Abstract
The present study compared the concentrations of different elements (Ca, P, Mg, Sr, Ba, K, S, Zn, Mn) as well as Ca/P, Ca/Mg, Sr/Ca, and Ba/Ca ratios in hard antler and pedicle bone of yearling red deer stags (n = 11). Pedicles showed higher concentrations of calcium and phosphorus and a higher Ca/Mg ratio than antlers, while antlers exhibited higher concentrations of potassium, sulfur, and manganese as well as higher Ca/P, Sr/Ca, and Ba/Ca ratios. The findings indicate that antlers are less mineralized and show less maturation of their bone mineral than pedicles. Antlers also showed a higher intrasample variation of mineralization than pedicles, which can be related to the shorter life span of the (deciduous) antlers compared to the (permanent) pedicles. It is suggested that antler bone formation is stopped before the theoretically possible degree of mineralization and mineral maturation is reached, resulting in antler biomechanical properties (high bending strength and work to fracture) that are well suited for their role in intraspecific fighting. It is further suggested that the differences in Sr/Ca and Ba/Ca ratios of antlers and pedicles are related to the dietary shift from milk to vegetation in combination with an increasing intestinal discrimination against Sr and Ba with age, resulting in a less marked difference in these ratios than would be expected based on the dietary shift alone. The findings of our study underscore the suitability of antlers and pedicles as models of bone mineralization and the influence of different animal-related and/or external factors on this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Four of the six extant families of ruminants possess bony cranial appendages. Among them, the antlers of deer (family Cervidae) have received the greatest scientific attention [1]. This is related to the facts that antlers constitute the fastest growing bones in mammals and are characterized by an annual cycle of loss and regrowth [2]. The rapidity of their formation combined with their easy accessibility compared to other (“internal”) bones makes antlers a valuable model for studying the histogenesis and mineralization of (primary) bone and the influence of hormonal as well as dietary and other environmental factors on these processes [3–11]. Antler regrowth moreover constitutes the only example of regeneration of a complex appendage in mammals [12–15]. Studying the mechanism of antler regeneration and comparing this process with appendage regeneration in “lower” vertebrates may therefore provide crucial insights about how to stimulate limb regeneration in humans [13, 14]. In its functional state, i.e., when used in fighting between males, antler bone is a dry structure, whose moisture content is in equilibrium with that of the atmosphere [16]. The biomechanical properties of the dry antlers have been demonstrated to be well suited for their function in combat, in that they show a very high work to fracture, which makes them very difficult to break on impact [16].
Antlers do not grow directly from the skull roof but are grown and cast from permanent bony outgrowths of the frontal bones called pedicles. Pedicle formation is triggered by a rise in circulating testosterone secreted by the testes at puberty [17, 18]. Growth of the first antlers starts from the pedicles when these have reached a threshold length that in red deer (Cervus elaphus) is 5–6 cm [19, 20]. Growth of the first antlers, which in red deer and other cervid species are unbranched spikes, occurs as a continuation of pedicle growth and therefore does not constitute a regenerative event.
The histogenesis and the histological structure of antler bone have been studied in greater detail in various species. These studies demonstrated that antlers elongate by the action of chondrogenic growth zones present at the tips of the main beam and tines. Antler bone is largely formed in an endochondral way, with some peripheral (cortical) bone being added by direct ossification from the perichondrium/periosteum [3, 21–23]. In the process of antler ossification, the initially formed cartilage is largely replaced by woven bone, and the spaces between the trabeculae of the woven bone scaffold are filled with primary osteons. In the outer antler regions, this infilling with primary osteons causes the formation of a compact cortex that surrounds the spongy antler core. Due to the short life span of antlers, there is almost no remodeling of the antler bone, and in consequence, only very few secondary osteons can be found [24–27]. The virtual lack of bone remodeling also explains the occasional presence of remnants of mineralized cartilage within the trabeculae of the woven bone scaffold [9, 27]. Antlers can thus histologically be characterized as developmentally young bones.
Compared to antlers, there is only limited information on the histological structure of pedicles. A recent study in the European roe deer (Capreolus capreolus) demonstrated that the pedicle is a heavily remodeled structure whose porosity undergoes large seasonal fluctuations, with the highest values around antler casting and low values during the rutting period when the antlers are used for fighting [27]. It has also been demonstrated that the pedicle increases in thickness by the formation of fibrolamellar bone that is later remodeled into secondary osteonal bone [27, 28].
As in the case of histology, there also exists a great discrepancy between antlers and pedicles regarding our knowledge of the elemental composition of the two types of bone. While several studies reported the concentrations of various major and minor (trace) elements in antler bone [8, 11, 29–37], there exists only very limited information on element levels in pedicle bone and on differences in elemental composition and degree of mineralization between antlers and pedicles [38].
From a biomechanical perspective, antlers and pedicles must be considered a functional unit, because forces acting upon the antlers are transferred to the skull via the pedicles [27]. A comparison of the mechanical properties of antlers and pedicles and their related structural and compositional features can therefore provide a better understanding of the structural adaptations of the cranial appendages of deer to the functional demands deriving from the use of antlers as weapons in intraspecific combat. The present study compares the concentrations of certain elements as well as element ratios in first antler and pedicle bone of free-ranging yearling red deer stags. Concentration ratios (Ca/P, Ca/Mg, Sr/Ca, Ba/Sr) are reported in addition to the concentrations of the respective elements because ratios are much less affected by variations in the degree of mineralization than absolute concentrations. Specifically, we addressed the question whether ontogenetic differences between the permanent pedicles and the subsequently formed short-lived first antlers are reflected in their composition.
