Abstract
Aluminum (Al) is the most widely distributed metal in the environment and is extensively used in daily life leading to easy exposure to human beings. Besides not having a recognized physiological role, Al may produce adverse effects through the interaction with the cholinergic system contributing to oxidative stress. The present study evaluated, in similar conditions of parenteral nutrition, whether the reaction of silicon (SiO2) with Al3+ to form hydroxyaluminosilicates (HAS) reduces its bioavailability and toxicity through intraperitoneal administrations of 0.5 mg Al/kg/day and/or 2 mg Si/kg/day in Wistar rats. Al and Si concentrations were determined in rat brain tissue and serum. Acetylcholinesterase (AChE) activity and lipid peroxidation (LPO) were analyzed in the cerebellum, cortex, hippocampus, striatum, hypothalamus, and blood. An increase in the Al concentration was verified in the Al + Si group in the brain. All the groups demonstrated enhanced Si compared to the control animals. Al3+ increased LPO measured by thiobarbituric acid reactive substances (TBARS) in cerebellum and hippocampus, whereas SiO2 reduced it when compared with the control group. An increase of AChE activity was observed in the Al-treated group in the cerebellum whereas a decrease of this enzyme activity was observed in the cortex and hippocampus in the Al and Al + Si groups. Al and Si concentrations increased in rat serum; however, no effect was observed in blood TBARS levels and AChE activity. SiO2 showed a protective effect in the hippocampus and cerebellum against cellular damage caused by Al3+-induced lipid peroxidation. Thus, SiO2 may be considered an important protector in LPO induced by Al3+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is the third most prevalent element in the Earth’s crust [1], and it is a non-essential element to which humans are often exposed to. This exposure may occur by dietary intake through feed or water [2, 3] as well as through drugs, parenteral nutrition [4, 5], and hemodialysis solutions [6].
Elevated levels of Al have been found in autopsied brain samples of patients with certain neurological disorders, such as Parkinsonism [7], amyotrophic lateral sclerosis [8], or Alzheimer’s disease (AD) [9]. Al has been reported as one of the most studied neurotoxicant affecting the central nervous system (CNS), including various regions of the brain [10]. Some experts believe that Al crosses the blood–brain barrier and plays a role in the formation of Alzheimer-like neurofibrillary tangles [11].
The central nervous system has a high rate of oxygen metabolism, and because of its strict aerobic glucose metabolism, it is totally dependent on an adequate arterial blood flow [12]. Cholinergic neurons and their projections are widely distributed throughout the CNS with an essential role in regulating many vital functions, such as learning, memory, cortical organization of movement, and cerebral blood flow control [13]. One of the most important mechanisms responsible for the correct cholinergic activity is performed by acetylcholinesterase (AChE) [14]. This enzyme hydrolyses the neurotransmitter acetylcholine (ACh) in the synaptic cleft of cholinergic synapses and neuromuscular junctions [15]. The inhibition of AChE causes the accumulation of ACh and interferes with the function of the nervous system, which may lead to respiratory failure and death [16].
Al is considered a potential etiologic factor in AD [17]. The precise molecular mechanism by which Al exerts its neurotoxic effects is still not completely understood. However, literature data suggest that Al interacts with the cholinergic system, acting as a cholinotoxin. Long-term administration of soluble salts of Al to rats may result in a neuropathological condition in which selective neuronal loss and impairment of cholinergic functions are evident [18]. The intensification of inflammations and the interference with cholinergic projection functions may represent the way by which Al contributes to pathological processes in AD leading to learning and memory deficits [19].
Literature data have demonstrated that the brain is susceptible to LPO because of its high rate of oxygen utilization, abundant supply of polyunsaturated fatty acids, deficient antioxidant defense, and high content of transition metals such as copper and iron, which may lead to the formation of reactive oxygen species (ROS) [20].
Despite Al is a non-redox active metal, it presents a pro-oxidant activity, which may be explained by the formation of an Al superoxide semireduced radical ion AlO• 2 2+ [21]. Al ions are not able to promote membrane LPO independently, although they may enhance Fe2+-dependent membrane LPO. Results indicate that Al toxicity is mediated through ROS production and iron accumulation [22]. In fact, Kaizer et al. [10] have observed increased LPO measured by thiobarbituric acid reactive substances (TBARS) in the hippocampus and cerebral cortex in mice after receiving Al by gavage.
In contrast, the health benefits of silicon (Si) regarding skeletal and neurological function and status have recently been recognized [23]. Bioavailable Si, which is Si in the form of silicic acid or orthosilicic acid, is mainly found in food rich in fiber and whole grains [24]. Si may also fulfill a biological role in mammals, and various studies have suggested that it is an essential trace element required in the development of bone cartilage and connective tissue collagen [25].
