Abstract
In India, endemic goitre is present in sub-Himalayan region and in pockets in states of Andhra Pradesh, Karnataka and Gujarat. Being a public health problem amenable for prevention, the assessment of prevalence of endemic goitre in an area helps in understanding whether the preventive strategies under National Iodine Deficiency Disorder Control Program (NIDDCP) have any impact on the control of endemic goitre. Hence, the current study was carried out to determine the prevalence, distribution and factors associated with iodine deficiency goitre among 6–12-year-old children in a rural area in south Karnataka. A cross-sectional study was conducted among 838 children, using a questionnaire adopted from Iodized Salt Program Assessment Tool and the tools prescribed by WHO for goitre survey. The prevalence of goitre in the study area was 21.9 % (95 % CI 19.2–24.8). There was higher prevalence of goitre among those having salt iodine <15 ppm than those with >15 ppm (P = 0.01; OR 1.59; 95 % CI 1.10–2.29). In 10 % of the children, urinary iodine excretion (UIE) was assessed and prevalence was higher among those with <100 μg/l of UIE than those with normal UIE, which was not statistically significant (P = 0.8, OR 1.36; 95 % CI 0.62–2.96). Multiple logistic regression revealed that gender (P = 0.002; OR 1.7; 95 % CI 1.21–2.35) was an independent variable associated with goitre. The study area was found to be moderately endemic for goitre based on the WHO criteria. Higher prevalence of goitre was found to be still associated with consumption of low iodized salt (<15 ppm) necessitating emphasis on monitoring of salt iodine levels in the study area. Though NIDDCP is being implemented since five decades in India, the burden of iodine deficiency disorders (IDDs) is still high demanding further impetus to the monitoring systems of the programme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Burden of iodine deficiency disorders (IDDs) is generally measured by estimating prevalence of goitre. In India, endemic goitre is present in sub-Himalayan region and in pockets in states of Andhra Pradesh, Karnataka and Gujarat. Prevalence of goitre in India is known to range from 0 % (no goitre) to 80 % (severe public health problem) [1] among school age (6–12 years) children; whereas in Karnataka, it is reported to be 1.7 % [1]. National Iodine Deficiency Disorder Control Program (NIDDCP) (previously known as National Goitre Control Programme from 1962 till 1992) was launched with goal of reducing the prevalence of endemic goitre in India [2]. Being a public health problem amenable for prevention, the assessment of the prevalence of endemic goitre in an area helps in understanding whether the preventive strategies under NIDDCP have any impact on the control of endemic goitre. Hence, the current study was carried out to determine the prevalence, distribution and factors associated with iodine deficiency goitre among 6–12-year-old children in a rural area in south Karnataka.
Methods
The study was conducted in sampled villages in a primary health centre (PHC) area near Bangalore, Karnataka, India. Cross-sectional study design was adopted and included 838 children in the age group of 6–12 years in the study villages.
Inclusion and Exclusion Criteria
All children aged 6–12 years residing in the study area for at least 6 months [3] were included as the subjects of the study and known cases of autoimmune thyroid diseases were excluded.
Estimation of Sample Size
The sample size for the present study was estimated based on the pilot study carried out in the neighbouring PHC area, which revealed prevalence of goitre among 6–12 year olds to be 24.1 % and the proportion of 6–12-year-old children in the village was found to be 16.6 %. Employing alpha level of 5 %, relative precision of 13 % [3], and adding 10 % sample for non-responders, the minimum required sample size for the study worked out to be 792 children in the age group of 6–12 years using nMaster2.0 [4].
Sampling Procedure
Multistage sampling design was adopted for selection of the sample. All 36 villages in the PHC area accounting for a total population of 34,355 as per PHC records (2009) were categorized into three strata based on population size of villages, as <500, 501–1500 and >1500. Employing probability proportional to sample size (PPS) technique [5] and using the data on proportion of 6–12-year-old children from pilot study, the population to be surveyed in each stratum was determined, based on which a sample of seven villages from all the three stratum were selected randomly and all children aged 6–12 years in selected villages were included in the study (Fig. 1).