Materials and Methods
The analyzed pedicle and hard antler samples were obtained from 11 yearling red deer stags that had been killed in the 1980s and early 1990s during normal hunting operations in western Germany (Eifel and Hunsrück mountains, n = 9) and the Netherlands (n = 2). All antlers had been completely cleaned of velvet by the stags. As velvet shedding from the first antlers of red deer occurs in September [39] and the hunting season for yearling stags in western Germany ends on January 31st, the animals’ ages at death can be assumed to have been between about 16 to 20 months for the deer from Germany. Ages at death for the two individuals from the Netherlands were probably in the same range. Following killing, the animals’ heads had been defleshed, and the crania routinely cleaned and dried by the hunters. The antlered skulls of the animals were stored at room temperature prior to analysis.
One antler and one pedicle bone sample per animal were analyzed. In nine animals, the samples were removed from the left cranial outgrowth, while in two deer, in which the left antler had been broken during life, the antler and pedicle samples were obtained from the right side. For analysis, about 1-cm-thick full-diameter cross-sectional disks were cut from the proximal antler and the distal pedicle using a fine-toothed saw. The cut surfaces were polished with a grinding stone made of silicon carbide to remove potentially adhering metallic particles derived from the saw. Antler and pedicle bone samples were then dried to constant mass at 130 °C, pulverized, and homogenized for 10 min in a grinding mill (HSM 100R, Herzog Maschinenfabrik, Osnabrück, Germany), and further ground (20 min) in an agate mortar with pestle. Subsequently, 4.6 g of bone powder per sample was thoroughly mixed with 1.035 g of Hoechst wax C micropowder (Merck, Germany), and 5.5 g of the mixture was pelleted using a pellet press (Herzog Maschinenfabrik, Osnabrück, Germany) at a pressure of 10 t for 20 min. The pellets were then stored at room temperature in a desiccator until analysis.
Element concentrations (Ca, P, Mg, Sr, Ba, K, S, Zn, and Mn) in the samples were analyzed by wavelength-dispersive spectrometry in a Siemens SRS 3000 sequential X-ray spectrometer (60 kV, 50 mA for Ca, Sr, Ba, and Mn; 50 kV, 60 mA for P, Mg, K, S, and Zn), with measurement times between 10 and 50 s. Antler bone standards used for calibration were prepared by the method of standard addition, using analytical grade substances (Merck, Germany) that were added to the bone powder. The homogenized bone powder plus added elements was then pelleted as described above. For matrix correction, additional elements were added to the homogenized samples [40]. Each bone standard was measured six times to obtain the calibration functions. Concentrations in the standards and count rates in the spectrometer were highly correlated (r values between 0.97 and 1.0). Concentrations are expressed on a dry matter basis as weight % (=g/100 g) for Ca and P, and as parts per million (ppm = mg/kg) for all other elements.
Differences in element concentrations as well as concentration ratios (on a weight basis) between antler and pedicle bone samples of the animals were analyzed with the Wilcoxon matched pairs test. Spearman rank order correlations were calculated to analyze the relationship between element concentrations. To correct for error accumulation due to running multiple statistical tests, we performed a sequential Bonferroni adjustment of P values [41]. Adjusted P values <0.05 were considered to indicate statistical significance. All statistical tests were performed with the software package Statistica version 8 (StatSoft, Tulsa, OK, USA).
Results
Significant differences (adjusted P values <0.05) between antlers and pedicles existed for the concentrations of calcium and phosphorus (higher in pedicles) as well as those of potassium, sulfur, and manganese (higher in antlers). Antlers also showed higher zinc concentrations than pedicles, but the difference between the two groups was not significant following sequential Bonferroni correction (adjusted P value = 0.051). No significant differences between antlers and pedicles existed for concentrations of magnesium, strontium, and barium (Table 1).
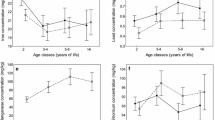
All analyzed element ratios differed significantly between antlers and pedicles, with antlers showing higher Ca/P, Sr/Ca, and Ba/Ca ratios, while the Ca/Mg ratio was higher in the pedicles (Table 2, Fig. 1). Intrasample variation (coefficients of variation) of calcium, phosphorus, and sulfur concentrations as well as Ca/P ratio in antlers markedly exceeded those in pedicles, while for the Mn content, the coefficient of variation in pedicles was more than two times that in antlers (Table 3).
For antlers, the statistical analysis (Spearman rank correlations) revealed significant positive correlations between calcium and phosphorus (r s = 0.96) and between potassium and sulfur (r s = 0.94) concentrations. Significant negative correlations (r s values between −0.85 and −0.98) existed for the relationships between Ca and K, Ca and S, P and K, P and S, and Mg and Mn concentrations in antlers (Table 4).
In the pedicles, only the (positive) correlation between calcium and phosphorus concentrations (r s = 0.87) was significant. The positive relationships between P and Mg (r s = 0.66) and Sr and Ba (r s = 0.69) as well as the negative relationships between Ca and Sr (r s = −0.70), Ca and Ba (r s = −0.62), Ca and K (r s = −0.69), and P and K (r s = −0.65) were not significant (adjusted P values >0.05; Table 5), although the correlation coefficients were quite high. The lack of statistical significance in these cases can be attributed to the small sample size and the rather conservative statistical approach used for data analysis.