Chemicals such as solutions for parenteral nutrition may have Al [4, 5, 26] and Si [27] contamination, especially if there is an affinity between the components and Al and/or Si [28]. The volume given to a patient in total parenteral nutrition (TPN) may reach 3000 mL per day, depending on the body weight. In addition, TPN is administered to critically ill patients or patients with compromised metabolic and organ function, aggravating the deleterious effects of contaminants [27].
Al3+ forms complexes with aqueous silicic acid in neutral or mildly acidic media, which are important in the protection of plant and animal life against Al toxicity [29]. Si and silicic acid may decrease Al bioavailability by blocking its uptake through the gastrointestinal tract and by impeding reabsorption [30]. The hypothesis of a protective effect of silica against the Al from drinking water was investigated [31–33]. The findings of Rondeau et al. [31] showed an effect of silica: subjects exposed to a high silica concentration (11.25 mg L−1) appeared to have a lower risk of developing AD than did those exposed to a low silica concentration. Jacqmin-Gadda et al. [32] indicate that if an association exists between cognitive impairment and Al, pH, and silica, the threshold for these effects are low (Al 3.5 μg L−1, silica 10.4 mg L−1, and pH 7.35). Other studies [34, 35] reported the Si intracellular mechanism for Al detoxification in aquatic invertebrate and the ameliorate of Al induced behavioral toxicity.
The intraperitoneal injection and a model of long-term exposure was used to simulate exposure through parenteral nutrition, utilizing low doses of Al and Si in similar concentrations found in these solutions. Taking into account the potential relationship between Al exposure, oxidative stress, AChE alterations, and neurological disorders, the aim of the present study was to verify the bioavailability of Al low doses and the protective effect of silicate anions as SiO2(OH)2 2− upon this. We also evaluated the oxidative alterations in the rat blood and cerebrum structures as well as the changes in the activity of AChE.
Materials and Methods
Chemicals
Acetylthiocholine iodide, ethopropazine, 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB), tris-(hydroxymethyl)-aminomethane GR, sodium dodecylsulfate (SDS), malondialdehyde (MDA), thiobarbituric acid (TBA), 5′-aminolevulinic acid, and Coomassie Brilliant Blue G were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The tetramethylammonium hydroxide (TMAH) (solution containing 250 g L−1) used in this study were supplied by Sigma-Aldrich (St. Louis, USA), Triton X-100 by Vetec (Brazil), and concentrated nitric acid, sodium hydroxide, aluminum chloride, sodium silicate, phosphoric acid, acetic acid, serum albumin, and ethylenediaminetetraacetic acid (EDTA) by Merck (Darmstadt, Germany). Nitric acid was further sub-boiling distilled in a Berghof Teflon apparatus (Eningen, Germany).
Animals
Adult male Wistar rats (60 days), weighing 200–300 g from the Central Animal House of the Federal University of Santa Maria (UFSM), were used in this experiment. Animals were maintained at a constant temperature (23 ± 1 °C) on a 12-h light/dark cycle with free access to food and water. All animal procedures were approved by the Institutional Ethical Committee of the Federal University of Santa Maria (Protocol number 71/2010).
Preparation of Doses and Treatment
Si 1.0 g L−1, AlCl3 0.25 g L−1, and Si 1.0 g L−1 + AlCl3 0.25 g L−1 solutions for rat administration were prepared by aluminum chloride or/and sodium silicate dissolution in water purified by a Milli-Q high-purity water device (electrical resistivity of 18.0 MΩ cm) (Millipore, Bedford, USA), stored in plastic containers previously decontaminated (48 h in a 10 % (v/v) HNO3/ethanol solution) and used for a period of 2 weeks. The pH of all solutions was adjusted to 7.4 with HCl 0.1 M or NaOH 0.1 M, accordingly.
In this study was tested one low dosage of Al chloride (0.5 mg/kg/day) for simulated parenteral nutrition contamination. Studies estimated that the daily aluminum intake by PN was 3–4 μmol/kg/day [36] (0.108 mg/kg/day of Al corresponding 0.5 mg/kg/day of AlCl3). Urinary analysis of trauma patients receiving PN indicated that they received only 1–3 mg Si/day [37]; thus, the dosage 2 mg/kg/day of Si was chosen.
Animals were subjected to intraperitoneal injection for 5 consecutive days followed by 2 days of no treatment each week, completing a total of 60 administrations (12 weeks). Rats were divided into four groups: (1) control animals, which received only ultrapure water (n = 5); (2) animals treated with 0.5 mg/kg/day of Al chloride (n = 5); (3) animals treated with 2 mg/kg/day of Si (n = 5); and (4) animals treated with Al 0.5 mg/kg/day of Al chloride plus 2 mg/kg/day of Si (n = 5). The total volume of the solution per treatment per day by injection was 400 μL by 200 g of the body weight.
