Abstract
In this study, we have investigated erianin, a natural phenolic drug that impedes proliferation and metastatic migration through suppression of STAT-3 phosphorylation in human esophageal cancer cells. Eca-109 cells were treated with different concentrations of erianin (4, 8, 12 µM) for 24 h, and then cell proliferation, apoptosis, and metastatic markers were evaluated. Erianin-induced cytotoxicity and cell proliferation were examined using MTT and crystal violet staining techniques. The measurement of reactive oxygen species (ROS) and the study of apoptotic changes were conducted through flow cytometry. Furthermore, protein expression analyses via western blotting included an evaluation of JAK-STAT3, cell survival, cell cycle, proliferation, and apoptosis-related proteins. Moreover, erianin treatment-associated MMP expressions were studied by RT-PCR. In this study, erianin treatment induces substantial cytotoxicity and ROS production based on the concentrations in Eca-109 cells. Moreover, erianin inhibits the MAPK phosphorylation, proliferation, and metastatic protein in Eca-109 cells. STAT-3 is a crucial transcriptional factor that regulates numerous downstream proteins, such as proliferation, anti-apoptosis, and metastatic proteins. In this study, erianin treatment inhibited the protein expression of IL-6, IL-10, JAK-1, and p-STAT-3 expressions leading to induce apoptosis in Eca-109 cells. Moreover, erianin inhibited the expression of proliferation, metastatic, and anti-apoptotic markers in Eca-109 cells. Hence, erianin suppressed the JAK/STAT-3 signaling pathway and demonstrates potential as a chemotherapeutic agent for the treatment of esophageal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal carcinoma (EC) is considered a significant malignant type of cancer that originates from the mucosal epithelium of the esophagus organ [1]. The survival rate of EC is very low due to the inadequate therapeutic approach and drug resistance. The incidence rate of EC has been documented as the sixth most common type that causes cancer-associated mortality observed in recent years. According to the International Agency for Research on Cancer (IARC), there are 572,034 new cases of esophageal cancer worldwide, or 3.2% of all new cases, and 508,585 deaths, or 5.3% of all cancer fatalities [2]. The main risk factor for EC reported changes in lifestyle activities such as drinking, frequent smoking, and unhealthy diets [3]. The treatment methods for EC include standard chemotherapy, radiotherapy, and surgical resection if chemo or radiotherapy fails [4]. Due to the high frequency of EC metastasis, the 5-year survival rate was 15–25% [5]. Hence, identifying pathological mechanisms underlying EC proliferation and metastasis is considered a crucial way for preventive and therapeutic strategies.
Reactive oxygen radicals (ROS) are by-products of cell metabolic processes, and their levels are controlled to maintain homeostasis [6]. However, an imbalance in intracellular ROS levels can lead to oxidative stress and DNA damage, leading to many illnesses [7]. ROS can also trigger apoptosis and cell cycle arrest in EC cells by activating mitogen-activated protein kinases (MAPK) families, including c-Jun N terminal kinases (JNK), extracellular signal-regulated kinase-1 (ERK1), and p38 pathways [8, 9]. These MAPK families are responses to activate and phosphorylate numerous transcriptional factors that lead to cell differentiation, proliferation, and metastasis [10]. Signal transducer and activating transcription-3 (STAT-3), a major protein that acts as the transcriptional role and regulates numerous downstream elements [11]. Several researches have proved that the subsequent activity and phosphorylation of STAT-3 are implicated in the progression of esophageal cancer. Hence, inhibition of STAT-3 could be a possible therapeutical target for inhibiting EC proliferation and enhancing the chemotherapy [12].
STAT3 has been directly regulating cellular proliferation and metastasis by enhancing the expressions of factors such as cyclins and matrix metalloproteinase (MMPs) [13]. Moreover, STAT-3 expressions stimulate the G1 phase of the cell cycle, which leads to cell proliferation. The constitutive activation of STAT3 upregulates cyclin D1, vascular endothelial growth factors (VEGF), MMP-2, MMP-9, and anti-apoptotic proteins are paying to accelerated cancer cell progression [14]. Chemotherapy represents the primary therapeutic choice, yet the challenges of insufficient treatment approaches and drug resistance pose significant obstacles to its success [15]. To address these challenges, natural products can serve as promising chemotherapeutic agents owing to their multifaceted signaling capabilities [16].
Erianin is a bis benzyl compound derived from the herb Dendrobium chrysotoxum Lindl and has been used in Chinese traditional medicine as an antipyretic and analgesic [17]. Erianin possesses several pharmacological roles such as antioxidant, antiviral, improving diabetic nephropathy, antibacterial, relaxing bronchial smooth muscle, and anti-tumor [18]. Erianin has suppressed the proliferation and migration of liver cancer by regulating pyruvate carboxylase in HePG2 cells [18]. Moreover, erianin obstructs bladder cancer cell progression by stimulating the ferroptosis mechanism and NrF2 signaling [19]. Erianin also inhibits MAPK family protein, thereby impeding the growth of human cervical cancer cells by regulating p53 [20]. However, erianin treatment on the STAT-3-associated signaling axis in EC has not yet been studied. Therefore, in this study, we investigated the role of erianin impedes the proliferation and metastatic role of esophageal cancer by inhibiting STAT-3 and associated signaling in Eca-109 cells.
