Abstract
Although exposure to arsenic (As) induces neurotoxic changes, there is a lack of data regarding its specific effects on neurotransmission, particularly dopaminergic neurotransmission. In this study, the dopamine content and expression of tyrosine hydroxylase (TH) and dopamine receptors (DRs) were examined in the striatum and cerebral cortex of the mouse brain following the administration of As (1–100 mg/L NaAsO2 in drinking water). After 3 weeks, significantly decreased TH expression and dopamine content, both in the striatum and the cerebral cortex of mice treated with 100 mg/L As, were observed when compared with controls. Although DR expression was similar in the cerebral cortex of As-treated mice, DRD1 to DRD4 expression significantly increased in the striatum of 100 mg/L As-exposed mice. These data indicate that altered dopaminergic neurotransmission may contribute to As-induced neurotoxic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Millions of people worldwide are exposed to arsenic (As) in drinking water at concentrations that exceed the allowable limit set by the World Health Organization [1, 2]. As is a toxic metalloid, and chronic exposure to toxic As levels is associated with a variety of diseases, including cancer, cardiovascular diseases, respiratory disease, and immune system dysfunction [3–5]. In addition, there is growing interest in the toxic effects of As exposure on the central nervous system (CNS), since increasing evidence has shown that the CNS is one of the major targets of As [6, 7]. Epidemiological studies reported that chronic exposure to As via drinking water results in reduced intellectual function in children and deteriorated sensory and motor function [8–10]. Animal experimental studies also demonstrated the presence of deficits in learning tasks, as well as behavioral alterations after As treatment [11, 12].
Although the mechanisms underlying the neurotoxic effects of As are unclear, research on the neurobiological consequences of As exposure has focused largely on neurotransmitter systems in the CNS. In this regard, studies in rodents showed that As alters the dopaminergic system [12–14]. Rats treated for 1 year with 50 mg/L As were hypoactive and had increased striatal dopamine content, and following 1 year of treatment with only 0.5 mg/L As, striatal dopamine receptor (DR) D1 messenger RNA (mRNA) levels were upregulated in rats [15]. These observations suggest that As may exert its neurotoxic effects, at least in part, by disturbing dopaminergic neurotransmission.
The neurotransmitter dopamine plays important roles in many brain functions, including the control of locomotion, cognition, emotion, and endocrine regulation [16]. Dopamine biosynthesis is regulated by tyrosine hydroxylase (TH), a rate-limiting enzyme in catecholamine synthesis. Meanwhile, dopamine affects neurons through dopamine receptors (DRs), which are classified into one of two receptor subtype families according to their pharmacological and biochemical properties: D1-like and D2-like. The Dl-like family includes dopamine receptors D1 (DRD1) and D5 (DRD5), whereas the D2-like family consists of dopamine receptors D2 (DRD2), D3 (DRD3), and D4 (DRD4) [17]. The D1-like receptors interact with G proteins that stimulate adenylate cyclase activity, whereas D2-like receptors inhibit adenylate cyclase activity [18].
To assess the effects of As on dopaminergic neurotransmission in the mouse brain, we investigated the changes in dopamine content and the expression of TH and DRs in As-exposed mouse brain. Dopamine content was analyzed to examine potential effects on dopamine synthesis, and we also examined the expression of TH and DR subtypes in the striatum and cerebral cortex following exposure to drinking water containing As.
