Abstract
Poor micronutrient levels are associated with an increased risk of progression to AIDS and are also suggested to influence outcome of highly active antiretroviral therapy (HAART), though existing data are inconclusive to support the latter. Few published data are available on micronutrient levels in Ethiopian HIV/AIDS patients taking HAART. The objective of the study was to determine the association of micronutrient levels and response to HAART (CD4+ T cell count) among adult HIV/AIDS patients attending a teaching Hospital in Addis Ababa. CD4+ T cell counts and micronutrient (retinol, zinc, and iron) levels for 171 subjects were determined using standard procedures. Some proportions of the study participants were found deficient for retinol (14.03 %), zinc (47.3 %), and iron (2.8 %). Patients who were deficient in retinol had a significantly lower median CD4+ T cell counts (P = 0.002) compared to non-deficient subjects. Association of micronutrient quartiles with CD4+ T cell count was assessed using adjusted multivariate regression by taking quartile 4 as a reference category. Accordingly, patients who had retinol levels in quartile 4 had a significantly lower mean CD4+ T cell count compared to quartile 3 (P = 0.02). The significantly higher CD4+ T cell counts in patients who were non-deficient in retinol imply the role of retinol in improving the production of CD4+ T cells. However, both lower and higher retinol levels were associated with suppressed immunity (CD4 < 200 cells/mm3), suggesting an adverse effect of higher retinol levels. Thus, retinol may be potentially harmful depending on the dose, emphasizing the need for optimized level of retinol in nutrient supplements in patients taking HAART.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deficiency in micronutrients has been extensively reported in HIV-1-infected individuals even in HAART era and further correlated with CD4+ T cell count, HIV-1 plasma viral load, advanced disease progression, and mortality [1–3]. For example, low vitamin A levels were correlated with increased mortality [4, 5]. It was also shown that vitamin A deficiency, independent of body mass index and CD4+ T cell count, predicts adult mortality [6]. Furthermore, retinol levels were lower in patients on highly active antiretroviral therapy (HAART) compared with non-HAART controls, while the levels of retinol-binding proteins were increased [7]. Conversely, other studies found no differences in vitamin A and E levels between patients on HAART and those not receiving therapy [8, 9].
Studies have also documented a relationship between antiretroviral therapy (ART) and zinc deficiency. Zidovudine (AZT)-treated patients not deficient in zinc had significant mitogen response while treated patients who were zinc-deficient did not [10]. This has been supported by a recent study which demonstrated an association between zinc supplementation and a reduction in the likelihood of immunological failure, compared with placebo [11, 12]. On the other hand, a study on HIV-positive adults found no significant difference in zinc concentrations between those taking HAART and those not receiving any HIV medications [13].
Iron may also have adverse effects in HIV and other viral infections. For example, according to an in vitro study, iron increases and iron chelation reduces HIV replication [14]. A longitudinal study which assessed serum iron of patients found that iron concentrations are not significantly different between those receiving HAART for ≤3 years and those not receiving HAART [8].
Very limited data existing on the association of HIV and micronutrient levels in Ethiopia also reported a significantly lower retinol levels in HIV-infected patients compared to HIV non-infected controls [15, 16]. However, the impact of micronutrient status on HAART outcome is poorly described. While, studies elsewhere indicated the effect of micronutrients on response to HAART are still inconclusive [17, 18]. Therefore, this study aims to investigate the association between some selected serum micronutrient levels and the response of HAART, as measured by CD4+ T cell count, among HIV-infected patients under ART.
Materials and Methods
Study Design, Site, and Period
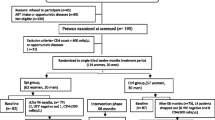
The St. Paul General Specialized Hospital, Addis Ababa, Ethiopia, has a separate ART unit which provides basic tests to provide antiretroviral (ARV) therapy. A cross-sectional study on patients receiving ART at the hospital was conducted between December, 2007, and January, 2008, to determine the association of micronutrient levels and the response to HAART as measured by CD4+ T cell count.