Materials and Methods
Reagents and Chemicals
The components for cell cultures, including DMEM medium, penicillin-streptomycin mix, 0.25% trypsin-EDTA, PBS, and FBS, were sourced from Invitrogen Life Technologies (Carlsbad, CA, USA). Chemicals such as erianin, DCFH-DA, MTT, EtBr, RIPA buffer, and AO were obtained from Sigma (St. Louis, MO, USA). Monoclonal antibodies targeting cyclin D1, cyclin-E1, p-p38, PCNA, VEGF, p-Jnk1, IL-6, IL-10, JAK-1, p-ERK-1, p38, p-STAT-3, and β-actin, along with secondary antibodies, were procured from cell signaling technology. The study necessitated the use of analytical and molecular-grade chemicals and solvents.
Cell Culture
The American Type Culture Collection (ATCC) was used to obtain the human esophageal cancer Eca-109 cell lines. These cell lines were shown to be mycoplasma-free. Eca-109 cell lines were grown in culture flasks with DMEM media (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/L penicillin, 100 U/L streptomycin (Gibco), and 2 mM L-glutamine (Gibco) in a humidified atmosphere at 37 °C with 5% CO2. The erianin was purchased from Sigma-Aldrich, USA, dissolved in 0.05% dimethyl sulfoxide (DMSO).
Cytotoxicity Assessment
The cytotoxicity of erianin against Eca-109 cell lines was evaluated using the MTT assay as described by Wang et al., 2022 [21]. Eca-109 cells were cultured in microtiter plates with 100 µl of MEM medium, seeded at a density ranging from 5000 to 10,000 cells per well, and incubated for 24 h. Following this, the cells were treated with various concentrations of erianin (ranging from 0.078 to 100 µM) and allowed to incubate for 24 and 48 h. Afterward, 100 µg of MTT solution was added to each well and incubated at 37 °C for 4 h. The resulting purple formazan was dissolved using 100 µl of DMSO after removing the MTT reagent, and the absorbance was measured at 570 nm using an ELISA plate reader.
Measurement of Reactive Oxygen Species (ROS)
The assay for cellular ROS detection utilized the DCFH-DA method [22]. Cells were seeded at a density of 5 × 105 cells/well in 6-well cell culture plates and incubated overnight. Subsequently, cells were treated with varying doses of erianin (4, 8, and 12 µM) for 24 h. Following treatment, cells were exposed to 10 µM DCFDA for 30 min at 37 °C. Afterward, cells were removed and washed with DPBS, and their ROS levels were assessed using a FACS Calibur flow cytometer.
Cell Cycle Analysis
Cells were first seeded into separate Petri dishes and treated with varying concentrations of erianin (4, 8, and 12 µM) for a 24-h period. Following treatment, the cells were harvested using Trypsin-EDTA and washed with PBS. Subsequently, they were placed in 15 ml conical tubes containing a growth medium. After collection, the cells were centrifuged at 300 g for 10 min at 4 °C and fixed with 70% ethanol for 30 min. Post-fixation, the cells were cleaned with PBS and stained using a PI cocktail (50 g/ml PI and 50 g/ml RNase) in darkness at room temperature (20–25 °C) for 30 min. The cell cycle analysis was performed using a FACS Calibur flow cytometer (BD Biosciences; Franklin Lakes, NJ, USA).
Apoptosis Assessment
Eca-109 cells were seeded in a 6-well cell culture plate at a density of 5 × 105 cells per well. Subsequently, the cells were exposed to varying concentrations (4, 8, and 12 µM) of Erianin. After a 24-h incubation period, the cells were harvested, washed twice with PBS, and subjected to staining in the presence of FITC, Annexin V, and 7-AAD for 15 min. This staining procedure was performed in a light-protected environment at a temperature of 20–25 °C. The assessment of apoptotic levels was conducted using the FACS Calibur flow cytometer.
Western Blot Analysis
Erianin treatment mediated numerous protein expressions were studied by western blot [23]. Cellular samples were disrupted using RIPA buffer at 4 °C for 30 min, followed by centrifugation at 15,000 g and 4 °C for 20 min to obtain protein-rich supernatant. The Bradford protein assay (Bio-Rad, Hercules, CA, USA) determined the total protein concentration of the supernatant. Protein samples were triplicate-loaded onto SDS-PAGE gels (10–12%) for electrophoretic separation and subsequently transferred to a nitrocellulose membrane. Following a 30-min blocking step in TBS-T (0.1%) Tween 20 solution with 5% skim milk, membranes were incubated overnight at 4 °C with the primary antibody. Post dual TBS-T rinses, membranes were exposed to an HRP-linked secondary antibody at room temperature for an hour. Chemiluminescence detection utilized Immobilon western chemiluminescent HRP substrate (Merck Millipore, Burlington, MA, USA).
