. So, this novel approach supports the use of MgO NPs as antiapoptotic, antioxidative, and antidiabetic compound in rat pancreatic islets during an islet transplantation procedure for improving its effectiveness.
Abbreviations
- AO:
-
Acridine orange
- BSA:
-
Bovine serum albumin
- DCFH-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- EB:
-
Ethidium bromide
- FRAP:
-
Ferric reducing antioxidant power
- LPO:
-
Lipid peroxidation
- MgO NPs:
-
Magnesium oxide nanoparticle
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NPs:
-
Nanoparticles
- ROS:
-
Reactive oxygen species
- TAP:
-
Total antioxidant power
- TBA:
-
Thiobarbituric acid
- TEM:
-
Transmission electron microscope
- TPTZ:
-
2,4,6-Tripyridyl-s-triazine
- UV-Vis:
-
Ultraviolet visible
References
Mody VV, Siwale R, Singh A, Mody HR (2010) Introduction to metallic nanoparticles. Int J Pharm Bio Sci 2(4):282–289
Shoae-Hagh P, Rahimifard M, Navaei-Nigjeh M, Baeeri M, Gholami M, Mohammadirad A, et al. (2014) Zinc oxide nanoparticles reduce apoptosis and oxidative stress values in isolated rat pancreatic islets. Biol Trace Elem Res 162(1–3):262–269
Rahimifard M, Navaei-Nigjeh M, Mahroui N, Mirzaei S, Siahpoosh Z, Nili-Ahmadabadi A, et al. (2014) Improvement in the function of isolated rat pancreatic islets through reduction of oxidative stress using traditional Iranian medicine. Cell J 16(2):147–163
Hosseini A, Baeeri M, Rahimifard M, Navaei-Nigjeh M, Mohammadirad A, Pourkhalili N, et al. (2013) Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum Exp Toxicol 32(5):544–553
Kiranmai G, Reddy AR (2013) Antioxidant status in MgO nanoparticle-exposed rats. Toxicol Ind Health 29(10):897–903
Jahangiri L, Kesmati M, Najafzadeh H (2013) Evaluation of analgesic and anti-inflammatory effect of nanoparticles of magnesium oxide in mice with and without ketamine. Eur Rev Med Pharmacol Sci 17(20):2706–2710
Patel MK, Md Z, Rizvi Moshahid Alam M, Agrawal VV, Ansari ZA, Malhotra BD, et al. (2013) Antibacterial and cytotoxic effect of magnesium oxide nanoparticles on bacterial and human cells. J Nanoeng Nanomanuf 3(2):162–166
Kumaran RS, Choi YK, Singh V, Song HJ, Song KG, Kim KJ, et al. (2015) In vitro cytotoxic evaluation of MgO nanoparticles and their effect on the expression of ROS genes. Int J Mol Sci 16(4):7551–7564
Carranza K, Veron D, Cercado A, Bautista N, Pozo W, Tufro A, et al. (2015) Cellular and molecular aspects of diabetic nephropathy and the role of VEGF-A. Nefrologia 35(2):131–138
Mohseni Salehi Monfared SS, Larijani B, Abdollahi M (2009) Islet transplantation and antioxidant management: a comprehensive review. World J Gastroenterol 15(10):1153–1161
Kanter M, Coskun O, Korkmaz A, Oter S (2004) Effects of Nigella sativa on oxidative stress and beta-cell damage in streptozotocin-induced diabetic rats. Anat Rec A: Discov Mol Cell Evol Biol 279(1):685–691
Gilbert K, Godbout R, Rousseau G (2016) Caspase-3 activity in the rat amygdala measured by spectrofluorometry after myocardial infarction. J Vis Exp 107:e53207
Astaneie F, Afshari M, Mojtahedi A, Mostafalou S, Zamani MJ, Larijani B, et al. (2005) Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch Med Res 36(4):376–381
Jowzi N, Rahimifard M, Navaei-Nigjeh M, Baeeri M, Darvishi B, et al. (2016) Reduction of chlorpyrifos-induced toxicity in human lymphocytes by selected phosphodiesterase inhibitors. Pestic Biochem Physiol 28:57–62
Rahimifard M, Navaei-Nigjeh M, Baeeri M, Maqbool F, Abdollahi M (2015) Multiple protective mechanisms of alpha-lipoic acid in oxidation, apoptosis and inflammation against hydrogen peroxide induced toxicity in human lymphocytes. Mol Cell Biochem 403(1–2):179–186