Study Participants
The study participants were HIV/AIDS patients who were having ART follow-up visits in St. Paul General Specialized Hospital, Addis Ababa. Using the formula for an estimate of a single population mean [19], a total of 172 individuals were included in to a cross-sectional study between December, 2007, and January, 2008. Since one individual failed to submit blood sample, all analysis was restricted to 171 participants. All study participants were selected by using the following criteria: the participant was taking HAART for ≥6 months, was 18 years of age or older, and has consented to participate. Individuals who failed to consent, chew khat, smoke cigarette, take immune-suppressive drugs, HIV negative, HIV-positive HAART naïve, <18 years of age, pregnant women, and patients with malignancies were excluded from the study.
Data Collection
A pre-designed structured questionnaire was used to collect relevant clinical data on the patients (previous and current clinical data) and information related to date of drug initiation.
Venous blood was collected by qualified nurses using a 10-mL syringe as a routine laboratory procedure to assess the status of patients on HAART. The collected blood was transferred into two tubes: a 5-mL tube containing EDTA for CD4+ T cell count and a non-anticoagulated tube to extract serum. Blood collection and serum separation were carried out under reduced light to prevent oxidation of retinol and in dust-free environment to avoid contamination with other trace elements. Leftover serum samples from the routine blood chemistry analysis were aliquoted into Eppendorf tubes, the tubes coded and kept at −70 °C until further determination of retinol, zinc, and iron [20].
Laboratory Analyses
Alanine Aminotransferase
Alanine aminotransferase (ALT) level was determined using chemistry analyzer (BT-2000, Biotechnica, Rome, Italy) according to the manufacturer’s specifications. As a quality control, animal serum-based HUMATROL was used.
CD4+ T Cell Count
Measurements of CD4+ T cell count were done on EDTA whole blood on the same day of blood collection using a specific monoclonal antibody and fluorescence-activated cell sorter (FACScount) (Model 339010, Becton Dickinson Bioscience, San Jose, CA, USA). BD FACSCount controls were run with every new batch of CD4 reagent kits (1 kit = 50 tests). Moreover, St Paul’s General Specialized Hospital laboratory is participating in an external quality assessment scheme for ART monitoring tests (CD4, hematology, and chemistry).
Retinol
Retinol was quantified by using reverse-phase HPLC (Prostar 310, Varian chromatography systems, California, USA) at the Ethiopian Health and Nutrition Research Institute (EHNRI) laboratory. The extract was injected and chromatographed on a 250 mm × 4.6 mm ID SUPERCOIL LC-18 5 μm and 250 × 3 mm ID Nucleosil 5 μm (CA, USA) by using a solvent system comprising 100 % methanol CHROMASOLV for high-performance liquid chromatography (Sigma-Aldrich, Germany) and detected by a UV/Vis detector (Varian chromatography systems, CA, USA) programmed at 325 nm (both for retinol and retinol acetate) with a retention time of 5.98 min. Pooled serum retinol and retinol acetate reference standards (28 μg/dL) were obtained from National Institute of Standard Technology (NIST) (Gaithersburg, MD, USA), and the recovery was found to be 95.45 %. All extraction procedures were carried out under reduced light to prevent oxidation of the compounds. Retinol was considered low if levels were 30 mg/dL [20].
Zinc and Iron
Zinc and Iron were measured by flame atomic absorption spectrometry (SpectraAA-10/20, Varian, Victoria, Australia). To quantify zinc and iron, 200-μL serum samples were diluted with 2 mL of butanol-1 (6 % v/v) and deionized water. Then, the diluted solution was mixed well with Vortex Mixer for 10 s. To verify the assay accuracy and to maintain quality, the standard solutions were run for every ten-test sample. A software package (SpectrAA worksheet oriented AA 10/20; version 01.30.203) was used to calculate the concentrations of zinc and iron. The measurement was automatically carried out, and the results were the average of three replicates. The precaution for both collection and subsequent handling of serum was taken in order to avoid or minimize trace element contamination. Standard solutions of 5 μmol/L zinc nitrate and iron nitrate, respectively, were used as an internal quality control. Deficient zinc and iron were defined as a serum level of <10.25 [20] and <8.92 μmol/L [21], respectively.