Discussion
It is well known that antlers have a lower mineral content than other types of mammalian bone [42, 43]. The latter studies compared antler bone with “internal bones,” mostly femora, from deer and other mammals. Thus far, only one study compared the permanent (pedicle) and the deciduous (first antler) portions of the cranial appendages grown by juvenile deer, showing that pedicle bone is more highly mineralized (higher ash content) than that of the first antler [38]. The results of the present study extend these findings by reporting elemental concentrations and ratios for first antlers and pedicles.
The mineral constituent of bone is a poorly crystalline, carbonated apatite whose chemical composition differs from that of stoichiometric hydroxylapatite, which has a Ca/P weight ratio of 2.15 and a molar ratio of 1.67. Bone apatite has much fewer hydroxyl groups than pure hydroxylapatite and furthermore contains numerous elements and molecular species that substitute for calcium (Ca2+), phosphate (PO4 3−), and hydroxyl (OH−) ions in the lattice [44–47]. Such substitutions cause structural changes in the bone mineral and can have major effects on bone mechanical and other properties [44].
The antlers of the yearling red deer showed lower calcium and phosphorus concentrations as well as higher sulfur concentrations compared to pedicles. Calcium and phosphate are the main constituents of the bone mineral, whereas sulfur in the bone is predominantly present as part of various constituents of the organic matrix [48, 49]. In line with earlier findings, the results of the present study thus clearly indicate that the antlers were less mineralized and had a higher relative proportion of organic matrix than the pedicles.
Our results also show that the degree of mineralization was much more variable among the antlers than the pedicles. Thus, the coefficients of variation for calcium, phosphorus, and sulfur concentrations as well as Ca/P ratio in the antlers all more than doubled the corresponding values for the pedicles. This suggests that bone formation and mineralization were of a more homogeneous nature during pedicle than during antler growth. The higher intrasample variability in mineralization of antlers compared to pedicles is also reflected by the significant negative correlations between the concentrations of both calcium and sulfur (r s = −0.98) and phosphorus and sulfur (r s = −0.95) in antlers, while no significant correlations of calcium and phosphorus concentrations with those of sulfur were observed in the pedicles (Tables 4 and 5).
The overall lower and more variable degree of mineralization of antlers compared to pedicles is hypothetically attributed to different factors. First, pedicle growth occurs at a slower rate than first antler growth [50]. Second, antlers are short-lived structures that in their functional (hard antler) state consist of dry, dead bone [16], whereas pedicles are permanent structures. It has been discussed previously that because of the short life span of antlers, there is no opportunity for a compensation of delays in the onset and progression of the mineralization process of antler bone as is principally possible in long-lived “internal” bones [28]. Thus, antlers are considered to be less “buffered” than pedicles against external or animal-related factors affecting bone growth and mineralization, which is reflected by the more variable mineralization of antlers compared to pedicles. An influence of nutrition and related growth parameters on the chemical composition, structure, and mechanical properties of antlers has been demonstrated repeatedly [10]. For example, it was shown that body weight and growth rate of yearling red deer stags influenced the chemical composition of their first antlers [36] and that the milk supply of the yearlings was positively correlated with the ash, calcium, and phosphorus contents of the first antlers [37].
Mineralization of the organic bone matrix is a gradual process that consists of two successive steps, viz. a rapid primary mineralization and a much slower secondary mineralization [46, 51]. Primary mineralization starts relatively shortly (5 to 10 days in humans) after deposition of the bone matrix, reaching 50 to 70 % of the maximal mineralization within about 3 weeks. During secondary mineralization, a slow and gradual maturation of the mineral component occurs that involves both an increase in crystal number and crystal size [46, 51, 52]. The normal duration of the secondary mineralization phase in humans is unknown [46]. In special cases (unremodeled primary lamellar bone), the process may be decades long [53]. In a study on ewes, secondary mineralization in bone structural units was completed after 30 months [52].
Pedicle growth in juvenile male red deer starts at an age of about 6 to 8 months (January/February), while first antler formation from the pedicles usually commences at around 12 months of age (May/June) and ends with velvet shedding in September (months apply to red deer from western and central Europe) [20, 39, 54]. Thus, first antler growth in red deer occurs over a period of only about 4 months. The period of bone formation and mineralization in the pedicles of the studied individuals was much longer, ranging from the onset at 6 to 8 months to the animals’ death at an age of 16 to 20 months.
A recent fluorochrome labeling study revealed a rapid mineralization process in cortical bone of regenerated antlers of red deer, with average mineral apposition rates of 2.15 μm/day in early stages and of 1.56 μm/day in later stages of primary osteon formation [26]. Corresponding data for pedicle mineralization are not available. It is suggested that the rapid mineralization observed in the red deer antlers corresponds to the primary mineralization process and early stages of the secondary mineralization described in other (internal) bones [46, 52]. The short life span of antlers further suggests that the process of secondary mineralization is not completed in antler bone, i.e., that the antlers die off before the physiological limit of their mineralization is reached. Their low degree of mineralization makes antlers less brittle than other bones and very difficult to break on impact [16], which is clearly an adaptive trait for a structure used in intraspecific fighting. Thus, the short-lived nature of the antler bone that results from the linking of the antler cycle to the annual fluctuations in the levels of circulating androgens may be viewed as a means to produce a type of “headgear” [1] that is biomechanically optimized for its role in intraspecific combat. The higher mineral content of pedicles compared to antlers most likely reflects the longer time available for mineralization of the pedicles, although also the pedicle bone of the yearling stags analyzed in this study had probably not yet achieved its theoretically possible maximum degree of mineralization. The effects of their higher mineral content, in combination with their different bone micromorphology [27], on the biomechanical properties of pedicles in comparison to antlers remain to be elucidated.