Real-Time PCR Analysis
The isolation of total RNA from Eca-109 cells was conducted using the TRIZOL reagent (Invitrogen, Carlsbad, CA, USA), following the guidelines provided by the manufacturer. The obtained RNA exhibited an A260/280 ratio ranging from 1.8 to 2.0, as measured using the NanoDrop2000 spectrophotometer (Thermo Fischer, USA). For quantitative real-time PCR (qRT-PCR), the Stratagene Mx 3000PTM qPCR system (Stratagene, La Jolla, CA, USA) was employed. The SYBR RT-PCR kit from Takara Bio (Hilden, China) was used for the qRT-PCR assay. The thermal cycling protocol included an initial step of 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 6 s. Subsequently, a melting curve analysis was performed with temperature settings of 95 °C for 1 min, 55 °C for 30 s, and a final step at 95 °C for 30 s, as outlined in the experimental procedure.
Statistical Analysis
The data is presented as mean values ± with standard deviation (SD) and was computed utilizing GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). A one-way analysis of variance (ANOVA) was employed to detect statistically significant distinctions among the groups. Statistical significance was acknowledged at a p-value of less than 0.05. All experiments were conducted in triplicate.
Results
Erianin Elicits Cytotoxicity Effects and Induces ROS Generation in Esophageal Cancer Cell Line
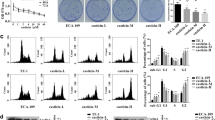
In this investigation, the impact of erianin on esophageal cancer cell was examined. Erianin exhibited a potent cytotoxic effect on the tested esophageal cancer cell lines; Fig. 1a demonstrates a concentration-dependent decrease in Eca-109 cell viability. The escalating concentrations of erianin (ranging from 0.39 to 50 µM) exhibited an enhanced cytotoxic effect on Eca-109 cells. The half-maximal inhibitory concentration (IC50) ranged within the 7 µM respectively, highlighting the potential efficacy of erianin as an anti-cancer agent. Furthermore, erianin toxicity was more pronounced in the range of 0.39–50 µM concentrations on Eca-109 cells. Subsequently, concentrations of 4, 8, and 12 µM were chosen for subsequent investigations. Moreover, the nontoxic concentration of erianin against normal NIH-3T3 cells was studied by MTT cytotoxicity assay. Figure 1b shows that erianin did not exhibit cytotoxicity in up to 100 µM concentrations against NIH-3T3 cells. Furthermore, the subsequent study explored the influence of erianin treatment led to a marked increase in the generation of reactive oxygen species (ROS) in Eca-109 cells, employing DCFH-DA staining. The results indicated a substantial increase in DCF fluorescence in Eca-109 cells following erianin treatment. The histogram presented a quantitative assessment of ROS production in terms of fluorescence intensity, as shown in Fig. 1B. This phenomenon was observed in a dose-dependent manner, suggesting a correlation between ROS production and erianin-induced cytotoxicity. Notably, the highest fluorescence intensity was observed in Erianin-treated Eca-109 cells at the concentration of 12 µM, surpassing other tested concentrations. These results emphasize erianin’s pronounced cytotoxic effects on esophageal cancer cells and its ability to trigger ROS production, suggesting its potential as a therapeutic agent for esophageal cancer treatment.
Erianin elicits cytotoxicity effects and induces ROS generation in the esophageal cancer cell line. a Erianin different concentrations ranging from 0.39 to 50 µM serially diluted, and cell cytotoxicity was evaluated for 24 h of incubation in Eca-109 cells. b Erianin and their toxicity in normal NIH-3T3 cells were studied by MTT assay. c Flow cytometry analysis indicated that erianin treatment mediated intracellular ROS measurement by DCFH-DA staining. In this investigation, results were presented as the mean accompanied by the standard deviation obtained from three separate experiments. To discern significant differences among the groups, a one-way analysis of variance (ANOVA) was applied. Statistical significance was acknowledged when the p-value was below 0.05
Erianin Induces G2/M Phase Cell Cycle Arrest in Esophageal Cancer Cells
The impact of erianin treatment on distinct stages of cell cycle arrest in Eca-109 cells was investigated via flow cytometry analysis. Figure 2 shows that erianin administration led to an accumulation of cells within the G1 phase of the cell cycle for Eca-109 cells. Furthermore, erianin treatment exhibited a concentration-dependent (4 to 12 µM) augmentation in the proportion of cells within the G1 phase, elevating the percentage from 42.72 to 51.47%. Additionally, the G2/M phase was reduced in Eca-100 cells following erianin treatment (p < 0.05). These findings suggest that erianin influence on the cell cycle might result in the arrest of cells, specifically at the G2/M phase.
Erianin Induces Apoptosis in Esophageal Cancer Cells
Erianin treatment’s impact on apoptosis in Eca-109 cells was assessed using Annexin V and PI double staining to quantify its role. In this study, the pro-apoptotic effects of erianin were explored in esophageal cancer cells over a 24-h period, utilizing concentrations spanning the range of 4, 8, and 12 µM. Erianin treatment led to a conspicuous induction of apoptosis in the targeted cancer cells shown in Fig. 3. Furthermore, the application of erianin was associated with a significant elevation in the proportions of apoptotic cells among the Eca-109 cell population (p < 0.05).