Statistical Analysis
Data were entered using excel spreadsheet and analyzed using SPSS software version 15. Student t test was used for comparisons of normally distributed continuous variables and the non-parametric test, Mann-Whitney-U test, for non-normally distributed independent variables. The median test was used for comparison of micronutrients between deficient and non-deficient groups. The HIV disease status indicator (CD4+ T cell count <200 cells/mm3) for odds ratio was analyzed using the stepwise logistic regression analysis. Mean trends were done for the test of linearity. Multivariate associations of serum micronutrient quartiles with CD4+ T cell count were used. For multivariate comparisons, only patients with complete data for the nutritional parameters and covariates were included. Adjustment for active liver disease was made making the confounding effect by liver disease less likely. That is, inclusion of ALT levels in the final regression model did not change the interpretation about the association of micronutrients with outcomes. Values were considered to be statistically significant when P values were less than or equal to 0.05.
Ethical Issues
The ethical aspect of this study was approved, and clearance was obtained from the Ethical Committee of the Department of Biology, Addis Ababa University. The objective of the study was explained to the study participants, HIV/AIDS patients, at the time of specimen collection prior to seeking their consent, including the confidentiality of the information as to be strictly kept in the best interest of the participants. This was also done under the strict observance of international protocols on human subject studies and national protocol on HIV/AIDS patients counseling and testing. Informed consent was obtained from all study participants.
Results
To study the association between micronutrients and ART outcome as measured by CD4+ T cell counts, a total of 171 individuals aged 18–65 years were included in this study.
As indicated in Table 1, the majority of the study participants (67.2 %) were females and the rest (32.8 %) were males. The mean age (and standard deviation (SD)) for males was 34.3 years (7.4), while for females, it was 39.2 years (8.8). Moreover, the majority of the study participants had formal education (78.4 %). Most of the study participants have been on HAART at least for 1 year (83.1 %), including those with ≥2-year treatment (31 %). According to WHO clinical staging criteria at the initiation of HAART, a significant majority of the patients (81.3 %) were classified under stages III and IV during HAART initiation.
Table 1 also shows that zinc was the highest deficient nutrient (46.8 %) followed by retinol deficiency (14.03 %). Moreover, study participants who had CD4+ T cell counts <200 cells/mm3 at the time of the study were significantly lower (P < 0.05) when compared to the baseline proportions of patients with CD4 < 200 cells/mm3. The baseline CD4 data was obtained from the patients’ medical records. In addition, 22.8 % of the study participants had evidence of liver disease (elevated ALT). However, the mean serum micronutrient levels were not significantly different (P < 0.05) between subjects with ALT level > 40 IU/L compared with subjects with ALT level <40 IU/L.
Micronutrient Deficiency and CD4+ T Cell Count
Micronutrient analysis revealed that patients with deficient retinol level had a significantly lower CD4+ T cell count compared to those with normal retinol level (186 versus 277 cells/mm3; P = 0.002) (Fig. 1). On the other hand, the overall inter-quartile ranges of CD4+ T cell count for those who had normal retinol level (171–382 cells/mm3) were also greater than the retinol-deficient ones (146.7–237.7 cells/mm3).
As shown in Fig. 2, the median CD4+ T cell count in patients deficient with zinc (248.5 cells/mm3) was lower than those that had normal zinc levels (284 cells/mm3). However, the difference in the median levels of zinc between the two groups was not statistically significant (P = 0.246). The inter-quartile CD4 ranges of patients with deficient zinc levels (157.3–379.8 cells/mm3) and normal zinc levels (170–393 cells/mm3) were also reasonably similar.
Figure 3 shows CD4+ T cell count between iron deficient and normal levels. In contrast to the normal iron levels, those deficient with iron had a slightly lower median CD4+ T cell count (271 versus 251 cells/mm3). However, the median differences in the levels of CD4+ T cell count for the two groups did not reach a statistically significant level (P = 0.559).