Ca/P weight ratios in the studied antlers and pedicles were relatively close to the value for hydroxylapatite (2.15). Values were more variable in the antlers, and in nine of 11 cases, the Ca/P ratio in the antler bone exceeded that in pedicle bone. The latter finding is suggestive of a generally higher degree of substitution of phosphate (PO4 3−) by carbonate (CO3 2−) in the bone mineral of the pedicles compared to antlers. Carbonate accounts for about 6 to 7 % of the total mineral ions in bone [46], and CO3 2− can substitute for either PO4 3− (type B carbonate) or OH− (type A carbonate) in the apatite lattice [46, 47], with the substitution for PO4 3− being the dominant one in bone [44, 55]. Occupancy at the B site in the apatite lattice is dependent on the competition between PO4 3−, HPO4 2−, and CO3 2− ions [56]. A third site of carbonate in bone mineral is labile CO3 2− at the mineral surface [46, 55].
Different studies have reported an increase in CO3 2− content and/or CO3 2−/PO4 3− ratio in bone with increasing age [57, 58]. More recent studies performed at the osteonal level provided conflicting results, reporting either a decreasing [59] or an increasing [60] CO3 2−/PO4 3− ratio from the center (youngest bone) to the periphery (most mature bone) within an osteon. The observed difference in Ca/P ratios between antlers and pedicles is principally consistent with the view that the developmentally younger antler bone exhibits a lower degree of carbonate substitution in the PO4 3− sites of the bone mineral (type B substitutions) than the developmentally older pedicle bone. However, further studies are needed to substantiate this assumption.
About 60 % of the magnesium in the human body is found in bone, both in the organic matrix and the mineral component, and the element plays a key role in bone metabolism [61]. Magnesium is essential for the biological activity of ATP and acts as a cofactor for many enzymes, including alkaline phosphatase [62]. An increase in the Ca/Mg ratio has been reported to occur with increasing mineralization of the bone [57]. Substitution of magnesium for calcium in bone mineral is, however, limited, which is related to the considerably smaller size of the Mg2+ ion compared to the Ca2+ ion and the resulting distortion of the apatite lattice structure caused by the replacement of Ca2+ by Mg2+. In consequence, binding of Mg2+ retards the nucleation and growth of apatite crystals [46, 63]. There is discrepancy in the literature about whether Mg2+ is preferentially incorporated in Ca [I] sites [46] or Ca [II] sites [63] of the apatite lattice.
Bone mineral crystals exhibit a hydrated layer with loosely bound ions (including Mg2+), which constitute the so-called nonapatitic mineral domain [45, 46]. During mineral maturation, the hydrated layer is reduced whereas the stable apatite domain grows [46]. As the incorporation of magnesium into the apatite lattice is limited, the loss of loosely bound magnesium present in the labile mineral domain, i.e., of magnesium adsorbed to the crystal surface, will lead to an increase in Ca/Mg ratio with increasing bone mineral maturation. The lower Ca/Mg ratio in antler bone compared to pedicle bone is therefore, like the higher Ca/P ratio of antlers, attributed to the fact that antler bone is developmentally younger than pedicle bone.
Strontium and barium are nonessential trace elements that tend to mimic calcium in metabolic pathways [64]. In biological apatites, Sr2+ and Ba2+ ions, which both have a higher ionic radius than Ca2+, can substitute for the latter [65]. As organisms discriminate against Sr and Ba in favor of Ca, Sr/Ca and Ba/Ca ratios diminish during metabolic processes, a phenomenon known as biopurification of calcium [64]. In the mammalian organism, discrimination of Sr and Ba occurs at five main locations, the intestines, kidneys, placenta, mammary glands, and sites of apatite formation [65]. As the efficiency of the process of biopurification at these sites changes throughout ontogeny, there is variation in the Sr/Ca and Ba/Ca ratios of apatites formed during different ontogenetic stages in mammals [65]. Thus, yearling male white-tailed deer (Odocoileus virginianus) were shown to exhibit less discrimination against uptake of strontium relative to calcium than 2-year old males [66]. In line with these early results, a later study observed that in wild white-tailed deer and in farm-raised steers (Bos taurus), discrimination against Sr and Ba early in ontogeny was relatively lower than during later life [65].
Variation in Sr/Ca and Ba/Ca ratios of biological apatites is also strongly influenced by dietary changes, which are often likewise linked to age and therefore difficult to separate from ontogenetic changes in discrimination [65]. In mammals, especially the change from milk to adult diet is associated with a marked increase in dietary Sr/Ca and Ba/Ca ratios. For lactating dairy cows, an average Sr/Ca ratio in the milk of 0.15 relative to the animals’ diet was observed [67]. A later study likewise found that in cattle, Sr/Ca and Ba/Ca ratios for milk are much lower than those of other dietary items [65]. An increase in Sr/Ca and Ba/Ca ratios of bone apatite with increasing proportion of forage in the diet is also expected in deer.