Materials and Methods
Animals
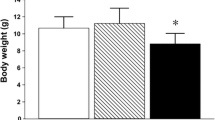
C57BL/6 mice (n = 40, 7-week-old males weighing 17.7 ± 0.5 g) were divided into four groups: water-treated control and 1, 10, and 100 mg/L As-treated groups. The mice (n = 10 per group) received 0, 1, 10, or 100 mg/L As in the form of NaAsO2 in their drinking water for 3 weeks. Control animals were given distilled deionized water only. Water consumption was measured throughout the study period, and the mice were weighed weekly. Mice and NaAsO2 were purchased from Samtako Laboratories (Osan, Korea) and Wako Chemical Co. (Osaka, Japan), respectively. Other reagents were of the highest quality available and were obtained from commercial sources. Experiments were approved by the Institutional Animal Care and Use Committee of Keimyung University, Korea. Experiments were conducted according to NIH guidelines for the care and use of laboratory animals. Animals were housed in a specific pathogen-free (SPF) facility, with free access to food and water. At the end of the treatment regimen, mice were sacrificed by CO2 asphyxiation, and the brains were harvested immediately. Striatum and cerebral cortex samples were dissected from the brains and stored at −70 °C until use.
Brain As Content
To measure the As concentration, the striatum and cerebral cortex samples were placed into a Teflon beaker, to which 1 mL HNO3 and 0.5 mL H2O2 were added. After soaking for 30 min, digestion took place in a microwave acid digestion system (MARS6; CEM, Matthews, NC, USA). The digested samples were cooled to room temperature and diluted to the final sample volume (5 mL) with ultra-pure water. As contents were measured using an inductively coupled plasma mass spectrometer (ELAN DRC-e; Perkin Elmer, Concord, Canada), and analytical performance was assessed periodically through participation in external quality assessment schemes, including the National Institute of Environmental Research (NIER) proficiency testing program for trace elements. Internal quality control was performed using a rhodium standard solution (Kanto Chemicals, Tokyo, Japan) as an internal standard material. Moreover, the digestion and analysis methods for the samples were validated using a gelatin multicomponent trace element reference material (Acros Organics, Fair Lawn, NJ, USA).
Measurement of Dopamine
To analyze dopamine content, the tissues were suspended in ice-cold 0.1 mol/L perchloric acid containing 1.34 mmol/L ethylenediaminetetraacetic acid (EDTA) and 0.05 % w/v sodium bisulfite at 10 μL/mg tissue, followed by sonication for 2 × 15 s. After simple protein precipitation using ZnSO4, the homogenates were centrifuged at 13,500 rpm for 5 min at 4 °C, and the supernatants were analyzed for dopamine content. Ultra-performance liquid chromatography (UPLC) analysis was performed using an Acquity UPLC system (Waters, Milford, MA, USA) equipped with a Waters Xevo TQ MS mass spectrometer. Chromatographic separation was performed on the Acquity UPLC BEH C18 column (2.0 mm i.d. × 50 mm, 1.7 μm) maintained at 40 °C. The mobile phase consisted of water (A) and acetonitrile (B), each containing 0.1 % formic acid. The proportion of mobile phase B was increased gradually from 5 to 100 % over 5 min. The injection volume was 5 μL, and the flow rate was 0.40 mL/min. The mass spectrometer conditions were as follows: capillary voltage, 3.0 kV; source temperature, 120 °C; dissolved nitrogen gas flow rate, 600 L/h; temperature, 350 °C; and cone gas flow rate, 50 L/h. Argon was used as the collision gas at a flow rate of 0.12 mL/min. Cone voltage and collision energy were optimized automatically to obtain the most intense fragmentation signal.
Western Blot Analysis
For Western blotting, the striatum and cerebral cortex were homogenized in radioimmunoprecipitation assay (RIPA) buffer (Sigma, MO, USA) containing 1 % protease inhibitor cocktail and a phosphatase inhibitor cocktail. The homogenate was centrifuged (14,000 rpm, 40 min, 4 °C), and the supernatant was collected. The protein concentration was estimated using the Bradford method, with bovine serum albumin (BSA) as the standard. An aliquot of 5 μg protein was subjected to 10 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the fractionated proteins were then transferred onto a nitrocellulose membrane. For immunoblotting, the following primary antibodies were used: mouse anti-TH monoclonal antibody (1:130,000 dilution; Chemicon, Temecula, CA, USA), goat anti-DRD1 polyclonal antibody (1:130 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-DRD2 monoclonal antibody (1:150 dilution; Santa Cruz Biotechnology), rabbit anti-DRD3 polyclonal antibody (1:200 dilution; Santa Cruz Biotechnology), rabbit anti-DRD4 polyclonal antibody (1:500 dilution; Novus Biologicals, Littleton, CO, USA), and goat anti-DRD5 polyclonal antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham Biosciences, Piscataway, NJ, USA), the immunoreactive bands were visualized using enhanced chemiluminescence (ECL) Western blotting detection reagents (Amersham Biosciences) and X-ray film. Band intensity was measured using the ImageJ program (NIH, Bethesda, MD, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control for immunoblotting.