Association of Micronutrient Quartiles with CD4+ T Cell Count
Table 2 shows the mean CD4 count versus inter-quartile range (IQR) of micronutrients. It also shows mean CD4+ T cell count and odds ratio within each micronutrient quartiles compared to the upper quartile (quartile 4). As shown in the table, patients who had retinol levels in the upper quartile (quartile 4) had a significantly lower mean CD4+ T cell count compared to quartile 3 (P = 0.02).
Discussion
Immune recovery, as measured by increase in CD4+ T cell count, can be achieved following successful suppression of viral replication by using HAART. Some studies pointed that nutritional status of patients would affect the outcome of HAART [1, 17, 22]. This study has attempted to assess the association between some micronutrients levels and the immune response among adult HIV-infected patients receiving HAART in a resource-poor setting.
A relatively low prevalence of retinol deficiency (14 %) was observed in the present study compared with the 29.3 % reported in asymptomatic HIV-infected blood donors from the northern part of Ethiopia [16]. However, the present finding was in the range of what has been reported for subjects taking HAART in the “Nutrition for Healthy Living cohort study” in USA [20]. There have been reports that some drugs like colchicine, for example, could regulate vitamin A release and antioxidant system in serum of patients with Behçet disease and family Mediterranean fever [23, 24]. Apparently, HAART also increases the concentrations of retinol or retinoic acid by increasing the activity of retinal dehydrogenase, by altering the mechanism of retinol signaling, or by inducing the gene expression of retinal dehydrogenase [9]. This observation suggests the possible benefit of HAART on increasing the levels of retinol.
The 46.8 % zinc deficiency rate observed in the current study was lower than the 64 % rate documented for zidovudine (AZT)-treated patients in USA [10], suggesting the possible effect of ARVs on zinc deficiency which could lead to decreased effectiveness of the drug in zinc-deficient patients. Our finding is inconsistent with a study on HIV-positive adults that found no significant difference in zinc concentrations between those taking HAART and those who were not receiving any HIV medications [13]. This shows that zinc deficiency in HIV-infected patients has not grossly changed after the introduction of ARV triple therapy in 1996.
Moreover, low prevalence of iron deficiency (1.8 %) was observed compared to pre-HAART studies on HIV/AIDS patients in France (19 %). It is important to note that the lower prevalence of iron among HIV patients receiving HAART may indicate the effectiveness of the HAART therapy in reducing viral load and improving hematocrit values, and it has been reported that HAART increases hemoglobin concentration and decreases the prevalence of iron deficiency anemia [8, 25, 26].
The role of retinol in stimulating the production of CD4+ T cells was indicated by the finding that CD4+ T cell counts were significantly lower in patients who were deficient in retinol. Such an association was also demonstrated over an 18-month period between the development of vitamin A deficiency and a significant decrease in CD4+ T cell count [27]. Similar association of other nutrients (zinc, iron) with an improvement on the immune status of HIV/AIDS patients on HAART was suggested by the increasing pattern in their concentrations. Therefore, micronutrient supplementation, if provided to undernourished populations taking HAART regimen, may result in a significant immunologic and antioxidant benefit [28].
On the other hand, for retinol, a significant reduction was noted with advanced HIV disease in non-ARV-treated Ethiopians [15]. However, the present finding is in agreement with another study who reported no significant differences in the vitamin concentrations between the levels of CD4+ T cell count in HIV-positive men receiving HAART [29].
In the present study, assessment was made to show if there is any significant difference in mean CD4+ T cell counts of patients among the different quartiles for nutritional parameters by taking the upper quartile (quartile four) as a reference category. The study found no significant association between mean CD4+ T cell counts and serum iron quartiles. In addition, there were no significant associations between zinc quartiles and mean CD4+ T cell counts whereas higher serum retinol levels in quartile 4 were associated with a lower level of CD4+ T cell count compared with retinol levels in quartile 3. In line with this interesting finding of low CD4+ T cell count in the highest retinol quartile, others have found a high log viral load levels in this same quartile [20]. Another study also found a U-shaped relationship between vitamin A intake and progression to AIDS in a Multicenter AIDS Cohort Study (MACS) suggesting an adverse effect of higher retinol levels [30]. Retinol is known to increase lymphoid cell differentiation, which leads to an increase in CCR5 receptors. These receptors are essential for attachment of HIV to the lymphocytes, and therefore, an increase in their number is likely to increase HIV replication and decrease CD4+ T cell counts [31]. Hence, dose-dependent effects should be taken into consideration in nutritional interventions to these groups of populations.