Erianin treatment associated apoptosis assessment in esophageal cancer. Eca-109 cells were treated with varying concentrations (4, 8, and 12 µM) of erianin for a 24-h incubation period, and it was subjected to staining in the presence of FITC, Annexin V to confirm the apoptosis nature of cells. The assessment of apoptotic levels was evaluated by the FACS Calibur flow cytometer
Erianin Suppressed the Phosphorylation of MAPK Proteins in Esophageal Cancer Cells
The present study investigated the modulation of MAPK (JNK, p38, and Erk1) protein signaling pathways in mediating oxidative stress. Western blot analysis assessed the phosphorylation status of JNK, p38, and ERK1 proteins in esophageal cancer cells (Eca-109). As depicted in Fig. 4, untreated Eca-109 cells exhibited enhanced levels of phosphorylated JNK, p38, and Erk1. Notably, treatment with erianin resulted in a pronounced suppressive effect on the phosphorylation status of JNK, p38, and Erk1 in Eca-109 cells (p < 0.05). This effect was evident across a spectrum of erianin concentrations, including 4, 8, and 12 µM. The concentration at which this suppressive effect was observed provides insight into the dose-dependent nature of the interaction between erianin and the MAPK signaling cascade in esophageal cancer cells. These findings suggest that erianin treatment exerts inhibitory effects on oxidative stress pathways.
Erianin suppressed the phosphorylation of MAPK proteins in esophageal cancer cells. a Western blot analysis evaluating the phosphorylated expression of Erk1, Jnk1, p38 in erianin treatment and without treated Eca-109 cells. b–d The bar diagram indicated Erk1, Jnk1, and p38 protein expression band intensities normalized by the internal control β-actin. In this investigation, results were presented as the mean accompanied by the standard deviation, obtained from three separate experiments. To discern significant differences among the groups, a one-way analysis of variance (ANOVA) was applied. Statistical significance was acknowledged when the p-value was below 0.05
Erianin Attenuated the Translocation of STAT-3 in Esophageal Cancer Cells
STAT3 is a pivotal transcription factor activated by specific upstream stimuli like ROS, interleukins, and tyrosine kinases, which modulate adverse effects on various proliferation and oncogenic proteins. Using western blotting, the impact of erianin treatment on IL-6, IL-10, JAK1, and p-STAT-3 expression was investigated in esophageal cancer cells (Eca-109). Figure 5 illustrates that untreated Eca-109 cells exhibited enhanced levels of IL-6, IL-10, JAK1, and p-STAT-3. However, treatment with erianin exhibited a notable attenuation of the expression of IL-6, IL-10, JAK1, and p-STAT-3 in Eca-109 cells (p < 0.05) across a range of erianin concentrations, including 4, 8, and 12 µM. These findings suggest that erianin treatment hinders the translocation of STAT-3, leading to the inhibition of proliferation and oxidative stress in Eca-109 cells. Notably, erianin demonstrated a particularly substantial attenuation of STAT-3 translocation at the higher concentration of 12 µM. This observation underscores the concentration-dependent nature of erianin effect on the subcellular disruption of STAT-3 in esophageal cancer cells.
Erianin attenuated the translocation of STAT-3 in esophageal cancer cells. a Western blot analysis evaluating the protein expression of IL-6, IL-10, JAK-1, and p-STAT-3 in erianin treatment and without treated Eca-109 cells. b–d The bar diagram indicated IL-6, IL-10, JAK-1, and p-STAT-3 protein expression band intensities normalized by the internal control β-actin. In this investigation, results were presented as the mean accompanied by the standard deviation, obtained from three separate experiments. To discern significant differences among the groups, a one-way analysis of variance (ANOVA) was applied. Statistical significance was acknowledged when the p-value was below 0.05
Erianin Inhibits Proliferation and Mitigates Anti-Apoptotic Protein Expression in Eca-109 Cells
The study investigated the impact of erianin treatment on the expression of key proliferative proteins (Cyclin-D1, Cyclin-E, and VEGF) in Eca-109 esophageal cancer cells through Western blotting analysis. Figure 6 indicates untreated Eca-109 cells exhibited elevated levels of these proliferative proteins. However, treatment with erianin led to the downregulation of markers (p < 0.05) associated with cell proliferation across concentrations of 4, 8, and 12 µM. Additionally, the research examined the effect of erianin treatment on apoptotic protein expression (Mcl-1 and Bcl-2) in the Eca-109 cells. Furthermore, untreated Eca-109 cells exhibited elevated levels of these anti-apoptotic proteins. Remarkably, erianin treatment showed a discernible effect on the expression of anti-apoptotic markers, specifically Mcl-1 and Bcl-2. Erianin’s application led to a marked reduction in the expression of these proteins, highlighting its potential role in counteracting cellular resistance to apoptosis in Eca-109 cells.