Animals were euthanized 24 h after the last dose, and the brains were dissected and collected immediately in vials and maintained on ice (5 °C).
Aluminum and Silicon Analysis of Serum and Brain Tissue
Sample brain aliquots of about 250 mg were weighed and mixed with 0.5 ml of a 25 % m/v TMAH solution. The mixture was kept in a water bath at 90 °C for 1 h. After solubilization, the volume was made up to 10 ml for the analysis. Serum samples were diluted with purified water 1:1 for Al and Si determination by graphite furnace atomic absorption spectrometry (GFAAS). Measurements were carried out in an Analytik Jena AG (Jena, Germany) ZEEnit 600 atomic absorption spectrometer according to Noremberg et al. [38].
Brain and Blood AChE Activity Assay
The brain was excised rapidly and the structures were isolated (hippocampus, striatum, cerebellum, cortex, and hypothalamus). The brain regions were then homogenized in a glass flask in a solution of 320 mM sucrose, 0.1 mM EDTA, 5 mM Tris–HCl, pH 7.5, at a proportion of 1:10 (g/v). AChE activity was determined according to [39], modified by [40]. The reaction mixture (2-mL final volume) was composed of 100 mM phosphate buffer pH (7.5) and 1 mM 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB). The method is based on the formation of a yellow anion, 4,4′-dithio-bis-acid nitrobenzoic, which absorbance is measured at 412 nm in a spectrophotometer UV–visible (HP 8453-Agilent) after 3 min incubation at 25 °C. The enzyme (40–50 μg of protein) was pre-incubated for 2 min at 25 °C. The reaction was initiated by adding 0.8 mM acetylthiocholine iodide. The enzyme activity was expressed in moles AcSCh/h/mg of protein.
Erythrocyte AChE activity was determined by the modification of [39] method as described by Worek et al. [41]. Whole blood dilutions were prepared by adding 100 μL blood to 10 mL sodium/potassium phosphate buffer 0.1 mM, pH 7.4, containing Triton X-100 (0.03 %). After being carefully mixed, the samples were frozen immediately and kept until analysis. The hemolizate (500 μL), phosphate buffer 0.1 mM pH 7.4, DTNB 0.3 mM, and ethopropazine 0.02 mM, a selective butyrylcholinesterase (BChE) inhibitor, were pre-incubated for 10 min at 37 °C. The reaction was started by the addition of substrate AcSCh 0.45 mM, and the color development was measured at 436 nm. The specific activity of erythrocyte AChE was calculated from the quotient between AChE activity and hemoglobin content. Results were expressed as milliunit per micromole Hb.
Brain and Blood TBARS Measurement
Brain TBARS levels were determined by the method described previously by [42] with a few modifications [43]. In short, the reaction mixture contained 200 μL of brain homogenates or standard (MDA-malondialdehyde 0.03 mM), 200 μL of 8.1 % sodium dodecylsulfate (SDS), 750 μL of acetic acid solution (2.5 M HCl, pH 3.5), and 750 μL of 0.8 % TBA. Since sucrose interferes in the TBARS assay, a portion of the brain was weighed and homogenized at a proportion of 1 g of tissue to 10 mL of buffer Tris/HCl 10 mM pH 7.4 plus 10 % of sodium dodecylsulfate (SDS) 10 %. Mixtures were heated at 95 °C for 90 min. After centrifugation at 1700g for 5 min, the absorbance was measured at 532 nm. TBARS tissue levels were expressed as nmol MDA per milligram of protein.
The malondialdehyde (MDA) level in serum was determined by the method described previously by [44]. An aliquot (0.2 mL) of serum was added to 0.250 mL of 0.11 mol/L 2-thiobarbituric acid and 1 mL of 0.2 M phosphoric acid. The mixture was heated at 90 °C for 45 min and the absorbance measured at 532 nm in a spectrophotometer UV–visible. Results were expressed as nanomole MDA per milliliter of serum.
Protein Determination
Protein was measured by the Coomassie Blue method according to [45] using bovine serum albumin as a standard.
Statistical Analysis
Data are expressed as means ± S.E.M. Data comparisons were carried out using analysis of variance (two-way ANOVA) followed by Duncan’s multiple range tests, where p < 0.05 was considered to represent a significant difference in all experiments. All analyses were performed using the GraphPad InStat software.