Instruments
A pre-tested semi-structured questionnaire adapted from Iodized Salt Program Assessment Tool (ISPAT) was used in the present study [6]. The presence and grades (grade 0, 1 and 2) of goitre were assessed through a thorough clinical examination using standard WHO goitre assessment tool adopted by the NIDDCP [5], as for any community-based goitre prevalence studies, clinical assessment of goitre is recommended by WHO over ultrasound assessment of goitre. The Investigator was trained in clinical assessment of goitre, and a single investigator assessed all the children. The questionnaire contained questions relating to socio-demographic particulars along with those relating to factors influencing salt iodine content and goitre. Salt iodine content was assessed using spot testing kits (STKs) (MBI KITS International; 85, G. N. Chetty Road, III Floor, T. Nagar, Chennai – 600017, India), supplied by the Government of Karnataka, in the households of all the study subjects during the house visits. Among 10 % of the study subjects (that is among all 79 children of Hulugummanahalli village), urine iodine concentration was measured using iodometric titration method (that is ammonium persulphate method a.k.a method B) and iodine content in salt samples from households was assessed by analytical method [2] and using STKs. Both urinary and quantitative salt iodine levels were estimated in the Government of India accredited State Public Health Institute laboratory in Bangalore by a single laboratory technician.
Data Collection
Data was collected between March to August 2010 by house to house visit, examination of the target population, interviewing their parents and testing salt sample using STKs for iodine levels. Salt and urine samples among 79 children from village 4 were simultaneously collected in clean and dry plastic covers, and plastic containers with toluene reagent respectively and were transported to the Public Health Institute laboratory within 4 h of collection. Urine samples were transported in cold boxes.
Statistical Analysis
Frequencies and percentages were used to express qualitative variables like various grades of goitre. Prevalence rate of goitre was estimated with 95 % CI. Point prevalence of goitre was estimated considering the ratio of 6–12-year-old children with goitre to the total number of 6–12-year-old children in the selected villages. Chi-square test, odds ratio (OR) and linear trend analysis were used to assess association between goitre and associated factors. All the prevalent cases of goitre were considered as case group and the rest of the children as controls. Univariate odds ratios were computed for various risk factors. Multiple logistic regression analysis was carried out to find out independent factors associated with goitre. SPSS version 18.0 [7] was used for statistical analysis of data. P < 0.05 was considered for statistical significance. Ethical clearance was obtained from the institution’s ethics committee. Informed written consent was obtained from the parents/guardians of all the children before examination. Each parent was explained about the purpose of the study and clinical examination procedures. Children requiring further management were appropriately advised.
Results
There were a total of 842 children from all the seven selected villages, of which 838 children participated in the survey (response rate = 99.5 %). The overall prevalence of goitre in the study area among 6–12-year-old children was 21.9 % (95 % CI 19.2, 24.8). Girls were found to have higher prevalence (26.7 %) as compared to boys (17.8 %) (Table 1), and this difference was found to be statistically significant (P = 0.002); the odds ratio (OR) was found to be 1.7 (95 % CI 1.21, 2.35). There was also higher prevalence of goitre among those having salt iodine <15 ppm than those with >15 ppm (OR 1.59; 95 % CI 1.10, 2.29). No increasing trend in the prevalence rate of goitre was observed with the increasing age, and linear trend analysis also revealed similar findings (P = 0.1). The prevalence of goitre among study villages ranged from 14.1 to 42.9 %. Of a total prevalence of 21.9 %, 19.3 % belonged to grade 1 goitre (88.0 %) and the remaining to grade 2. There was no significant difference in the prevalence of various grades of goitre between genders (grade 1 goitre among boys = 88.6 %; grade 1 goitre among girls = 87.61 %). Multiple logistic regression analysis was carried out to assess the independent factors associated with goitre, and the findings revealed that gender (P = 0.002) was an independent variable associated with goitre.