Erianin attenuated the proliferative protein expressions in Eca-109. a Western blot analysis evaluating the protein expression of cyclin-D1, cyclin-E1, VEGF, Mcl-1, and Bcl-2 in erianin treatment and without treated Eca-109 cells. b–f Bar diagram indicated cyclin-D1, cyclin-E1, VEGF, Mcl-1, and Bcl-2 protein expression band intensities normalized by the internal control β-actin. This study provided data using mean ± standard deviation from 3 independent experiments. A one-way analysis of variance (ANOVA) was employed to detect statistically significant distinctions among the groups. Statistical significance was acknowledged at a p-value of less than 0.05
Erianin Inhibits the Metastatic Gene Expressions in Esophageal Cancer Cells
The investigation focused on elucidating the impact of erianin on the expression of metastatic genes within esophageal cancer cells through mRNA analysis. Figure 7 demonstrated that untreated Eca-109 cells exhibited elevated levels of these metastatic gene expressions. However, treatment with erianin exhibited a discernible inhibitory effect on the expression of genes associated with metastatic processes. This effect was consistently observed across a range of erianin concentrations, including 4, 8, and 12 µM. The observed attenuation in the expression of metastatic genes underscores erianin’s potential as a regulator of cellular pathways implicated in the metastatic cascade in Eca-109 cells.
Erianin impedes metastatic gene expressions in Eca-109. RT-PCR analysis evaluating the mRNA expression of MMP-2, MMP-9, and MMP-12 in erianin treatment and without treated Eca-109 cells. The MMP-2, MMP-9, and MMP-12 mRNA expression were normalized by the internal control GAPDH. This study provided data using mean ± standard deviation from 3 independent experiments. A one-way analysis of variance (ANOVA) was employed to detect statistically significant distinctions among the groups. Statistical significance was acknowledged at a p-value of less than 0.05
Discussion
In this study, we examined the molecular mechanisms underlying the anti-cancer effects of erianin, a naturally occurring dibenzyl compound, on esophageal cancer cells. We observed that erianin could block the STAT-3 signaling pathway, leading to apoptosis in esophageal cancer cells. One of the deadliest cancers, esophageal cancer, has a poor prognosis and few available treatments [24]. STAT-3 is frequently activated in esophageal cancer, promoting tumor survival, growth, angiogenesis, and metastasis [25]. Hence, STAT-3 overexpression is thought to be a crucial target for the identification of new therapeutical molecules against esophageal cancer. Erianin belongs to A dibenzyl drug extracted from Dendrobium officinale and Dendrobium chrysotoxum, commonly used in traditional Chinese medicine [17]. In this investigation, we elucidated the capacity of erianin to enhance cellular toxicity in esophageal carcinoma cells in a dose-dependent manner. Notably, erianin exhibited an IC50 value of 7.0 µM. Moreover, the viability of human osteosarcoma and EJ bladder cancer cells diminished significantly upon exposure to erianin. This effect was observed to be both time- and dose-dependent, with an IC50 value of 65.04 mM/L after 48 h [26]. The research findings underscore that erianin’s robust anti-proliferative properties against Eca-109 cancer cells.
In response to genotoxic stress induced by various agents such as ultraviolet irradiation, alkylating agents, biphenyls, and ionizing radiation, cells undergo apoptosis and arrest in the cell cycle [27]. A multitude of phytochemicals have been identified to elicit cell cycle arrest and apoptosis in neoplastic cells [28]. Erianin, in particular, has demonstrated its ability to provoke early and late-stage apoptosis and impede cell cycle progression at the G2/M checkpoint in diverse cancer cell types [29]. Flow cytometric analyses revealed that erianin-induced G2/M phase arrest in Eca-109 cells was characterized by heightened proportions of cells in the G0/G1 and S phases, coupled with a reduction in the population of cells in the G2/M phase.
Cyclin D1 and Cyclin E1 are recognized as proliferative indicators, orchestrating the activity of cyclin-dependent kinases 4 and 6 (CDK4/CDK6) to govern the transition of the cell cycle from G1 to the S phase. Enhanced expression or activation of these proteins has been correlated with growth factor induction in human malignancies [30]. In this present investigation, our observations unveil that erianin induces a reduction in the levels of cyclin D1, cyclin E1, and VEGF within Eca-109 cells. Previously, erianin administration induced a substantial upregulation in the expression of p53, cyclin B1, p21, and p27, while concurrently leading to a significant downregulation in the mRNA levels of CDK1 and CDK7 [31]. These observations underscore the capability of erianin to enhance the expression of p21, p27, and related genes, resulting in the suppression of cyclin-CDK complex activation and the subsequent induction of G2/M phase cell cycle arrest [31].
The MAPK pathway represents a conserved signaling cascade governing cellular responses to extracellular cues such as stress, cytokines, and growth factors. Dysregulation of this pathway has been implicated in various pathologies, notably cancer [32]. Inhibition of MAPK pathway in esophageal cancer cells using erianin was observed to impede MAPK protein phosphorylation, potentially via oxidative stress attenuation. Western blot analysis indicated significant reductions in phosphorylated JNK, p38, and ERK-1 proteins. These results concur with earlier studies demonstrating erianin capacity to hinder ERK pathway activation in diverse cancer cells, yielding various biological effects, notably anti-tumor properties [33]. Prior research supports erianin apoptosis induction, along with its capability to curb the proliferation and migration of diverse cancer cells [18, 19]. Given these findings, erianin emerges as a promising candidate for esophageal cancer therapy through MAPK pathway modulation.