Results
Al and Si Concentration in the Brain Tissue and Serum
Al concentration in the brain was significantly higher in Al + Si-treated rats (about threefold) than in control animals (p < 0.05, Fig. 1a). Al serum content was significantly higher in Al and Al + Si groups (by 50- and 30-fold, respectively) than in control and Si groups (p < 0.0001). Concomitant treatment with Si was associated with a significant reduction, about 30 %, in serum Al content (p < 0.0005, Fig. 1b).
Brain tissue Si content was significantly increased (about fourfold) in all groups treated compared to the control animals (p < 0.0001, Fig. 2a). In serum, animals that received Si and Al + Si presented an accumulation of this element by 70- and 62-fold, respectively, in relation to control and Al group (p < 0.00001, Fig. 2b).
TBARS in Different Brain Structures and Serum
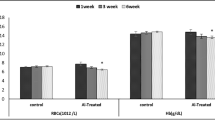
The effect of Al and Si on TBARS production from the cerebellum, cortex, striatum, hippocampus, and hypothalamus were analyzed. The post hoc comparisons by Duncan’s test revealed that the cerebellum MDA levels in the Al group were significantly higher (approximately 20 %) than those found in the other groups (p < 0.05, Fig. 3a). In the hippocampus, Al increased (54 %) TBARS production and is significantly different from the control and Al + Si groups (p < 0.05, Fig. 3b). Treatment with Si reversed the TBARS production to the control level in these structures. In the cortex, striatum, and hypothalamus, there was no difference in TBARS production between the groups (p > 0.05, data not shown).
Thiobarbituric acid reactive substances (TBARS) production in supernatant of cerebellum (a) and hippocampus (b) of Al-exposed rats and co-administered with Si. Different lowercase letters indicate a significant difference between groups. Bars represent means ± S.E.M. Two-way ANOVA–Duncan’s Test (p < 0.05)
No effect of Al and Si exposure (individually or in combination) was noted regarding TBARS production in rat serum (p > 0.05, data not shown).
Activity of AChE in Different Brain Structures and Blood
AChE activity was modified by Al in the cerebral cortex, hippocampus, and cerebellum. The enzyme activity was significantly higher in the group that received Al when compared to the control (24 %) in the cerebellum (p < 0.05, Fig. 4a). The treatment with Si had no significant effect in restoring the enzyme activity to the control levels. Treatment with Al individually as well as its combination with Si decreased significantly the AChE activity in the cortex by about 55 % (p < 0.05, Fig. 4b), and in the hippocampus by about 15 % (p < 0.01, Fig. 4c), when compared to the control levels. When Al was co-administered with Si, the enzyme activity was lower (20 %) than in the control and Si groups in the hippocampus, (p < 0.01, Fig. 4c). In the striatum and hypothalamus, there was no alteration in the enzyme activity between the groups (p > 0.05, data not shown).
When Al and Si were administered, the AChE activity in blood remained unchanged in all the groups (data not shown).
Discussion
The present study is the first to show that intraperitoneal administration of Si in concentrations similar to those found in parenteral nutrition reduces the harmful effects of increased LPO in rat brain induced by long-term Al exposure. This finding is relevant because increased levels of oxidative stress and products of LPO in cerebral tissue are the major contributing factors in the development of neurodegenerative diseases [22]. The reduction of TBARS levels by the administration of Si strongly suggests its neuroprotective effect.
Al and Si administration resulted in a significant increase in the brain Al content. In this case, Si seems not to act as a protector, increasing metal concentration compared to the control group. However, a number of biological sites have been identified in which Si and Al are co-deposited or co-localized. One of the areas investigated is the senile plaque cores in the cerebral cortex of patients suffering from senile dementia/Alzheimer’s type (SDAT). High-resolution solid-state nuclear magnetic resonance measurements on the central regions of these plaques have shown that Si and Al are present as an aluminosilicate species and it has been suggested that Si ameliorates Al toxicity [46].
There are few studies reporting about the presence of Si in the human body and experimental animals. In the present study, we showed that this element may be deposited in the rat brain, also appearing in high concentrations in the serum after a long-term exposure to low doses. Si concentrations in the brain of Al group animals were higher than those of the animals in the control group. Previously, Gonzalez-Muñoz et al. [47] reported that the treatment with oral Al nitride resulted in a significant Si accumulation in mice brain. There is evidence that Al is able to produce free radicals that cause LPO, thereby damaging neuronal membranes and increasing blood–brain barrier permeability [48], allowing more Si to enter in the brain tissue.