Statistical Analysis
As or dopamine brain content and densitometric measurements of TH, DRD1, DRD2, DRD3, DRD4, or DRD5 protein expression in As-treated animals were compared with those of controls using one-way analysis of variance (ANOVA), followed by a post hoc Duncan test. p values <0.05 were deemed statistically significant. The data are presented as the means ± standard error of the mean (SEM). All statistical analyses were conducted using SAS v. 9.3 computer software (SAS Institute Inc., Cary, NC, USA).
Results
Brain As Content
After 3 weeks of As treatment, total As content in the brain was evaluated using inductively coupled plasma mass spectrometry (ICP-MS) analysis (Table 1). The total As level was increased dose-dependently in mice treated with 1, 10, or 100 mg/L compared with saline-treated controls. Specifically, total As content was increased significantly in mice treated with 100 mg/L As when compared with control mice (p < 0.01).
Dopamine Content in the Striatum and Cerebral Cortex
To understand the effect of As on dopamine synthesis, we examined striatal and cerebral cortex dopamine levels following exposure to 1, 10, or 100 mg/L As. Although not statistically significant, the dopamine level was decreased slightly in the striatum of mice treated with 100 mg/L As (Fig. 1a). Furthermore, treatment with 100 mg/L As induced a significant decrease in dopamine levels in the cerebral cortex of treated mice (p < 0.05; Fig. 1b).
Tyrosine Hydroxylase Expression in the Striatum and Cerebral Cortex
Based on the dopamine content data, we further examined the effects of treatment with 1, 10, or 100 mg/L As on modulation of TH expression in the striatum and cerebral cortex. In the striatum, TH expression was decreased significantly in mice treated with 100 mg/L As compared with saline-treated controls (p < 0.01; Fig. 2a). Similarly, TH expression was decreased significantly in the cerebral cortex of mice treated with 100 mg/L As compared to the cerebral cortex of control mice (p < 0.01; Fig. 2b).
Relative expression levels of tyrosine hydroxylase (TH) in the striatum (a) and cerebral cortex (b) of mice treated with As. Each panel shows a representative Western blot (top) and densitometric analysis of bands in the control and As-treated groups (bottom). Values represent the means ± SEM of each group (n = 5). **p < 0.01, compared with the control group
Effects of As on the Expression of DRs in the Striatum and Cerebral Cortex
To further elucidate the effects of As on the striatum and cerebral cortex, striatal and cerebral cortex dopamine receptor (DRD1, DRD2, DRD3, DRD4, and DRD5) expression was compared between control and As-treated mice. In the striatum, exposure to a higher dose of As induced a significant increase in DRD1, DRD2, DRD3, and DRD4 expression, whereas the expression of DRD5 was not changed significantly by As treatment (Fig. 3a). However, unlike DR expression in the striatum, As had no significant overall effect on DR expression in the cerebral cortex of treated mice (Fig. 3b).
Discussion
As elicits both intellectual and behavioral effects in the CNS by altering neurotransmitter systems [19–21]. In this study, we demonstrated that treatment with 100 mg/L As decreased dopamine content both in the striatum and cerebral cortex. These results are consistent with a previous study showing that whole brain dopamine concentration was decreased in rats exposed to As for 16 weeks [22]. In addition, a recent study showed that 60 days of As exposure resulted in significantly decreased dopamine concentration in the cerebrum and cerebellum of mice through ultra-structural changes in synapses [19]. However, rats that received 50 mg/L As for 1 year reportedly had significantly increased striatal dopamine levels, but not prefrontal cortex or nucleus accumbens dopamine levels [15]. A possible explanation for these discrepancies may be related to the treatment duration, in that long-term exposure to As increases the production of dopamine as an adaptive mechanism in specific brain areas.