Most births in red deer from Germany occur during May and June [39]. Pedicle growth commences at an age of about 6 to 8 months, and growth of the first antlers from the top of the pedicles starts about 12 months after birth [20, 39, 54]. Weaning of red deer calves whose mothers have become pregnant again occurs at about 5 to 7 months after birth, with last sucking behavior typically observed before end of November [68]. However, barren mothers, i.e., hinds that did not become pregnant again during the rut (September/October) in the year of birth, often wean their calves later, although in this case, the frequency of sucking behavior drops to lower levels during winter [39, 68]. Pedicle growth in red deer thus starts close to the end of the period of milk feeding, and only early stages of pedicle formation and mineralization may take place preweaning. In contrast, first antler growth starts some months after weaning when the animals feed entirely on vegetation.
The observed lower Sr/Ca and Ba/Ca ratios in pedicle bone compared to antler bone of the yearling red deer stags can therefore be attributed to the fact that pedicle growth occurs closer to the period of milk feeding (and partly overlaps with it) than antler growth. However, the magnitude of the observed differences is clearly less than would be expected if the ratios in bone apatite would only reflect the transition from milk (with low Sr/Ca and Ba/Ca ratios) to plant diet (with higher ratios). In line with the reasoning by Peek and Clementz [65] for apatite ratios in mineralized tissues of cattle and deer, we therefore assume that an increasing intestinal discrimination against strontium and barium with increasing age is also of influence here and that this factor somewhat dampens the effect of the dietary transition occurring at weaning.
In bone, zinc can be present bound to either the organic matrix (in metalloenzymes or collagen) or the bone mineral [9, 69, 70]. In the bone apatite, the Zn2+ ion is preferentially incorporated in Ca [I] sites [46]; however, replacement of Ca2+ with the smaller Zn2+ distorts the lattice and thus inhibits further crystal growth [71]. Substitution of calcium by zinc in lattice positions is therefore very limited, and most of the zinc in bone mineral is thought to be loosely bound, thereby matching the situation for magnesium [71]. There is evidence that alkaline phosphatase, a zinc-containing metalloenzyme [62], becomes incorporated as a bone matrix protein during initial mineralization [70]. In a recent study on red deer antlers, zinc staining was observed at the mineralization front of forming primary osteons, whereas completely formed and fully mineralized primary osteons showed no Zn-staining [9]. It was concluded that high zinc concentrations are found at sites of primary mineralization [9]. In the present study, the difference in zinc content between antlers and pedicles was not statistically significant (adjusted P value = 0.051); however, there was a clear tendency for higher values in the antlers, which is in line with the view that antler bone is less mature than pedicle bone.
Also, the K+ ion can substitute for Ca2+ in the apatite lattice [44, 47]. Using a histochemical approach in red deer antlers, Landete-Castillejos et al. [9] observed that potassium was abundant in the trabecular scaffold composed of woven bone, whereas it was not detected in primary osteons. Accordingly, antlers with a higher porosity due to incomplete infilling of osteons (incomplete mineralization), and thus a higher proportion of trabecular compared to osteonal bone, had a higher potassium content than more mature antlers with low porosity [9]. It was concluded that potassium content in antlers can serve as a marker of the ratio between trabecular (woven) bone and osteonal bone and thus of the degree of maturation of the antler bone. Extending this reasoning to the differences in potassium content observed between antlers (considerably higher values) and pedicles in the present study, this would again point to the more mature nature of pedicle compared to antler bone.
The present study revaled a marked difference in manganese content of antlers (mean 17.1 ppm dry wt) and pedicles (mean 1.4 ppm dry wt). Average manganese concentrations for antlers reported in the literature vary widely, ranging from 0.57 ppm dry wt for antlers grown by farmed Spanish red deer following a winter with late frosts [10] to 21.5 ppm dry wt for antlers of wild roe deer from Germany [30]. To the best of our knowledge, thus far, no manganese concentrations in pedicle bone have been published. However, for femora of female red deer, mean Mn concentrations of 0.26 ppm dry wt for animals feeding on natural vegetation and of 0.32 ppm dry wt for animals who in addition were supplementally fed with wholemeal feed were reported [72].
Manganese is required for the synthesis of glycosaminoglycans during bone matrix formation and is a cofactor for different enzymes in bone tissue [73]. In the apatite lattice, Mn2+ can substitute for Ca2+ [44, 47]. It may be speculated that the higher Mn content of the antlers is related to their relatively lower degree of mineralization and relatively higher proportion of organic matrix compared to the pedicles. However, our analytical approach did not allow for separating matrix-bound from mineral-bound manganese, and therefore, further studies are needed to clarify this issue. An increased frequency of antler breakage observed in red deer has been related to a reduced Mn content of the antler bone [74]. It does not seem likely that this reasoning can simply be extended to the differences in Mn content between antlers and pedicles observed in the present study, as there were two cases of antler fracture in our sample but no case of pedicle fracture.