The present study showed that the prevalence of goitre among those consuming salt with <15 ppm iodine was higher (24.5 %) than among those consuming salt with >15 ppm iodine (17 %), which was statistically significant (P = 0.013). The OR was found to be 1.59 (95 % CI 1.1, 2.29) among those consuming salt with <15 ppm iodine.
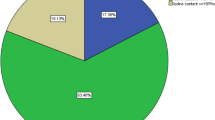
The median urinary iodine excretion (UIE) was found to be 150 μg/l (IQR 75, 200) among the study subjects examined for UIE. The normal UIE (100–199 μg/l) was found in 46.8 % of the children, excess UIE (>200 μg/l) was found in 26.6 % of the children, UIE below 100 μg/l was found in 26.6 % of the children and UIE below 50 μg/l was found in 11.4 % children. Out of 79 children, 50 (63.3 %) were found to have goitre. Among the children having UIE >200 μg/l and <100 μg/l, 14 (66.6 %) children each in both the groups were having goitre. However, this was not statistically significant (P = 0.8). It was observed that <100 μg/l UIE level was high (29.1 %) among those consuming salt with <15 ppm iodine as compared to those consuming salt with iodine level of >15 ppm (26.6 %). However, this difference was not statistically significant (P = 0.59).
Discussion
The problem of iodine deficiency goitre is ubiquitous in India, and there are many areas in the country where the burden of this disease is still unknown. The present study found the prevalence of iodine deficiency goitre in the study area to be 21.9 %. The finding appears to be similar to a study conducted by Kochupillai (2005) on prevalence of fluorosis [8] in one of the villages in the PHC area, where the prevalence of goitre was found to be 24 per 100 children aged 6–12 years. Similar pockets of mild to moderate endemic goitre areas were found in Karnataka in Belgaum (Kamath et al., 2009) where the prevalence of goitre was 16.6 % [9]; a study done at the coastal area of Karnataka in Udupi district among 8–10-year age group showed a prevalence of 30 % [10], whereas Sundaram et al. in 1997 estimated a low prevalence of goitre of 2.5 % in Belgaum district [11]. There was a significant increase in prevalence of goitre among girls than boys similar to the findings in a study conducted in Uttarkhand (Srivastava et al., 2008) which was statistically significant [12]. A study conducted in Belgaum (Kamath et al., 2009) also showed higher prevalence of goitre among girls (21.8 per 100 children) than boys (7.2 per 100 children) which was statistically significant [9]. There was no increase in trend of goitre with relation to age in present study, but a contradictory finding was made in a study conducted in Belgaum where the prevalence of goitre increased significantly with the advancement of age [13], and in a study in coastal Karnataka, in both sexes goitre rate increased with the advancement of age [10]. The prevalence of grade 1 and grade 2 goitres in the study area were neither related to the age nor sex of the study population. Though as per “criteria for monitoring progress towards sustainable IDD elimination” [14], the goal of UIE has been achieved, iodine intake was found to be still insufficient in 26.6 % children, which amounts to a considerable burden of disease in terms of the number of children affected. Population studies have shown that children with UIE >200 μg/l, if continue to consume the same levels of iodine in the diet for 5 to 10 years, may develop autoimmune thyroiditis, increasing the risk of overt hypothyroidism which results in goitre [15]. Though the lower UIE level among children consuming lower iodine through salt is self-explanatory, the lower levels of UIE among those consuming adequate iodine through salt may be due to the presence of goitrogens or iodine interaction with other trace elements, which needs to be further explored.
In areas of severe iodine deficiency (prevalence of goitre >30 %), cretinism is known to affect 5–15 % of the population and individuals living in areas of mild (prevalence of goitre 5–10 %) or moderate (prevalence of goitre 10–30 %) iodine deficiency would exhibit more number of mild neurological and intellectual deficits [16]. Hence the estimation of the prevalence of iodine deficiency goitre serves only as a tip of the iceberg of a huge underlying problem.