Higher level of Si and Al in serum than in the brain was expected, considering that the blood is the momentary situation which was directly administered. Al serum concentration in rats of the control group agrees to that found in most healthy subjects. According to [49], Al concentrations in the plasma of these individuals were approximately 2 μg L−1. Al values of animals of the Al + Si group were about 30 % lower than those of the Al group. The higher rate of excretion in the Si-co-treated animals could be a cause of the lower accumulation of Al in the serum. However, despite this, a decrease has occurred and the Al concentration present in the serum of Al + Si-treated animals remains elevated. This may account for not reducing the Al levels in the brain of this group, because this element is still available for co-deposition in this tissue.
Various studies have shown that Al is able to displace Fe in diverse biomolecules and increase intracellular Fe concentrations, thereby promoting the Fenton reaction [50–52]. This metal may also damage the mitochondrion directly and affect electron transport in the respiratory chain [53, 54]. In both cases, there is increased ROS production, which explains the increase in LPO. Although in this work no significant Al accumulation was observed in the Al-treated rats, we verified that this has effects on LPO in specific brain areas. Al could be deposited in specific cerebral areas, and perhaps for this reason, we did not observe a metal accumulation when whole brain was used.
Earlier studies have already shown that Al ingestion elevated its concentration in various regions of the brain [55, 56]. Gonzalez-Muñoz et al. [47] showed that the inclusion of Si in the diet in the form of beer or silicic acid reduced the harmful effects caused by lowering Al levels in the brain. Our results indicate a pattern of Al-induced LPO in the hippocampus and cerebellum, suggesting an Al-induced free radical generation in the brain. However, when silicon was co-administered, the MDA levels reduced equating the control group. Our results demonstrated that Si was effective in reducing the LPO since the formation of hydroxyaluminosilicates may reduce Al leading to the decreased availability of Al to ROS generation. In this case, Si could be considered a protector against Al-associated neurological diseases.
The brain may be particularly vulnerable to oxidative damage, and it is known that one of the pathways of neuronal damage and death in AD is mediated by free radical injury, mainly because the brain is rich in peroxidizable fatty acids [57]. Regarding LPO, Al is not a transition metal and does not undergo redox reactions in vivo. However, increased ROS have been reported during Al exposure [58]. ROS subsequently attack almost all cell components including membrane lipids, producing LPO [59]. LPO is a hallmark of oxidative stress, which disrupts the structural integrity of cell membranes and may also lead to the formation of aldehydes, which in turn damage lipids, protein, and DNA [60]. LPO measured by TBARS was elevated in the hippocampus and cerebral cortex compared with the control in mice loaded by gavage with Al 2.7 mg/kg/day [10]. In our work, TBARS production was higher in the Al group in the cerebellum and hippocampus, demonstrating the different behaviors of Al depending on the route of administration and dosage.
Al exposure increased the level of TBARS in the hippocampus, which is in agreement with other recent results [61]. In recent years, a number of authors have demonstrated that Al administration increases LPO in the rat brain [10, 45, 62, 63].
One of the major neurochemical features of Alzheimer’s disease is the marked reduction of nicotinic acetylcholine receptors in relevant diseased brain regions such as the cerebral cortex and hippocampus [64]. Depletion of cholinergic markers such as choline acetyltransferase (ChAT) and AChE are among the earliest and most severe of the biochemical changes to occur, and loss of these enzymes correlates well with the degree of cognitive impairment [65]. As reported by [66], the decreased activity of AChE may be associated with cholinergic hyperactivity, convulsion, and status epilepticus. Moreover, Ecobichon [67] reported that in the central nervous system, AChE inhibition may precipitate symptoms such as confusion, headache, sleep disturbances, and memory lapses.
Results regarding AChE activity were not homogenous in the Al group (animals that received only Al intraperitoneally) for the different cerebral structures studied. An elevation was observed in the AChE activity in the cerebellum, a reduction in the cortex and hippocampus, and no alterations in the hypothalamus and striatum. Different results showing both potentiating and inhibitive effects of Al on the cholinergic system are likely to simply be a reflection of the known biphasic effects of Al [68]. Another important aspect is that the hippocampus and cortex, which receive cholinergic projections from the nucleus basalis of Meynert, presented similar results.
Enhanced Al levels have been detected in the brain areas of patients with senile plaques and neurofibrillary tangles, both of which are characteristic of AD. The hippocampus and cortex are considered AD vulnerable regions because they accumulate β-amyloid (Aβ) in mice and humans [69]. It has been described that in AD, AChE expression is substantially altered and its activity is decreased in most brain regions. However, the AChE activity is increased within and around the amyloid plaques [70]. Our study showed the contrary, that is, in the hippocampus and cortex there was a decrease of the AChE activity in the presence of Al, which would be a precursor of AD.