Conclusions
Using wavelength-dispersive X-ray fluorescence, the present study, for the first time, demonstrated marked differences in element concentrations and element ratios between first antlers and pedicles of yearling red deer stags. The results indicate that in comparison with pedicle bone, the antler bone of the yearling red deer was less mineralized and its mineral component less mature. This can be related to the rapid growth and short life span of the antler compared to the longer formation time and permanent nature of the pedicle. Our findings suggest that antler bone formation is stopped before the theoretically possible degree of mineralization and mineral maturation is reached. Interestingly, this is an advantageous feature, as it gives the antlers a high bending strength and work to fracture [16], thereby making them very well suited for their fighting function. Thus, the linking of the antler cycle to the reproductive cycle of deer and the associated seasonal demise of the antlers as a result of velvet shedding caused by a rise in circulating androgen concentrations produces a kind of cranial appendage that is biomechanically highly adapted for its role in intraspecific combat.
For first antlers of red deer, it has previously been reported that the concentrations of calcium and phosphorus as well as the ash content in the antler base were higher than those in the tip region while the potassium and zinc contents were lower [36]. These results are supportive of our above conclusion, since the later formed antler tips have less time to undergo mineralization than the more basal antler portions. Evidence for incomplete mineralization of antler bone has also been demonstrated histologically in red deer [26] and roe deer [27].
As a cautionary note, we want to emphasize here the fact that the present study analyzed antlers and pedicles of yearling stags. First, antlers are much smaller than those of adult stags and are formed during a period of ongoing body growth. It has previously been shown that the investment in antler growth by yearling red deer stags is much lower than that by older stags [75]. Moreover, yearling red deer males do not intensely participate in the rut and, in contrast to older males, do not use their antlers in fighting with other males over access to females. In addition, the pedicles of yearlings have not undergone the pronounced seasonal changes in porosity that are associated with antler casting and regeneration and make the pedicles of older deer heavily remodeled bone structures [27]. It therefore remains to be analyzed, whether the differences observed between antlers and pedicles of yearling red deer also hold for adult stags. It may, for instance, be assumed that the differences in Sr/Ca and Ba/Ca ratios between antlers and pedicles observed in yearling stags diminish with increasing animal age, as the initially formed pedicle bone becomes more and more replaced by secondary osteons in the process of remodeling and new bone is laid down along the pedicle periphery by periosteal apposition [27]. Further studies addressing the differences in chemical composition between regenerated antler bone and remodeled pedicle bone of older deer are thus clearly needed. Another field of high interest to antler biology is how the compositional and histological differences between antler and pedicle bone are reflected by their mechanical properties.
References
Davis EB, Brakora KA, Lee AH (2011) Evolution of ruminant headgear: a review. Proc R Soc B 278:2857–2865
Goss RJ (1983) Deer antlers: regeneration, function, and evolution. Academic Press, New York
Muir PD, Sykes AR, Barrell GK (1988) Changes in blood content and histology during growth of antlers in red deer (Cervus elaphus) and their relationship to plasma testosterone levels. J Anat 158:31–42
Brown RD (1990) Nutrition and antler development. In: Bubenik GA, Bubenik AB (eds) Horns, pronghorns and antlers. Springer, New York, pp 426–441
Bubenik GA (1990) Neuroendocrine regulation of the antler cycle. In: Bubenik GA, Bubenik AB (eds) Horns, pronghorns and antlers. Springer, New York, pp 265–297
Suttie JM, Fennessy PF, Lapwood KR, Corson ID (1995) Role of steroids in antler growth of red deer stags. J Exp Zool 271:120–130
Kierdorf U, Kierdorf H, Schultz M, Rolf HJ (2004) Histological structure of antlers in castrated male fallow deer (Dama dama). Anat Rec 281A:1352–1362
Landete-Castillejos T, Currey JD, Estevez JA, Gaspar-López E, Garcia A, Gallego L (2007) Influence of physiological effort of growth and chemical composition on antler biomechanical properties. Bone 41:794–803
Landete-Castillejos T, Currey JD, Ceacero F, Garcia AJ, Gallego L, Gomez S (2012) Does nutrition affect bone porosity and mineral distribution in deer antlers? The relationship between histology, mechanical properties and mineral composition. Bone 50:245–254
Landete-Castillejos T, Estevez JA, Ceacero F, Garcia AJ, Gallego L (2012) A review of factors affecting antler composition and mechanics. Front Biosci E4:2328–2339
Estevez JA, Landete-Castillejos T, Martinez A, Garcia AJ, Ceacero F, Gaspar-López E, Calatayud A, Gallego L (2008) Antler mineral composition of Iberian red deer Cervus elaphus hispanicus is related to mineral profile of diet. Acta Theriol 54:235–242