Intracellularly, cellular systems maintain homeostasis of reactive oxygen species (ROS). Elevated ROS levels, however, can induce oxidative stress, eliciting detrimental effects on cellular macromolecules such as proteins, lipids, and DNA [34]. The resultant cellular damage may lead to cell cycle arrest, programmed cell death, or apoptosis [35]. Erianin, a phytoconstituent, has been observed to enhance ROS generation [26]. Nonetheless, in osteosarcoma cells, the suppressive impact of erianin on cell proliferation, apoptosis, and autophagy can be significantly counteracted by pre-administration of the ROS-inhibiting agent, NAC [36]. Our experimental findings demonstrate that erianin treatment in esophageal cancer cells escalates levels of ROS in a dose-dependent manner, leading to diminished antioxidant capacity and heightened lipid peroxidation, ultimately culminating in a state of oxidative stress. Notably, Eca-109 cancer cells exhibited a pronounced elevation in ROS levels upon exposure to 12 µM erianin.
Tyrosine kinases, interleukins, and interferons are upstream activators of the oncogenic transcription factor STAT3. Proteins belonging to the MAPK family trigger the activation of STAT3 signaling in various cell types. Erk1 phosphorylates STAT3 at serine 727 (Ser727), promoting the translocation of STAT3 both in in vitro and in vivo models [37]. Negative regulators of STAT3 activation also play a role in controlling the transcription of various proliferative genes [14]. Previous study demonstrates that inhibiting the overexpression of IL-6 and JAK-1 in ovarian cancer cells, which initiate STAT-3 translocation, results in the modulation of proliferation, angiogenesis, and apoptosis [38]. In this study, we have observed that erianin treatment substantially inhibits the JAK/STAT-3 signaling by observing the decreased expression of IL-6, IL-10, JAK-1, and p-STAT-3 in Eca-109. Previously, plumbagin belonged to the natural drug that inhibits esophageal cancer proliferation by diminishing the expression of STAT-3 signaling. Hence, natural drugs can have a strong binding interaction with STAT-3, suppressing the expressions [39].
Matrix metalloproteinases (MMPs) hold a pivotal role in facilitating tumor metastasis, as they govern the degradation of the extracellular matrix and modulation of cell adhesion mechanisms, thereby promoting the invasiveness and metastatic potential of tumor cells [40]. The overexpression of MMPs has been regulated by the STAT-3, and numerous reports have documented that inhibition of STAT-3 plays to downregulates the expression of MMPs. In this study, we have obtained that erianin treatment effectively downregulated the mRNA expression of MMP-2, MMP-9, and MMP-12 in Eca-109 cells. Previously, STAT3 null does not induce the expression of VEGF, MMP-2, and MMP-9 in hepatocellular carcinoma cells [41]. Hence STAT-3 inhibitions are a crucial target for the treatment of esophageal cancer. Based on the above results, erianin, a natural product, substantially inhibits the phosphorylation of STAT-3, thereby impeding the proliferation, metastasis, and anti-apoptosis marker expressions in esophageal cancer. These qualities make erianin a promising and novel therapeutic option for esophageal cancer, addressing the current lack of treatment choices. As such, erianin emerges as a potential candidate for combating tumor invasion, metastasis, and anti-apoptotic processes, suggesting potential clinical applications in the future. Nevertheless, it’s important to note that further research using experimental animal models is necessary to delve deeper into erianin’s effects and recognize that STAT-3 inhibition does have its limitations. Additional investigations into erianin’s drug metabolism, pharmacokinetics, and pharmacodynamics are essential to determine its suitability for use in esophageal cancer models and its potential therapeutic value.
Conclusion
This study finding concluded that erianin exhibited significant inhibitory effects on the proliferation and migratory capabilities of the esophageal cancer cell line. We have observed that erianin treatment substantially induces cytotoxicity, ROS, and apoptosis in esophageal cancer cells. Moreover, erianin inhibits the proliferation, metastatic, and anti-apoptosis genes by diminishing the expression of IL-6/JAK1/STAT3 expression in esophageal cancer. The outcomes of this investigation suggest that erianin holds considerable promise as a prospective therapeutic agent for addressing esophageal cancer, a malignancy characterized by its aggressive nature and limited therapeutic avenues. Nonetheless, further comprehensive preclinical and clinical investigations are imperative to validate erianin therapeutic potential and ascertain its safety profile and efficacy in human subjects.
Data Availability
All data displayed in this publication, including Supporting Information, are available from the corresponding author upon request.
References
Li, J., Xu, J., Zheng, Y., Gao, Y., He, S., Li, H., Zou, K., Li, N., Tian, J., Chen, W., & He, J. (2021). Esophageal cancer: Epidemiology, risk factors and screening. Chinese Journal of Cancer Research, 33(5), 535.
He, F., Wang, J., Liu, L., Qin, X., Wan, Z., Li, W., & Ping, Z. (2021). Esophageal cancer: Trends in incidence and mortality in China from 2005 to 2015. Cancer Medicine, 10(5), 1839–1847.
Asombang, W., Chishinga, N., Nkhoma, A., Chipaila, J., Nsokolo, B., Manda-Mapalo, M., Montiero, J. F. G., Banda, L., & Dua, K. S. (2019). Systematic review and meta-analysis of esophageal cancer in Africa: Epidemiology, risk factors, management and outcomes. World Journal of Gastroenterology, 25(31), 4512–4533.