It is important to emphasize that since cerebral AChE is an important regulator of behavioral process, the decreased AChE activity found in the cortex and hippocampus may be an indicator of Al-induced damage in the brain, and Si is not a protector of this toxicity.
Kumar [71] reported that increased Al concentration and an increased LPO rate could affect the neurons, leading to the depletion of AChE [63]. However, we have observed this relationship only in the hippocampus: AChE was increased in the cerebellum and reduced in the cortex and hippocampus, whereas there was an increase in TBARS levels in the hippocampus and cerebellum structures. Kaizer et al. [10] reported a decrease of AChE activity in the hypothalamus, but verified an enhancement in the striatum and no alterations in the hippocampus, cortex, and cerebellum. These results demonstrate that Al acts differently depending on the dose and chemical form of Al3+ administration, the route of administration (oral or intraperitoneal), and the time of exposure.
In another study [72], it was shown that long-term Al feeding altered kinetic properties of cholinesterases, presenting a reduction in the maximum velocity or rate at which the enzyme catalyzed a reaction (Vmax) of brain AChE in the soluble as well as membrane-bound fraction. In our experiments, we observed mainly the reduction of AChE activity after a long-term exposure with a low Al intraperitoneal dose. When this enzyme is inhibited, ACh is not hydrolyzed and accumulates in cholinergic sites causing alteration in the normal nervous system function.
In conclusion, the results of the present study show a protective effect of Si in the hippocampus and cerebellum against cellular damage caused by Al-induced oxidative stress, by the measure of LPO. The effects of Al on AChE activity were contradictory, causing both increase and decrease in the cerebral structures. Moreover, Si did not present protective effect against them. Si can be considered beneficial; even though Si did not reverse the action of Al in the cholinergic system, it inhibited the oxidative damage induced by Al and reduces Al accumulation in serum. Blood cannot be considered an indicator of oxidative damage or change in the activity of AChE enzyme because no changes have been verified. Though Si does not decrease Al effects on AChE activity, it may be considered an important protector in the LPO induced by Al.
References
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82:789–802
de Oliveira SMR, Bertagnolli D, Bohrer D, et al (2005) Nível Sérico de Alumínio: Influência da Água e de Alimentos Ingeridos Por Pacientes Com Insuficiência Renal Crônica Mantidos em Hemodiálise. J Bras Nefrol 27:101–109
Priest ND, Talbot RJ, Newton D, et al (1998) Uptake by man of aluminium in a public water supply. Hum Exp Toxicol 17:296–301
Alvarez L, Rebollido M, Fernandez-Lorenzo JR, et al (2007) Electrothermal atomic absorption spectrometry determination of aluminium in parenteral nutrition and its components. J Trace Elem Med Biol 21(Suppl 1):29–30
Bohrer D, do Nascimento PC, Binotto R, et al (2002) Contribution of the raw material to the aluminum contamination in parenterals. JPEN J Parenter Enteral Nutr 26:382–388
Llopis LS, Díez FB (2002) Revisión de los estudios sobre exposición al aluminio y enfermedad de Alzheimer. Rev Esp Salud Pública 76:645–658
Alvarez EM, Otero RS, Ameijeiras AH, et al (2001) Effects of aluminum and zinc on the oxidative stress caused by 6-hydroxydopamine autoxidation: relevance for the pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1586:155–168
Golub MS, Han B, Keen CL (1996) Developmental patterns of aluminum and five essential mineral elements in the central nervous system of the fetal and infant Guinea pig. Biol Trace Elem Res 55:241–251
Savory J, Huang Y, Herman MM, Wills MR (1996) Quantitative image analysis of temporal changes in tau and neurofilament proteins during the course of acute experimental neurofibrillary degeneration; non-phosphorylated epitopes precede phosphorylation. Brain Res 707:272–281
Kaizer RR, Correa MC, Spanevello RM, et al (2005) Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem 99:1865–1870
Sharma D, Sethi P, Hussain E, Singh R (2009) Curcumin counteracts the aluminium-induced ageing-related alterations in oxidative stress, Na+, K+ ATPase and protein kinase C in adult and old rat brain regions. Biogerontology 10:489–502
Moro MA, Almeida A, Bolanos JP, Lizasoain I (2005) Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med 39:1291–1304