Price JS, Allen S, Faucheux C, Althnaian T, Mount JG (2005) Deer antlers: a zoological curiosity or the key to understanding organ regeneration in mammals. J Anat 207:603–618
Kierdorf U, Kierdorf H, Szuwart T (2007) Deer antler regeneration: cells, concepts, and controversies. J Morphol 268:726–738
Kierdorf U, Kierdorf H (2012) Antler regrowth as a form of epimorphic regeneration in vertebrates—a comparative view. Front Biosci E4:1606–1624
Li C (2012) Deer antler regeneration: a stem cell-based epimorphic process. Birth Defects Res (Pt C) 96:51–62
Currey JD, Landete-Castillejos T, Estevez J, Ceacero F, Olguin A, Garcia A, Gallego L (2009) The mechanical properties of red deer antler bone when used in fighting. J Exp Biol 212:3985–3993
Lincoln GA (1971) Puberty in a seasonally breeding male, the red deer stag (Cervus elaphus L.). J Reprod Fert 25:41–54
Suttie JM, Lincoln GA, Kay RNB (1984) Endocrine control of antler growth in red deer stags. J Reprod Fert 71:7–15
Fennessy PF, Suttie JM (1985) Antler growth: nutritional and endocrine factors. R Soc New Zeal Bull 22:239–250
Li C, Suttie JM (1994) Light microscopic studies of pedicle and early first antler development in red deer (Cervus elaphus). Anat Rec 239:198–215
Banks WJ (1974) The ossification process of the developing antler in the white-tailed deer (Odocoileus virginianus). Calcif Tissue Res 14:257–274
Kierdorf H, Kierdorf U, Szuwart T, Clemen G (1995) A light microscopic study of primary antler development in fallow deer (Dama dama). Ann Anat 177:525–532
Price JS, Oyajobi BO, Nalin AM, Frazer A, Russell RGG, Sandell LJ (1996) Chondrogenesis in the regenerating antler tip in red deer: expression of coallgen types I, IIA, IIB, and X demonstrated by in situ nucleic acid hybridization and Immunocytochemistry. Dev Dyn 205:332–347
Krauss S, Wagermaier W, Estevez JA, Currey JD, Fratzl P (2011) Tubular frameworks guiding orderly bone formation in the antler of the red deer (Cervus elaphus). J Struct Biol 175:457–464
Kulin RM, Chen PY, Jiang F, Vecchio KS (2011) A study of the dynamic compressive behavior of elk antler. Mater Sci Eng C31:1030–1041
Gomez S, Garcia AJ, Luna S, Kierdorf U, Kierdorf H, Gallego L, Landete-Castillejos T (2013) Labeling studies on cortical bone formation in the antlers of red deer (Cervus elaphus). Bone 52:506–515
Kierdorf U, Flohr S, Gomez S, Landete-Castillejos T, Kierdorf H (2013) The structure of pedicle and hard antler bone in the European roe deer (Capreolus capreolus): a light microscope and backscattered electron imaging study. J Anat 223:364–384
Kierdorf U, Kierdorf H, Boyde A (2000) Structure and mineralization density of antler and pedicle bone in red deer (Cervus elaphus L.) exposed to different levels of environmental fluoride: a quantitative backscattered electron imaging study. J Anat 196:71–83
Bernard R (1963) Specific gravity, ash, calcium and phosphorus content of antlers of Cervidae. Natur Canadien 90:310–322
Anke M, Brückner E (1973) Der Mengen- und Spurenelementgehalt verschieden frequentierter Äsungspflanzen des Rotwildes und des Rothirschgeweihes unterschiedlicher Qualität. Beitr Jagd Wildforsch 8:21–32
Hyvärinen H, Kay RNB, Hamilton WJ (1977) Variation in the weight, specific gravity and composition of the antlers of red deer (Cervus elaphus L.). Br J Nutr 38:301–311
Miller KV, Marchinton RL, Beckwith JR, Bush PB (1985) Variations in density and chemical composition of white-tailed deer antlers. J Mamm 66:693–701
Tataruch F, Wolsperger M (1995) Chemische Analysen an prähistorischen Rothirsch- und Riesenhirschgeweihen. Z Jagdwiss 41:225–228
Pathak NN, Pattanaik AK, Patra RC, Arora BM (2001) Mineral composition of antlers of three deer species reared in captivity. Small Rumin Res 42:61–65
Johnson HE, Bleich VE, Krausman PR (2007) Mineral deficiencies in Tule elk, Owens Valley, California. J Wildl Dis 43:61–74
Landete-Castillejos T, Garcia A, Gallego L (2007) Body weight, early growth and antler size influence antler bone mineral composition of Iberian red deer (Cervus elaphus hispanicus). Bone 40:230–235
Gomez JA, Landete-Castillejos T, Garcia AJ, Gaspar-López E, Estevez JA, Gallego L (2008) Lactation growth influences mineral composition of first antler in Iberian red deer Cervus elaphus hispanicus. Wildl Biol 14:331–338
Kierdorf U, Richards A, Sedlacek F, Kierdorf H (1997) Fluoride content and mineralization of red deer (Cervus elaphus) antlers and pedicles from fluoride polluted and uncontaminated regions. Arch Environ Contam Toxicol 32:222–227
Wagenknecht E (1988) Rotwild, 3rd edn. VEB Deutscher Landwirtschaftsverlag, Berlin
Stoffels D (1998) Analyse von Bioindikatoren im knöchernen Rosenstock und Primärgeweih mittels Röntgenfluoreszenzspektrometrie und Wasserdampfdestillation. University of Münster, Diploma thesis
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Statist 6:65–70
Currey JD (1999) The design of mineralized hard tissues for their mechanical functions. J Exp Biol 202:3285–3294
Currey JD, Brear K, Zioupos P (2004) Notch sensitivity of mammalian mineralized tissues in impact. Proc R Soc Lond B 271:517–522
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mat Sci Eng C25:131–143