Ilson, D. H. (2008). Esophageal cancer chemotherapy: Recent advances. Gastrointest Cancer Research, 2(2), 85–92.
Then, E. O., Lopez, M., Saleem, S., Gayam, V., Sunkara, T., Culliford, A., & Gaduputi, V. (2020). Esophageal cancer: An updated surveillance epidemiology and end results database analysis. World Journal of Oncology, 11(2), 55–64.
Balupillai, A., Kanimozhi, G., Khan, H. A., Alhomida, A. S., & Prasad, N. R. (2020). Opuntiol prevents photoaging of mouse skin via blocking inflammatory responses and collagen degradation. Oxidative Medicine and Cellular Longevity, 2020.
Trachootham, D., Alexandre, J., & Huang, P. (2009). Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nature Reviews Drug Discovery, 8(7), 579–591.
Wagner, E. F., & Nebreda, A. R. (2009). Signal integration by JNK and p38 MAPK pathways in cancer development. Nature Reviews Cancer, 9(8), 537–549.
Zhang, W., & Liu, H. T. (2002). MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research, 12(1), 9–18.
Peng, Q., Deng, Z., Pan, H., Gu, L., Liu, O., & Tang, Z. (2018). Mitogen-activated protein kinase signaling pathway in oral cancer. Oncology Letters, 15(2), 1379–1388.
Agilan, B., Rajendra Prasad, N., Kanimozhi, G., Karthikeyan, R., Ganesan, M., Mohana, S., Velmurugan, D., & Ananthakrishnan, D. (2016). Caffeic acid inhibits chronic UVB-induced cellular proliferation through JAK‐STAT 3 signaling in mouse skin. Photochemistry and Photobiology, 92(3), 467–474.
Roos, W. P., & Kaina, B. (2006). DNA damage-induced cell death by apoptosis. Trends in Molecular Medicine, 12(9), 440–450.
Zhang, F., Wang, Z., Fan, Y., Xu, Q., Ji, W., Tian, R., & Niu, R. (2015). Elevated STAT3 signaling-mediated upregulation of MMP-2/9 confers enhanced invasion ability in multidrug-resistant breast cancer cells. International Journal of Molecular Sciences, 16(10), 24772–24790.
Tolomeo, M., & Cascio, A. (2021). The multifaced role of STAT3 in cancer and its implication for anticancer therapy. International Journal of Molecular Sciences, 22(2), 603.
He, S., Xu, J., Liu, X., & Zhen, Y. (2021). Advances and challenges in the treatment of esophageal cancer. Acta Pharmaceutica Sinica B, 11(11), 3379–3392.
Singh, R. P., Dhanalakshmi, S., & Agarwal, R. (2002). Phytochemicals as cell cycle modulators a less toxic approach in halting human cancers. Cell Cycle, 1(3), 155–160.
Yan, L., Zhang, Z., Liu, Y., Ren, S., Zhu, Z., Wei, L., Feng, J., Duan, T., Sun, X., Xie, T., & Sui, X. (2022). Anticancer activity of erianin: Cancer-specific target prediction based on network pharmacology. Frontiers in Molecular Biosciences, 9, 862932.
Hong, J., Xie, Z., Yang, F., Jiang, L., Jian, T., Wang, S., Guo, Y., & Huang, X. (2022). Erianin suppresses proliferation and migration of cancer cells in a pyruvate carboxylase-dependent manner. Fitoterapia, 157, 105136.
Xiang, Y., Chen, X., Wang, W., Zhai, L., et al. (2021). Natural product erianin inhibits bladder cancer cell growth by inducing ferroptosis via NRF2 inactivation. Frontiers in Pharmacology, 12, 775506.
Li, M., He, Y., Peng, C., Xie, X., & Hu, G. (2018). Erianin inhibits human cervical cancer cell through regulation of tumor protein p53 via the extracellular signal-regulated kinase signaling pathway. Oncology Letters, 16(4), 5006–5012.
Wang, S., Jiang, K., Muthusamy, R., Kalaimani, S., Selvababu, A. P., Balupillai, A., Narenkumar, J., & Jeeva Jeevakaruniyam, S. (2022). Protosappanin-B suppresses human melanoma cancer cell growth through impeding cell survival, inflammation and proliferative signaling pathways. Process Biochemistry, 122, 78–85.
NilamberLal Das, R., Muruhan, S., Nagarajan, R. P., & Balupillai, A. (2019). Naringin prevents ultraviolet-B radiation‐induced oxidative damage and inflammation through activation of peroxisome proliferator‐activated receptor γ in mouse embryonic fibroblast (NIH‐3T3) cells. Journal of Biochemical and Molecular Toxicology, 33(3), e22263
Subramaniyan, S., Kamaraj, Y., Kumaresan, V., Kannaiyan, M., David, E., Ranganathan, B., Selvaraj, V., & Balupillai, A. (2022). Green synthesized zinc oxide nanoparticles induce apoptosis by suppressing PI3K/Akt/mTOR signaling pathway in osteosarcoma MG63 cells. Global Translational Medicine, 1(1), 1–2.