Mesulam MM, Guillozet A, Shaw P, et al (2002) Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 110:627–639
Appleyard ME (1992) Secreted acetylcholinesterase: non-classical aspects of a classical enzyme. Trends Neurosci 15:485–490
Soreq H, Seidman S (2001) Acetylcholinesterase-new roles for an old actor. Nat Rev Neurosci 2:294–302
Worek F, Reiter G, Eyer P, Szinicz L (2002) Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol 76:523–529
Pailler FM, Bequet D, Corbé H, Giudicelli CP (1995) Aluminum, hypothetic cause of Alzheimer disease. Presse Med 24:489–490
Bilkei-Gorzo A (1993) Neurotoxic effect of enteral aluminium. Food Chem Toxicol 31:357–361
Platt B, Fiddler G, Riedel G, Henderson Z (2001) Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull 55:257–267
Calabrese V, Bates TE, Stella AM (2000) NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role of oxidant/antioxidant balance. Neurochem Res 25:1315–1341
Exley C (2004) The pro-oxidant activity of aluminum. Free Radic Biol Med 36:380–387
Wu ZH, Du YM, Xue H, et al (2012) Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol Aging 33:199.e1–199.e12
Chumlea WC (2007) Silica, a mineral of unknown but emerging health importance. J Nutr Health Aging 11:93
Perez-Granados AM, Vaquero MP (2002) Silicon, aluminium, arsenic and lithium: essentiality and human health implications. J Nutr Health Aging 6:154–162
Pérez JCR, Mancilla CLA (2012) El Papel del Silicio en los Organismos y Ecosistemas. Conciencia Tecnológica 43:42–46
Bohrer D, do Nascimento PC, Binotto R, Becker E (2003) Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part III: interaction container-chemicals during the heating for sterilisation. J Trace Elem Med Biol 17:107–115
Bohrer D, Bortoluzzi F, Nascimento PC, et al (2008) Silicate release from glass for pharmaceutical preparations. Int J Pharm 355:174–183
Bohrer D, do Nascimento PC, Martins P, Binotto R (2002) Availability of aluminum from glass and an Al form ion exchanger in the presence of complexing agents and amino acids. Anal Chim Acta 459:267–27635
Swaddle TW (2001) Silicate complexes of aluminum(III) in aqueous systems. Coord Chem Rev 219:665–686
Reffitt DM, Jugdaohsingh R, Thompson RPH, Powell JJ (1999) Silicic acid: its gastrointestinal uptake and urinary excretion in man and effects on aluminium excretion. J Inorg Biochem 76:141–147
Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF (2000) Relation between aluminum concentrations in drinking water and Alzheimer’s disease: an 8-year follow-up study. Am J Epidemiol 152:59–66
Jacqmin-Gadda H, Commenges D, Letenneur L, et al (1996) Silica and aluminium in drinking water and cognitive impairment in the elderly. Epidemiology 7:281–285
Gillette-Guyonnet S, Andrieu S, Vellas B (2007) The potential influence of silica presents in drinking water on Alzheimer’s disease and associated disorders. J Nutr Health Aging 11:119–124
Desouky M, Jugdaohsingh R, Catherine R, et al (2002) Aluminum-dependent regulation of intracellular silicon in the aquatic invertebrate Lymnaea stagnalis. Proc Natl Acad Sci 99:3394–3399
White KN, Ejim AI, Walton RC, et al (2008) Avoidance of aluminum toxicity in freshwater snails involves intracellular silicon-aluminum biointeraction. Environ. Sci Technol 42:2189–2194
Gura KM, Pharm D (2010) Aluminum contamination in products used in parenteral nutrition: has anything changed? Nutrition 26:585–594
Klein CJ, Nielsen FH, Moser-Veillon PB (2008) Trace element loss in urine and effluent following traumatic injury. J Parenteral Enteral Nutr 32:129–139
Noremberg S, Veiga M, Bohrer D, et al (2015) Determination of aluminum and silicon in bovine liver by graphite furnace atomic absorption spectrometry after dissolution with tetramethylammonium hydroxide. Anal Methods 7:500–506
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Rocha JBT, Emanuelli T, Pereira ME (1993) Effects of early undernutrition on kinetic parameters of brain acetylcholinesterase from adult rats. Acta Neurobiol Exp 53:431–437
Worek F, Mast U, Kiderlen D, et al (1999) Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 288:73–90
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Rossato JI, Zeni G, Mello CF, et al (2002) Ebselen blocks the quinolinic acid-induced production of thiobarbituric acid reactive species but does not prevent the behavioral alterations produced by intra-striatal quinolinic acid administration in the rat. Neurosci Lett 318:137–140
Jentzsch AM, Bachmann H, Furst P, Biesalski HK (1996) Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med 20:251–256