Limitations of the Study
There are a few limitations encountered in the present study. It was not possible to study other parameters used to assess iodine deficiency goitre like thyroid ultrasound scanning due to logistic constraints. Trends in the prevalence of endemic goitre could not be commented upon due to lack of baseline data on goitre prevalence in the study area. Literature states that consumption of vegetables and other food rich in thiocyanates impairs the absorption of iodine; as the dietary intake of such food was not assessed in the present study, conclusion regarding the cause of greater prevalence of goitre and low UIE among children consuming adequately iodized salt in the study area needs to be further assessed.
Conclusion
In conclusion, the study area is found to be moderately endemic for goitre based on the WHO criteria. Higher prevalence of goitre was found to be still associated with consumption of low iodized salt (<15 ppm) necessitating emphasis on monitoring of salt iodine levels in the study area. Though NIDDCP is being implemented since five decades in India, the burden of IDDs is still high in the study area demanding further impetus to the monitoring systems of the programme.
All procedures performed in these studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
Ramachandran P (2007) Iodine deficiency disorders. In: Nutrition transition in India 1947–2007: Nutrition Foundation of India. Ministry of Women and Child Development, Government of India, pp 287–302. http://wcd.nic.in/research/nti1947/7.11.3%20Iodine%20deficiency%20%20pr%20%208.2%20new.pdf. Accessed 8 May 2013
National Iodine Deficiency Disorders Control Programme. National Institute of Health and Family Welfare, Government of India. http://www.nihfw.org/NDC/DocumentationServices/NationalHealthProgramme/NATIONALIODINEDEFICIENCYDISORDERS.html. Accessed 22 May 2013
Report of a Joint WHO/UNICEF/ICCIDD Consultation on Indicators for Assessing Iodine Deficiency Disorders and their Control Programmes (1993). World Health Organization, Geneva
nMaster2.0, sample size software [Computer Software] (2008) Vellore: Department of Biostatistics, Christian Medical College
Revised policy guidelines on National Iodine Deficiency Disorder Control Programme. IDD & Nutrition Cell (2006). DGHS, MoHFW, GoI, New Delhi
Partnership for tracking country progress in universal salt iodization programmes towards the sustainable elimination of IDDs by and beyond the year 2000—a manual for programme managers by ICCIDD and program against micronutrient malnutrition [draft] (1998). USAID/ OMNI, Micronutrient Initiative, UNICEF, WHO, Geneva
SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.
Kochupillai (2005) Science in preventive health care in India [abstract]. http://insaindia.org/insa/pillai.pdf. Accessed 9 Oct 2009
Kamath R, Vinod B, Rao RSP, et al (2009) Prevalence of goitre in rural area of Belgaum district, Karnataka. Ind J Commun Med 34(1):48–51
Rao RS, Kamath R, Das A, Nair NS, Keshavamurthy (2002) Prevalence of goitre among school children in coastal Karnataka. Indian J Pediatr 69(6):477–479
Sundaram J, Sugunammani BK, Chandrashekar K (1997) Goitre prevalence in Karnataka. Indian J Commun Health 3:98–105
Srivastava AK, Negi KS, Kishore S (2008). Prevalence of iodine deficiency disorders and utilization of iodized salts in rural districts of Uttarkhand. Ind J Prev Soc Med 39(1):76–79
Kamath R, Bhat V, Rao RS et al (2009) Prevalence of goitre among school children in Belgaum district. Indian J Pediatr 76(8):825–828
Assessment of iodine deficiency disorders and monitoring their elimination (2001), 2nd ed. Geneva: WHO, UNICEF, ICCIDD
Burgi H (2010) Iodine excess. Best Pract Res Clin Endocrinol Metab 24:107–115
Zimmermann M (2007) Gap analysis: key barriers to global IDD control: a summary. The USAID Micronutrient and Child Blindness Project. A2Z Project. http://www.a2zproject.org/pdf/Gap-Analysis-Iodine-Final.pdf. Accessed 14 Mar 2013
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjunath, B., Suman, G., Hemanth, T. et al. Prevalence and Factors Associated with Goitre among 6–12-year-old Children in a Rural Area of Karnataka in South India. Biol Trace Elem Res 169, 22–26 (2016). https://doi.org/10.1007/s12011-015-0398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0398-0