Attari F, Sepehri H, Delphi L, Goliaei B (2009) Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iran Biomed J 13(4):229–236
Ramkumar KM, Sekar TV, Bhakkiyalakshmi E, Foygel K, Rajaguru P, Berger F, et al. (2013) The impact of oxidative stress on islet transplantation and monitoring the graft survival by non-invasive imaging. Curr Med Chem 20(9):1127–1146
Karlsson HL, Gustafsson J, Cronholm P, Möller L (2009) Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size. Toxicol Lett 188(2):112–118
Arora S, Rajwade JM, Paknikar KM (2012) Nanotoxicology and in vitro studies: the need of the hour. Toxicol Appl Pharmacol 258(2):151–165
Chalkidou A, Simeonidis K, Angelakeris M, Samaras T, Martinez C, Boubeta, et al. (2011) In vitro application of Fe/MgO nanoparticles as magnetically mediated hyperthermia agents for cancer treatment. J Magn Magn Mater 323(6):775–780
Fukui H, Horie M, Endoh S, Kato H, Fujita K, Nishio K, et al. (2012) Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem Biol Interact 198(1):29–37
Jo DH, Kim JH, Lee TG, Kim JH (2015) Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 11(7):1603–1611
Heydary V, Navaei-Nigjeh M, Rahimifard M, Mohammadirad A, et al. (2015) Biochemical and molecular evidences on the protection by magnesium oxide nanoparticles of chlorpyrifos-induced apoptosis in human lymphocytes. J Res Med Sci 20:1021–1031
Hans CP, Chaudhary DP, Bansal DD (2002) Magnesium deficiency increases oxidative stress in rats. Indian J Exp Biol 40(11):1275–1279
Buha A, Bulat Z, Dukić-Ćosić D, Matović V (2012) Effects of oral and intraperitoneal magnesium treatment against cadmium-induced oxidative stress in plasma of rats. Arh Hig Rada Toksikol 63(3):247–254
Süzer T, Coskun E, Islekel H, Tahta K (1999) Neuroprotective effect of magnesium on lipid peroxidation and axonal function after experimental spinal cord injury. Spinal Cord 37(7):480–484
Ge S, Wang G, Shen Y, Zhang Q, Jia D, Wang H, et al. (2001) Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro. IET Nanotechnol 5(2):36
Würstle ML, Laussmann MA, Rehm M (2012) The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318(11):1213–1220
Zhou H, Ma Y, Zhou Y, Liu Z, et al. (2003) Effects of magnesium sulfate on neuron apoptosis and expression of caspase-3, bax and bcl-2 after cerebral ischemia-reperfusion injury. Chin Med J 116:1532–1534
Kaufmann SH, Hengartner MO (2011) Programmed cell death: alive and well in the new millennium. Trends Cell Biol 11:526–534
Graciano MF, Valle MM, Kowluru A, Curi R, Carpinelli AR (2001) Regulation of insulin secretion and reactive oxygen species production by free fatty acids in pancreatic islets. Islets 3(5):213–223
Alkaladi A, Abdelazim AM, Afifi M (2014) Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci 15(2):2015–2023
Acknowledgments
The authors thank the Pharmaceutical Sciences Research Center of TUMS for the partial support of this study (Grant No.: 94-01-45-28957). The assistance of Iran National Science Foundation (INSF) is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Shermineh Moeini-Nodeh and Mahban Rahimifard contributed equally to this work.
Rights and permissions
About this article
Cite this article
Moeini-Nodeh, S., Rahimifard, M., Baeeri, M. et al. Functional Improvement in Rats’ Pancreatic Islets Using Magnesium Oxide Nanoparticles Through Antiapoptotic and Antioxidant Pathways. Biol Trace Elem Res 175, 146–155 (2017). https://doi.org/10.1007/s12011-016-0754-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0754-8