Zhang, Y. (2013). Epidemiology of esophageal cancer. World Journal of Gastroenterology, 19(34), 5598–5606.
Zhang, N., Zhang, M., Wang, Z., Gao, W., & Sun, Z. G. (2020). Activated STAT3 could reduce survival in patients with esophageal squamous cell carcinoma by up-regulating VEGF and cyclin D1 expression. Journal of Cancer, 11(7), 1859–1868.
Wang, H., Zhang, T., Sun, W., Wang, Z., Zuo, D., Zhou, Z., Li, S., Xu, J., Yin, F., Hua, Y., & Cai, Z. (2016). Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death & Disease, 7(6), e2247.
Thin, T. H., Li, L., Chung, T. K., Sun, H., & Taneja, R. (2007). Stra13 is induced by genotoxic stress and regulates ionizing-radiation-induced apoptosis. Embo Reports, 8(4), 401–407.
Kaur, V., Kumar, M., Kumar, A., Kaur, K., Dhillon, V. S., & Kaur, S. (2018). Pharmacotherapeutic potential of phytochemicals: Implications in cancer chemoprevention and future perspectives. Biomedicine & Pharmacotherapy, 97, 564–586.
Zhang, Y., Zhang, Q., Wei, F., & Liu, N. (2019). Progressive study of effects of erianin on anticancer activity. Oncotargets and Therapy, 12, 5457–5465.
Fassl, A., Geng, Y., & Sicinski, P. (2022). CDK4 and CDK6 kinases: From basic science to cancer therapy. Science, 375(6577), eabc1495.
Xu, Y., Fang, R., Shao, J., & Cai, Z. (2021). Erianin induces triple-negative breast cancer cells apoptosis by activating PI3K/Akt pathway. Bioscience Reports, 41(6), 20210093.
Katz, M., Amit, I., & Yarden, Y. (2007). Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochimica Et Biophysica Acta, 1773(8), 1161–1176.
Trapika, I. G. M. G. S. C., Liu, X. T., Chung, L. H., Lai, F., Xie, C., Zhao, Y., Cui, S., Chen, J., Tran, C., Wang, Q., Zhang, S., Don, A. S., Li, G. Q., Hanrahan, J. R., & Qi, Y. (2021). Ceramide regulates anti-tumor mechanisms of erianin in androgen-sensitive and castration-resistant prostate cancers. Frontiers in Oncology, 11, 738078.
Balupillai, A., Prasad, R. N., Ramasamy, K., Muthusamy, G., Shanmugham, M., Govindasamy, K., & Gunaseelan, S. (2015). Caffeic acid inhibits UVB-induced inflammation and photocarcinogenesis through activation of peroxisome proliferator‐activated receptor‐γ in mouse skin. Photochemistry and Photobiology, 91(6), 1458–1468.
Qiao, Q., Du, Y., & Xie, L. (2022). Research advances of erianin: Source, production, biological activities, and pharmacological properties. Pharmacological Research-Modern Chinese Medicine, 2, 100059.
Niu, J., Yan, T., Guo, W., Wang, W., & Zhao, Z. (2019). Insight into the role of autophagy in osteosarcoma and its therapeutic implication. Frontiers in Oncology, 9, 1232.
Chung, J., Uchida, E., Grammer, T. C., & Blenis, J. (1997). STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Molecular and Cellular Biology, 17, 6508–6516.
Liang, R., Chen, X., Chen, L., Wan, F., Chen, K., Sun, Y., & Zhu, X. (2020). STAT3 signaling in ovarian cancer: A potential therapeutic target. Journal of Cancer, 11(4), 837–848.
Cao, Y. Y., Yu, J., Liu, T. T., Yang, K. X., Yang, L. Y., Chen, Q., Shi, F., Hao, J. J., Cai, Y., Wang, M. R., Lu, W. H., & Zhang, Y. (2018). Plumbagin inhibits the proliferation and survival of esophageal cancer cells by blocking STAT3-PLK1-AKT signaling. Cell Death and Disease, 9(2), 17.
Cabral-Pacheco, G. A., Garza-Veloz, I., Castruita-De la Rosa, C., Ramirez-Acuña, J. M., Perez-Romero, B. A., Guerrero-Rodriguez, J. F., Martinez-Avila, N., & Martinez-Fierro, M. L. (2020). The roles of matrix metalloproteinases and their inhibitors in human diseases. International Journal of Molecular Sciences, 21(24), 9739.
Wang, X. H., Liu, B. R., Qu, B., Xing, H., Gao, S. L., Yin, J. M., Wang, X. F., & Cheng, Y. Q. (2011). Silencing STAT3 may inhibit cell growth through regulating signaling pathway, telomerase, cell cycle, apoptosis and angiogenesis in hepatocellular carcinoma: Potential uses for gene therapy. Neoplasma, 58(2), 158–171.
Author information
Authors and Affiliations
Contributions
AH: investigation; methodology; writing; original draft, LK: conceptualization; funding acquisition; writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, A., Li, K. Erianin Impedes the Proliferation and Metastatic Migration Through Suppression of STAT-3 Phosphorylation in Human Esophageal Cancer Cells. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-023-04829-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04829-8