Dopamine is synthesized from tyrosine by the sequential actions of tyrosine hydroxylase, a rate-limiting enzyme [23]. Therefore, we determined whether As exposure modulates TH protein expression in the striatum and cerebral cortex using Western blotting with TH-specific antibodies. Western blot analysis of TH revealed that its expression in the striatum and cerebral cortex was decreased significantly in the 100-mg/L As exposure group. This finding is consistent with dopamine level findings, as well as previous studies showing that As exposure induced decreased striatal and cerebral TH mRNA expression [13, 19, 24]. On the other hand, lower concentrations (1 and 10 mg/L) of As exposure did not significantly affect dopamine content or TH expression in either the striatum or cerebral cortex. Overall, these results indicate that decreased dopamine concentration may be related to the downregulated expression of TH, and dopamine content may play an important role in the mechanism of neurotoxicity induced by relatively high doses of As.
To better understand the mechanism underlying As-induced neurotoxic effects, we further measured dopaminergic receptors in the striatum and cerebral cortex. We found that high-dose As exposure upregulated expression of striatal DRs, except DRD5. Previous studies showed that chronic As exposure significantly decreased locomotor activity [13, 15]. In addition, the dopaminergic nervous system is implicated in the regulation of motor behavior, and each dopamine receptor subtype has a distinct role in motor control, including locomotor activity. For example, DRD1 activation has a stimulatory effect on locomotor activity [16, 25], whereas activation of presynaptic class DRD2 or DRD3 autoreceptors generally causes a decrease in locomotor activity [16, 25–27]. Since DRs are known to mediate behavioral alterations, including locomotor activity, these results suggest that As-induced changes in motor function are mediated at least in part by striatal DR subtype expression.
In this study, As exposure affected DR expression differentially between the striatum and cerebral cortex. Other studies also showed differential DR expression in the striatum and cortex. For example, lead (Pb)-exposed morphine-administered rats in a morphine dependence experimental model had significantly increased DRD2 expression in the prefrontal cortex, but not in the striatum [20]. Additionally, dopamine has long been recognized as a key neuromodulator of basal ganglia function, essential for normal motor activity, and the striatum is one of the main functional targets of dopamine [28]. Therefore, our findings are consistent with the notion that DRs in the striatum have major functional roles in neurotoxicant-induced changes in motor function [29].
Taken together, As treatment resulted in significantly decreased dopamine content and TH expression after 3 weeks of treatment at a dose of 100 mg/L. Further, we observed significantly increased DRD1–4 expression in the striatum, whereas the expression of DRs did not change significantly in the cerebral cortex. These data indicate that altered homeostasis of dopamine or DRs may contribute to As-induced neurotoxicity. However, further studies are required to address fully the effects of As on dopaminergic neurotransmission, including its effects on receptor activity. Moreover, because low striatal dopamine levels are known to induce DR overexpression as a compensatory mechanism [30, 31], it is important to determine whether changes in dopamine receptor expression in the striatum are a primary or secondary effect of As treatment.