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Birchall JD, Chappell JS (1989) Aluminium, water chemistry, and Alzheimer’s disease. Lancet 29:953
Gonzalez-Munoz MJ, Meseguer I, Sanchez-Reus MI, et al (2008) Beer consumption reduces cerebral oxidation caused by aluminum toxicity by normalizing gene expression of tumor necrotic factor alpha and several antioxidant enzymes. Food Chem Toxicol 46:1111–1118
Srivastava RAK, Jain JC (2002) Scavenger receptor class B type I expression and elemental analysis in cerebellum and parietal cortex regions of the Alzheimer’s disease brain. J Neurol Sci 196:45–52
Bronner F (2008) Metals in bone: aluminum, boron, cadmium, chromium, silicon, and strontium lead. In: Bilezikian JP, Raisz LG, Martin TJ (eds) Principles of bone biology, 3rd edn. Academic Press, San Diego, pp. 515–531
Amador FC, Santos MS, Oliveira CR (2001) Lipid peroxidation and aluminium effects on the cholinergic system in nerve terminals. Neurotox Res 3:223–233
Dua R, Gill KD (2001) Aluminium phosphide exposure: implications on rat brain lipid peroxidation and antioxidant defence system. Pharmacol Toxicol 89:315–319
Yousef MI (2004) Aluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicology 199:47–57
Bondy SC, Cambell A (2001) Oxidative and inflammatory properties of aluminium possible relevance in Alzheimer’s disease. In: Exley C (ed) Aluminium and Alzheimer’s disease, 1st edn. Elsevier Science, Amsterdam, pp. 311–321
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Abubakar MG, Taylor A, Ferns GA (2004) Regional accumulation of aluminium in the rat brain is affected by dietary vitamin E. J Trace Elem Med Biol 18:53–59
Kaur J, Singh S, Sharma D, Singh R (2003) Aluminium-induced enhancement of ageing-related biochemical and electrophysiological parameters in rat brain regions. Indian J Biochem Biophys 40:330–339
Pratico D, Delanty N (2000) Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med 109:577–585
Campbell A, Prasad KN, Bondy SC (1999) Aluminum-induced oxidative events in cell lines: glioma are more responsive than neuroblastoma. Free Radic Biol Med 26:1166–1171
Yokel RA (2000) The toxicology of aluminum in the brain: a review. Neurotoxicology 21:813–828
da Silva AC, Rocha JB, Morsch AL, et al (2007) Oxidative stress and delta-ALA-D activity in chronic renal failure patients. Biomed Pharmacother 61:180–185
Gomez M, Esparza JL, Nogues MR, et al. (2005) Pro-oxidant activity of aluminum in the rat hippocampus: gene expression of antioxidant enzymes after melatonin administration. Free Radic Biol Med 38:104–111
Bhadauria M (2012) Combined treatment of HEDTA and propolis prevents aluminum induced toxicity in rats. Food Chem Toxicol 50:2487–2495
Flora SJ, Mehta A, Satsangi K, et al (2003) Aluminum-induced oxidative stress in rat brain: response to combined administration of citric acid and HEDTA. Comp Biochem Physiol C Toxicol Pharmacol 134:319–328
Oddo S, LaFerla FM (2006) The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J Physiol Paris 99:172–179
Perry EK, Perry RH (1980) In: Roberts PJ (ed) Biochemistry of dementia. John Wiley & Sons, Chichester, pp. 135–183
Olney JW, Collins RC, Sloviter RS (1986) Excitotoxic mechanisms of epileptic brain damage. Adv Neurol 44:857–877
Ecobichon DJ (1996) Toxic effects of pesticides. In: Klaassen CD, Doull J (eds) Casarett and Doull’s toxicology: the basic science of poisons, 5th edn. McGraw-Hill, New York, pp. 643–689
Exley C, Birchall JD (1992) The cellular toxicity of aluminium. J Theor Biol 159:83–98
Caccamo A, Oddo S, Sugarman MC, et al (2005) Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging 26:645–654
Arendt T, Bigl V, Tennstedt A, Arendt A (1984) Correlation between cortical plaque count and neuronal loss in the nucleus basalis in Alzheimer’s disease. Neurosci Lett 48:81–85
Kumar S (1999) Aluminium-induced biphasic effect. Med Hypotheses 52:557–559
Dave KR, Syal AR, Katyare SS (2002) Effect of long-term aluminum feeding on kinetics attributes of tissue cholinesterases. Brain Res Bull 58:225–233
Acknowledgments
This study was supported by CNPq (grant 477258/2010–7) and the Federal University of Santa Maria, RS, Brazil.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noremberg, S., Bohrer, D., Schetinger, M.R.C. et al. Silicon Reverses Lipid Peroxidation but not Acetylcholinesterase Activity Induced by Long-Term Exposure to Low Aluminum Levels in Rat Brain Regions. Biol Trace Elem Res 169, 77–85 (2016). https://doi.org/10.1007/s12011-015-0392-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0392-6