Taken together, the study has shade some light that the provision of simple, inexpensive micronutrient supplements as an adjunct to HAART may have benefit to HIV-infected patients receiving HAART. However, it should be noted that intervention studies thus far have given conflicting results whether to recommend or refute the benefit of providing micronutrient supplements to HIV-positive persons receiving HAART [17]. Secondly, it has been shown that both deficiency and excess levels of retinol has been linked with declining CD4+ T cell counts and reduced survival [32], signifying the need for careful design and interpretation of such studies.
There are some limitations to this study. For instance, specific inflammation markers such as c-reactive proteins were not measured. However, as adjustment for active liver disease was made, the confounding effect by liver disease was made less likely. That is, inclusion of alanine aminotransferase (ALT) levels in the final regression model did not change the interpretation about the association of micronutrients with outcomes. Most importantly, this study is a cross-sectional and does not involve follow-up. Hence, finding an association does not necessarily imply causation. Therefore, longitudinal description of changes in micronutrient concentrations after HAART initiation, with adjustment for acute inflammatory markers (c-reactive proteins) would be valuable. Furthermore, assessment of micronutrients in HIV-positive persons receiving HAART is crucial in order to optimize the role of nutrition on drug efficacy.
References
Faintuch J, Soeters PB, Osmo HG (2006) Nutritional and metabolic abnormalities in pre-AIDS HIV infection. Nutrition 22:683–690
Campa A, Baum MK (2010) Micronutrients and HIV infection. HIV Ther 4(4):437–469
Nunnari G, Coco C, Pinzone MR, Pavone P et al (2012) The role of micronutrients in the diet of HIV-1-infected individuals. Front Biosci 4:2442–2456
Semba RD, Graham NM, Caiaffa WT et al (1993) Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch Intern Med 153:2149–2154
Semba RD, Lyles CM, Margolick JB et al (1998) Vitamin A supplementation and human immunodeficiency virus load in injection drug users. J Infect Dis 177:611–616
Semba RD, Caiaffa WT, Graham NM et al (1995) Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus-infected injection drug users. J Infect Dis 171:1196–1202
Toma E, Devost D, Chow N et al (2001) HIV protease inhibitors alter retinoic acid synthesis. AIDS 15:1979–1984
Rousseau MC, Molines C, Moreau J et al (2000) Influence of highly active antiretroviral therapy on micronutrient profiles in HIV-infected patients. Ann Nutr Metab 44:212–216
Kaio DJK, Rondo PHC, Souza JMP et al (2013) Vitamin A and beta-carotene concentrations in adults with HIV/AIDS on highly active antiretroviral therapy. J Nutr Sci Vitaminol 59:496–502
Baum MK, Javier JJ, Mantero-Atienza E et al (1991) Zidovudine-associated adverse reactions in a longitudinal study of asymptomatic HIV-1 infected homosexual males. J Acquir Immune Defic Syndr 4:1218–1226
Baum MK, Lai S, Sales S et al (2010) Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV infected adults. Clin Infect Dis 50:1653–1660
Asdamongkol N, Phanachet P, Sungkanuparph S (2013) Low plasma zinc levels and immunological responses to zinc supplementation in HIV-infected patients with immunological discordance after antiretroviral therapy. Jpn J Infect Dis 66:469–474
Wellinghausen N, Kern WV, Jöchle W et al (2000) Zinc serum level in human immunodeficiency virus-infected patients in relation to immunological status. Biol Trace Elem Res 73:139–149
Mamdooh G, Magda S (2010) MRN-100, an iron-based compound, possesses anti-HIV activity in vitro. Evid based complement. Altern Med 7(4):427–432
Abuye C, Tsegaye A, West CE et al (2005) Determinants of CD4 counts among HIV-negative Ethiopians: role of body mass index, gender, cigarette smoking, khat (Catha edulis) chewing, and possibly altitude? J Clin Immunol 25(2):127–133