References
Kinniburgh DG, Kosmus W (2002) Arsenic contamination in groundwater: some analytical considerations. Talanta 58:165–180
Del Razo LM, Corona JC, García-Vargas G, Albores A, Cebrián ME (1993) Fluoride levels in well-water from a chronic arsenicism area of Northern Mexico. Environ Pollut 80:91–94
Dangleben NL, Skibola CF, Smith MT (2013) Arsenic immunotoxicity: a review. Environ Health 12:73
Farzan SF, Karagas MR, Chen Y (2013) In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol 272:384–390
Axelson O, Dahlgren E, Jansson CD, Rehnlund SO (1978) Arsenic exposure and mortality: a case-referent study from a Swedish copper smelter. Br J Ind Med 35:8–15
Tyler CR, Allan AM (2013) Adult hippocampal neurogenesis and mRNA expression are altered by perinatal arsenic exposure in mice and restored by brief exposure to enrichment. PLoS ONE 8:e73720
Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 21:132–147
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH (2004) Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112:1329–1333
Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC (2006) Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology 27:210–216
Rodríguez VM, Jiménez-Capdeville ME, Giordano M (2003) The effects of arsenic exposure on the nervous system. Toxicol Lett 145:1–18
Nagaraja TN, Desiraju T (1994) Effects on operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intake. Hum Exp Toxicol 13:353–356
Rodríguez VM, Carrizales L, Jiménez-Capdeville ME, Dufour L, Giordano M (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55:301–308
Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM (2009) Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol 239:169–177
Itoh T, Zhang YF, Murai S, Saito H, Nagahama H, Miyate H, Saito Y, Abe E (1990) The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol Lett 54:345–353
Rodríguez VM, Limón-Pacheco JH, Carrizales L, Mendoza-Trejo MS, Giordano M (2010) Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol Teratol 32:640–647
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225
Lachowicz JE, Sibley DR (1997) Molecular characteristics of mammalian dopamine receptors. Pharmacol Toxicol 81:105–113
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24:125–132
Zhang J, Liu X, Zhao L, Hu S, Li S, Piao F (2013) Subchronic exposure to arsenic disturbed the biogenic amine neurotransmitter level and the mRNA expression of synthetase in mice brains. Neuroscience 241:52–58
Listos J, Baranowska-Bosiacka I, Talarek S, Listos P, Orzelska J, Fidecka S, Gutowska I, Kolasa A, Rybicka M, Chlubek D (2013) The effect of perinatal lead exposure on dopamine receptor D2 expression in morphine dependent rats. Toxicology 310:73–83
Zhang C, Li S, Sun Y, Dong W, Piao F, Piao Y, Liu S, Guan H, Yu S (2014) Arsenic downregulates gene expression at the postsynaptic density in mouse cerebellum, including genes responsible for long-term potentiation and depression. Toxicol Lett 228:260–269
Tripathi N, Kannan GM, Pant BP, Jaiswal DK, Malhotra PR, Flora SJ (1997) Arsenic-induced changes in certain neurotransmitter levels and their recoveries following chelation in rat whole brain. Toxicol Lett 92:201–208
Zhou QY, Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adiposic, and aphagic. Cell 83:1197–1209
Liu X, Piao F, Li Y (2013) Protective effect of taurine on the decreased biogenic amine neurotransmitter levels in the brain of mice exposed to arsenic. Adv Exp Med Biol 776:277–287
Sibley DR (1999) New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu Rev Pharmacol Toxicol 39:313–341
Wolf ME, Roth RH (1990) Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci 604:323–343
Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, Caron MG, Wightman RM (2002) Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience 112:39–49
Smith Y, Villalba R (2008) Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord 23(Suppl 3):S534–S547
Ball KT, Budreau D, Rebec GV (2003) Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res 994:203–215
Wakamatsu M, Iwata S, Funakoshi T, Yoshimoto M (2008) Dopamine receptor agonists reverse behavioral abnormalities of alpha-synuclein transgenic mouse, a new model of Parkinson’s disease. J Neurosci Res 86:640–646
Ebadi M, Srinivasan SK, Baxi MD (1996) Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog Neurobiol 48:1–19
Acknowledgments
This research was supported by the Bisa Research Grant of Keimyung University in 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M., Seo, S., Sung, K. et al. Arsenic Exposure in Drinking Water Alters the Dopamine System in the Brains of C57BL/6 Mice. Biol Trace Elem Res 162, 175–180 (2014). https://doi.org/10.1007/s12011-014-0145-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0145-y