Rey C, Combes C, Drouet C, Glimcher MJ (2009) Bone mineral: update on chemical composition and structure. Osteoporos Int 20:1013–1021
Farley D, Boivin G (2012) Bone mineral quality. In: Dionyssiotis Y (ed) Osteoporosis. InTech, available from http://www.intechopen.com/books/osteoporosis/bone-mineral-quality, pp 1–32
Skinner HCW (2013) Mineralogy of bones: In Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P (eds) Essentials of medical geology, revised edn. Springer, Dordrecht, pp 665–687
Robey PG (2008) Noncollagenous bone matrix proteins. In: Bilezikian JP, Raisz LG, Martin TJ (eds) Principles of bone biology, 3rd edn. Elsevier, Amsterdam, pp 335–349
Robey PG, Boskey AL (2008) The composition of bone. In: Primer on the metabolic bone diseases and disorders of mineral metabolism, 7th edition. ASBMR, Washington, pp 32–38
Suttie JM, Fennessy PF, Crosbie SF, Corson ID, Laas FJ, Elgar HJ, Lapwood KR (1991) Temporal changes in LH and testosterone and their relationship with the first antler in red deer deer (Cervus elaphus) stags from 3 to 15 months of age. J Endocrinol 131:467–474
Burr DB, Akkus O (2014) Bone morphology and organization. In: Burr DB, Allen MR (eds) Basic and applied bone biology. Elsevier Academic Press, Amsterdam, pp 3–25
Bala Y, Farlay D, Delmas PD, Meunier PJ, Boivin G (2010) Time sequence of secondary mineralization and microhardness in cortical and cancellous bone from ewes. Bone 46:1204–1212
Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB (2003) Aging of microstructural compartments in human compact bone. J Bone Min Res 18:1012–1019
von Raesfeld F, Reulecke K (1988) Das Rotwild, 9th edn. Verlag Paul Parey, Hamburg
Ou-Yang H, Paschalis EP, Mayo WE, Boskey AL, Mendelsohn R (2001) Infrared microscopic imaging of bone: spatial distribution of CO3 2−. J Bone Min Res 16:893–900
Rey C, Renugopalakrishnan V, Collins B, Glimcher MJ (1991) Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif Tissue Int 349:251–258
Burnell JM, Teubner EJ, Miller AG (1980) Normal maturational changes in bone matrix, mineral, and crystal size in the rat. Calcif Tissue Int 31:13–19
Legros R, Balmain N, Bonel G (1987) Age-related changes in mineral of rat and bovine cortical bone. Calcif Tissue Int 41:137–144
Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL (1996) FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int 59:480–487
Petra M, Anastassopoulou J, Theologis T, Theophanides T (2005) Synchrotron micro-FT-IR spectroscopic evaluation of normal paediatric human bone. J Mol Struct 733:101–110
Bonjour J-P, Guéguen L, Palacios C, Shearer MJ, Weaver CM (2009) Minerals and vitamins in bone health: the potential value of dietary enhancement. Br J Nutr 101:1581–1596
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 21:441–483
Laurencin D, Almora-Barrios N, de Leeuw NH, Gervais C, Bonhomme C, Mauri F, Chrzanowski W, Knowles JC, Newport RJ, Wong A, Gan Z, Smith ME (2011) Magnesium incorporation into hydroxyapatite. Biomaterials 32:1826–1837
Balter V (2004) Allometric constraints on Sr/Ca and Ba/Ca partitioning in terrestrial mammalian trophic chains. Oecologia 139:83–88
Peek S, Clementz MT (2012) Ontogenetic variations in Sr/Ca and Ba/Ca ratios of dental bioapatites from Bos taurus and Odocoileus virginianus. J Trace Elem Med Biol 26:248–254
Cowan RL, Hartsook EW, Whelan JB (168) Calcium-strontium metabolism in white tailed deer related to age and antler growth. Proc Soc Exp Biol Med 129:733–737
Cragle RG, Demott BJ (1959) Strontium and calcium uptake and excretion in lactating dairy cows. J Dairy Sci 42:1367–1372
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer—behavior and ecology of two sexes. University of Chicago Press, Chicago
Spadaro JA, Becker RO (1970) The distribution of trace metal ions in bone and tendon. Calc Tissue Res 6:49–54
Gomez S, Rizzo R, Pozzi-Mucelli M, Bonucci E, Vittur F (1999) Zinc mapping in bone tissues by histochemistry and synchrotron radiation-induced X-ray emission: correlation with the distribution of alkaline phosphatase. Bone 25:33–38
Bigi A, Foresti E, Gandolfi M, Gazzano M, Roveri N (1995) Inhibiting effect of zinc on hydroxylapatite crystallization. J Inorg Biochem 58:49–58
Olguin CA, Landete-Castillejos T, Ceacero F, Garcia AJ, Gallego L (2013) Effects of feed supplementation on mineral composition, mechanical properties and structure in femurs of Iberian red deer hinds (Cervus elaphus hispanicus). PLoS One 8(6):e65461
Palacios C (2006) The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr 46:621–628
Landete-Castillejos T, Currey JD, Estevez JA, Fierro Y, Calatayud A, Ceacero F, Garcia AJ, Gallego L (2010) Do drastic weather effects on diet influence changes in chemical composition, mechanical properties and structure in deer antlers? Bone 47:815–825
Gomez JA, Ceacero F, Landete-Castillejos T, Gaspar-López E, Garcia AJ, Gallego L (2012) Factors affecting antler investment in Iberian red deer. Anim Prod Sci 52:867–873
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kierdorf, U., Stoffels, D. & Kierdorf, H. Element Concentrations and Element Ratios in Antler and Pedicle Bone of Yearling Red Deer (Cervus elaphus) Stags—a Quantitative X-ray Fluorescence Study. Biol Trace Elem Res 162, 124–133 (2014). https://doi.org/10.1007/s12011-014-0154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0154-x