Kassu A, Andualem B, Nhien VN et al (2007) Deficient serum retinol levels in HIV-infected and uninfected patients with tuberculosis in Gondor, Ethiopia. Nutr Res 16:323–328
Drain PK, Kupka R, Mugusi F et al (2007) Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am J Clin Nutr 85:333–345
Semba RD, Tang AM (1999) Micronutrients and the pathogenesis of human immunodeficiency virus infection. Br J Nutr 81(3):181–189
Hinders DC (2003) AP Statistics. McGraw-Hill Professional, USA
Jones CY, Tang AM, Forrester JE et al (2006) Micronutrient levels and HIV disease status in HIV-infected patients on highly active antiretroviral therapy in the nutrition for healthy living cohort. J Acquir Immune Defic Syndr 43(4):475–482
Rodak BF, Fristma GA, Doig K (2007) Hematology: clinical principles and applications. Elsevier, St. Louis
Jerene D, Næss A, Lindtjørn B (2006) Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther 3:10
Korkmaz S, Erturan I, Nazıroğlu M et al (2011) Colchicine modulates oxidative stress in serum and neutrophil of patients with Behçet disease through regulation of Ca2+ release and antioxidant system. J Membr Biol 244(3):113–120
Şahin M, Uğuz AC, Demirkan H, Nazıroğlu M (2011) Colchicine modulates oxidative stress in serum and leucocytes from remission patients with family Mediterranean fever through regulation of Ca2+ release and the antioxidant system. J Membr Biol 240:55–62
Belperio PS, Rhew DC (2004) Prevalence and outcome of anemia in individuals with human immunodeficiency virus: a systematic review. Am J Med 116:27–43
Omoregie R, Egbeobauwaye A, Ogefere H, Omokaro EU, Ekeh CC (2008) Prevalence of antibodies to HAART agents among HIV patients in Benin City, Nigeria. Afr J Biomed Res 11:33–37
Akkuş S, Nazıroğlu M, Eris S, Yalman K et al (2009) Levels of lipid peroxidation, nitric oxide, and antioxidant vitamins in plasma of patients with fibromyalgia. Cell Biochem Funct 27:181–185
Baum MK, Shor-Posner G, Lu Y et al (1995) Micronutrients and HIV-1 disease progression. AIDS 9:1051–1056
Tang AM, Smit E, Semba RD et al (2000) Improved antioxidant status among HIV-infected injecting drug users on potent antiretroviral therapy. J Acquir Immune Defic Syndr 23:321–326
Mehta S, Fawzi W (2007) Effects of vitamins, including vitamin A, on HIV/AIDS patients. Vitam Horm 75:355–383
Tang AM, Graham NMH, Kibry AJ et al (1993) Dietary micronutrient intake and risk of progression to AIDS in HIV-1 infected homosexual men. Am J Epidemiol 138:937–951
Baum MK, Shor-Posner G, Bonvehi P et al (1993) Influence of HIV infection on vitamin status and requirements. Ann N Y Acad Sci 669:165–173
Acknowledgments
This study was funded mainly by a grant from the Management Sciences for Health (RPM plus) and by Addis Ababa University. We are very grateful to Dr. Alemayehu Worku for his valuable assistance with statistical analysis. We also gratefully acknowledge St. Paul’s General Specialized Hospital (VCT and ART unit) and its physicians, nurses, and laboratory technicians/technologists for their invaluable help in obtaining the blood samples and additional data from patients’ clinical records. The Ethiopian Health and Nutrition Research Institute (EHNRI) is acknowledged for allowing the facility and the staff for their assistance with the laboratory analysis. We are very thankful to all the study participants who willingly participated in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eshetu, A., Tsegaye, A. & Petros, B. Selected Micronutrient Levels and Response to Highly Active Antiretroviral Therapy (HAART) Among HIV/AIDS Patients Attending a Teaching Hospital in Addis Ababa, Ethiopia. Biol Trace Elem Res 162, 106–112 (2014). https://doi.org/10.1007/s12011-014-0095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0095-4